Research Article - Archives of General Internal Medicine (2023) Volume 7, Issue 6
The Effectiveness of Different Current Interventions for Managing Symptoms of Irritable Bowel Syndrome (IBS): A Systematic Review
Mihirkumar P. Parmar1, Raja Hamsa Chitturi2, Lyluma Ishfaq3, Mahek Fatima4, Hima Varsha kandepi5, Roopeessh vempati6, Mayank Jaitwar7, Khushi Dharmendra Mehta8, Anubhuti Vashyani9, Vishal Venugopal101Gujarat Medical Education and Research Society, Vadnagar, Gujarat, India
2Great Eastern Medical School, Srikakulam, India
3Government Medical college, Srinagar, India
4Osmania Medical College, Hyderabad, India
5Nri Medical College of Sciences, India
6Gandhi Medical College, Secunderabad, India
7Indira Gandhi Government Medical College, Nagpur, India
8Rajarshee Chhatrapati Shahu Maharaj Government Medical College, Kolhapur, India
9Indira Gandhi Medical College, Shimla, India
10Bhaarath Medical College and Hospital, Chennai, India
- *Corresponding Author:
- Mihirkumar P. Parmar
Department of Internal Medicine
Gujarat Medical Education and Research Society, Vadnagar, India
E-mail: mmihir981@gmail.com
Received: 23-Nov-2023, Manuscript No. AAAGIM-23-119274; Editor assigned: 27- Nov -2023, PreQC No. AAAGIM-23-119274(PQ); Reviewed: 12-Dec-2023, QC No. AAAGIM-23-119274; Revised: 15- Dec -2023, Manuscript No. AAAGIM-23-119274(R); Published: 22- Dec-2023, DOI:10.35841/aaagim-7.6.201
Citation: Parmar M.P., Chitturi R. H., et al. The effectiveness of different current interventions for Managing symptoms of Irritable Bowel Syndrome (IBS): A systematic review. Arch Gen Intern Med. 2023;7(6):201
Abstract
Chronic stomach pain and irregular bowel motions are symptoms of Irritable Bowel Syndrome (IBS), a functional gastrointestinal disorder. It affects 20% of the general population, primarily women, and significantly influences healthcare expenses and quality of life. Diarrhea-related IBS, constipation-related IBS, constipation-and-diarrhea-related IBS mixed, and unclassified IBS are the different IBS types. The pathophysiology of IBS is currently believed to be influenced by changes in neurohumoral mechanisms, psychosocial factors, visceral hypersensitivity, bacterial overgrowth, hereditary factors, gastrointestinal motility, and immune system variables. One or more of these etiologic factors may interact. The therapeutic approach includes both non-pharmacologic therapies and pharmacotherapy. The choice of intervention depends on the predominant symptom, and a prescribed time point should be planned for effectiveness evaluation and dose adjustment. The most recent developments in the various interventions for IBD are discussed in this systematic review.
Keywords
Irritable bowel syndrome, constipation, diarrhea, stomach pain, hereditary.
Introduction
Between 7 to 10% of the general population worldwide suffers from gastrointestinal functional illnesses, with Irritable Bowel Syndrome (IBS) having the highest frequency [1]. Function problems of the digestive tract are thought to be the cause of approximately 40% of gastroenterology consultations [2]. The majority of IBS treatment recommendations are still centered on symptom control, which is frequently ineffective. Research on the subject indicates that fewer than 50% of patients are thought to be satisfied with the mainstream care they receive, with many turning to alternative medical remedies [3].
Over the years, researchers and clinicians have explored various interventions to alleviate the distressing symptoms experienced by individuals with IBS. These interventions span a diverse spectrum, including dietary modifications, pharmacological treatments, psychological therapies, and complementary and alternative therapies [4-7]. However, the effectiveness of these interventions remains variable, and their comparative benefits and limitations are not always well established.
This systematic review addresses the need to comprehensively evaluate the interventions available for managing IBS symptoms. By rigorously assessing the existing body of literature, we aim to synthesize the evidence surrounding the efficacy of various interventions in relieving individuals suffering from IBS. This review will delve into randomized controlled trials, cohort studies, and clinical trials that have investigated the impact of interventions on symptom severity, quality of life, and overall well-being. Through a meticulous literature analysis, we seek to shed light on the relative merits of different interventions, acknowledging their potential mechanisms of action and the patient populations that might benefit most from them. By critically appraising the strengths and limitations of each intervention, we hope to guide clinicians and patients in making informed decisions about suitable management strategies for IBS.
The insights gained from this systematic review can potentially contribute to refining clinical guidelines for IBS management and pave the way for further research in the field. As we delve into the nuances of each intervention’s impact on IBS symptoms, we aim to provide a comprehensive resource for healthcare professionals and individuals navigating the complex landscape of IBS management. Through our endeavor, we aim to enhance the understanding of effective interventions and ultimately improve the quality of life for those living with IBS.
Methodology
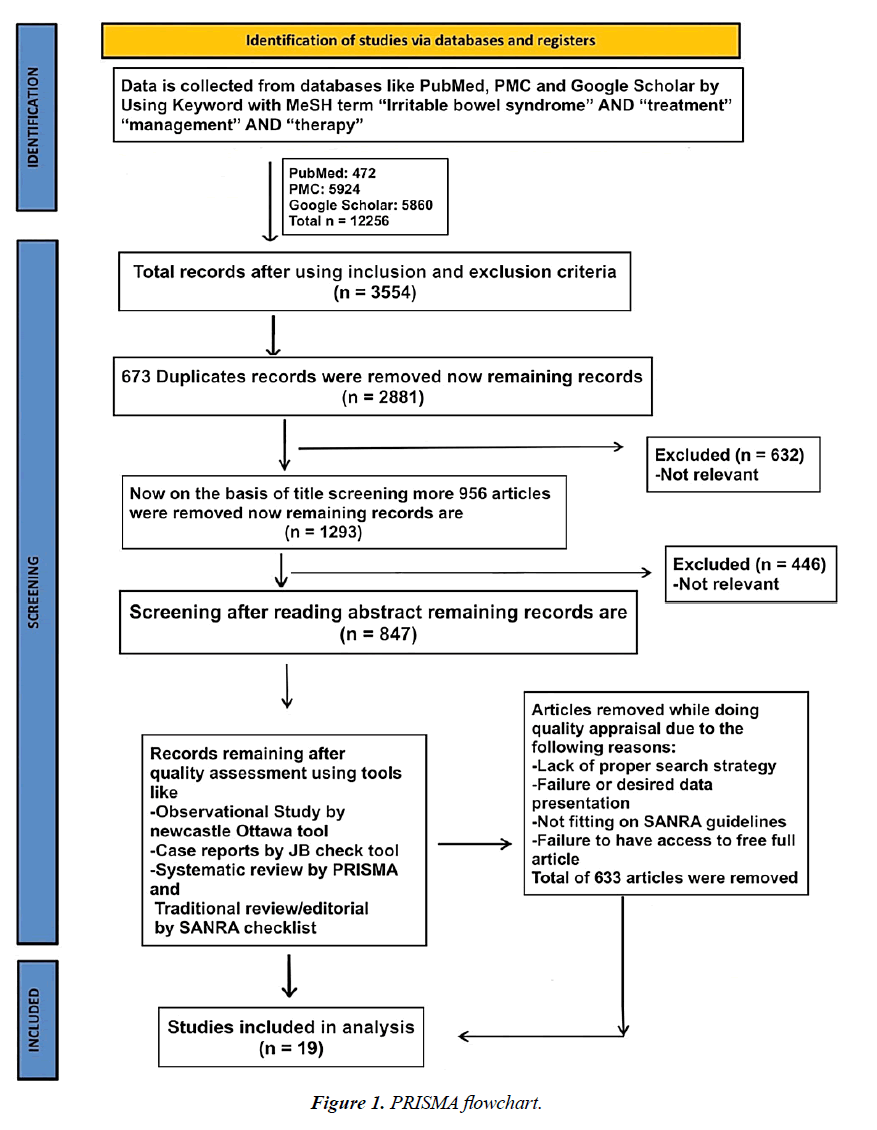
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting our systematic review (Figure 1).
Data Extraction and Quality Assessment
Two authors independently extracted data from the National Library of Medicine (PubMed), PubMed Central (PMC), and Google Scholar using the following Medical Subject Headings (MeSH) terms with keywords: “irritable bowel syndrome” AND “treatment,” “management” AND “therapy,” and similar phrases. The total number of articles found in electronic databases was 12,256. Studies included clinical trials, controlled clinical trials, randomized controlled trials, observational studies, case-control studies, prospective cohort studies, population-based cohort studies, and cross-sectional studies. The Newcastle-Ottawa Scale was used to evaluate the listed studies' quality. Three key factors are used to assign stars on this scale: the choice of study groups, group comparability, and exposure/outcome determination. The scale provides a uniform assessment of study quality across various research papers, making it easier to identify bias risks in studies (Table 1-2).
| Author(s) | WHO region | Country | The focus of the study | Findings | Key observations |
|---|---|---|---|---|---|
| Howell et al. | United Kingdom | This randomized controlled multicenter trial sought to ascertain enterosgel’s (polymethylsiloxane polyhydrate) effectiveness and safety in IBS-D patients | Stool frequency (treatment impact = -0.32 (-0.62 to -0.02)), urgency (treatment = effect -0.59 (-0.85 to -0.33)), stool consistency (48.5% vs. 32.5%, p = 0.0001), stomach pain (53.3% vs. 40.2%, p = 0.003), and consistency (treatment = effect -0.32 (-0.62 to -0.02). After receiving open-label care, 60% of patients reported adequate symptom alleviation | Enterosgel offers an alternative to the few available treatments for IBS-D and is safe and effective for this condition | |
| El Salhy et al. | European region | Norway | The goal of the study was to determine whether patients with severe and moderate IBS symptoms responded differently to FMT | After FMT, patients with S-IBS-S showed lower levels of Eubacterium rectale and greater levels of Eubacterium siraeum than patients with Mo-IBS-S | Compared to patients with Mo-IBS-S, individuals with S-IBS-S had a higher response rate to FMT and noticeably better quality of life and fatigue |
| Oh et al. | East Asian region | Korea | The study aimed to examine how a synbiotic formulation containing Lactobacillus paracasei DKGF1 and Opuntia humifusa affects the gastrointestinal symptoms of elderly IBS patients | In the synbiotic group, responder rates were substantially greater (51.5%) than in the placebo group (23.5%) (p = 0.017). Both psychological well-being (26.4% vs. 60.6%) and abdominal discomfort (58.8% vs. 81.8%) significantly improved in the synbiotic group (p = 0.038 and p = 0.004, respectively). Compared to the placebo, the synbiotics considerably reduced the symptoms of constipation and stomach pain (responder rates = 22.2% vs. 85.7%, p = 0.04). In neither group were there any unfavorable incidents | This new synbiotic supplement containing Lactobacillus paracasei DKGF1 and Opuntia humifusa can potentially relieve abdominal symptoms in elderly IBS patients |
| Wang et al. | Western Pacific region | China | To compare the effects of MM with those of placebo moxibustion in the treatment of IBS-D. | The response rate in the MM group was higher than the PM group following therapy at week six (81.58% vs. 36.84%), with an estimated difference of 44.74 (95% CI = 23.46 to 66.02, p = 0.001). No participant had any serious negative effects | The results imply that modest moxibustion, with benefits lasting up to 12 weeks, may be more effective than placebo moxibustion for the treatment of IBS-D |
| Ricci et al. | European region | Italy | Effectiveness of geraniol treatment for IBS | When compared to the placebo group, patients receiving LAGS had significantly fewer IBS symptoms, as measured by the IBS-SSS (195 vs. 265, p = 0.001). Due mostly to the IBS mixed subtype, the rate of responders according to the IBS-SSS (reduction of 50 points) was substantially greater in the geraniol group compared to the placebo group (52.0% vs. 16.7%, p = 0.009). After geraniol administration, the microbiota composition underwent noticeable changes | In addition to improving the gut microbiota composition, LAGS was successful in treating the full range of IBS symptoms, particularly for the IBS mixed subtype |
| Mourey et al. | European region | France | To verify Saccharomyces cerevisiae I-3856’s effectiveness in treating IBS-C’s gastrointestinal symptoms | The overall QOL score was substantially higher in the probiotic group than in the placebo group after eight weeks of supplementation (p = 0.047, 95% CI = 3.86 (0.52; 7.20)). Additionally, exploratory analyses revealed statistically significant and clinically pertinent improvements in QOL scores for abdominal pain responders compared to non-responders | The findings of this clinical trial supported S. cerevisiae I-3856’s ability to reduce stomach pain in IBS-C patients. Relief from abdominal pain was linked to a higher quality of life |
| D'Silva et al. | Region of the Americas | Canada | Effectiveness, viability, and security of yoga instruction given online to IBS patients | IBS-SSS considerably declined in the treatment group but not in the control group (change = 22.6, p = 0.277; change = 54.7, p = 0.028) | Yoga that is virtually provided is secure, practical, and helpful for easing IBS symptoms. The intervention did not outperform a control group that merely received guidance in terms of the primary endpoint |
| Lövdahl et al. | Region of the Americas | USA | Individual versus group hypnotherapy for IBS | Without obvious variations between the groups, extracolonic symptoms, psychological symptoms, and QOL also improved. After treatment, 69% of patients receiving solo hypnosis and 57% of those receiving group hypnotherapy were responders (p = 0.25). At the follow-up, improvements in symptoms were also noted | Individual or group sessions of nurse-administered gut-directed hypnotherapy reduce IBS symptoms and enhance psychological symptoms and QOL. A powerful substitute that would allow more patients to benefit from the therapy is group hypnosis |
| Carbone et al. | European region | Belgium | The impact of OB versus a smartphone FODMAP-lowering diet on IBS symptoms in general practice | After eight weeks, the responder rate with diet was higher than that with OB (71% (155/218) vs. 61% (133/217), p = 0.03, and it was even more pronounced in Rome (77% (118/153) vs. 62% (98/158), p = 0.004 | A FODMAP-lowering diet was more effective than a spasmolytic medication in treating IBS symptoms in people receiving primary care. In primary care, a FODMAP-reduced diet ought to be the first line of treatment for IBS |
| Ebrahimloee et al. (2022) | Eastern Mediterranean region | Iran | This investigation examined the impact of the Benson relaxation technique on the degree of IBS symptoms and QOL in young patients | Prior to the intervention, the experimental group had lower mean values for QOL (p = 0.05). In the experimental group, children with IBS had a mean symptom severity score of 13.88; this changed to 9.83 in the post-test, showing a significant difference (p = 0.000) | Children with IBS may benefit from the non-pharmacological Benson relaxation technique to reduce the severity of their symptoms and enhance their QOL |
| Huang et al. | Western Pacific region | China | To examine the potential causes and long-term consequences of TEA in individuals with IBS-C | TEA reduced abdominal pain and constipation. Following the treatment, the TEA group experienced more CSBMs per week than the sham-TEA group did (3.5 1.6 vs. 2.3 0.6, p = 0.002). Both the IBS-SSS score (p = 0.025) and the visual analog scale pain score (p = 0.002) showed similar results. Additionally, the QOL for patients with constipation significantly improved | By increasing colon transit and decreasing rectal feeling, TEA reducing constipation and IBS symptoms. The use of autonomic systems may achieve this effect |
| Menon et al. | Southeast Asian region | India | To compare the effectiveness of psyllium husk to a placebo in treating children with IBS | At four weeks, there was a significant difference between psyllium and placebo in terms of the median interquartile range (IQR) of total IBS-SSS (75 (42.5–140) vs. 225 (185-270); p = 0.001). Similar to how 9.7% of Group B achieved remission, 43.9% of Group A did (IBS-SSS 75 (p = 0.0001)). The risk ratio between Group A and Group B was 0.64, and the absolute risk reduction was 32% (NNT = 3). The mean difference in IBS-SSS between Group A and Group B was -122.85 | When used as a short-term treatment for pediatric IBS, psyllium husk is superior to a placebo |
| Jamalizadeh et al. | Eastern Mediterranean region | Iran | Zataria multifloraBoiss and Trachyspermum copticum (L.) have been clinically evaluated for their effects on individuals with IBS | When compared to the placebo and mebeverine groups after the fourth week of the intervention, the ZT group’s symptoms of pain, bloating, and reflux showed a significant decrease (p = 0.05). In addition, compared to the other two groups, individuals in the ZT group reported significantly less weariness (p = 0.05) | The favorable impact of ZT on IBS symptoms, including discomfort, bloating, constipation, and bowel motions, was confirmed by this study |
| Touny et al. | European region | Sweden | IBS ROSE-010, a GLP-1 receptor agonist, pain alleviation, and pain intensity response | Maximum pain alleviation from ROSE-010 was achieved at 300 mg compared to 100 mg and placebo at 120 minutes after injection. Age and body mass index had no effect on how well the treatment worked; females experienced a higher pain reduction than males. In contrast to diarrhea-dominant and unspecified IBS, IBS-C, and IBS-M showed the greatest improvement from IBS pain | Female participants are more likely than male participants to respond to ROSE-010 100 and 300 mg to achieve significant IBS pain relief. The greater dose provided the most pain alleviation after 120 minutes, but it was also associated with an increased incidence of nausea. IBS-C and IBS-M subgroups showed the greatest improvement in IBS pain attacks, indicating that they are the best ROSE-010 responders |
| IBS-D: irritable bowel syndrome with diarrhea; FMT: fecal microbiota transplantation; KGF: keratinocyte growth factor; MM: mild moxibustion; IBS-C: irritable bowel syndrome with constipation; QOLS: quality of life scale; FODMAP: fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; TEA: transcutaneous electrical acustimulation; IQR: interquartile range; ZT: Zataria multiflora boiss and Trachyspermum copticum; GLP-1: glucagon-like peptide-1; IBS-M: irritable bowel syndrome mixed type. | |||||
Table 1. Effectiveness of different interventions for managing symptoms of IBS.
| Citation | Selection | Exposure | Outcome | Overall |
|---|---|---|---|---|
| Howell CA et al. | 3 | 1 | 2 | Fair |
| El Salhy M. et al. | 3 | 2 | 3 | Good |
| Oh JH et al. | 2 | 1 | 3 | Fair |
| Wang Z. et al. | 3 | 2 | 2 | Good |
| Chiara Ricci et al. | 2 | 2 | 3 | Good |
| Florian Mourey et al. | 3 | 2 | 3 | Good |
| Adrijana D'Silva et al. | 3 | 2 | 3 | Good |
| Jenny Lövdahl et al. | 2 | 2 | 3 | Good |
| Florencia Carbone et al. | 3 | 1 | 2 | Fair |
| Saba Ebrahimloee et al. | 2 | 2 | 3 | Good |
| Zhihui Huang et al. | 2 | 2 | 3 | Good |
| Jagadeesh Menon et al. | 2 | 1 | 3 | Fair |
| Hossein Jamalizadeh et al. | 3 | 2 | 3 | Good |
| Aya, Touny, et al. | 3 | 1 | 3 | Good |
Table 2. Quality Assessment.
Inclusion and Exclusion Criteria
We reviewed all full-text papers, human subject studies, and English-language papers for our analysis. The last three years, from 2021 to 2023, saw the publication of papers examining the efficacy of various treatments for treating the symptoms of IBS.
Articles that did not include people or whose full text could not be retrieved were excluded. Clinical reviews, meta-analyses, and systematic reviews were not taken into consideration.
Results
Each author reviewed the papers, and those irrelevant to our topic were manually removed. Table 1 provides a summary of studies showing the effectiveness of different interventions for managing symptoms of IBS.
Discussion
Irritable Bowel Syndrome (IBS) is a common gastrointestinal disorder characterized by abdominal pain, bloating, and changes in bowel habits. Various interventions have been employed to manage its symptoms, with varying degrees of effectiveness. Some of these interventions include emerging interventions, dietary modifications, pharmacological treatments, psychological therapies, and lifestyle changes like the following:
Enterosgel: Enterosgel can improve stool consistency, stomach pain, stool frequency, and urgency. So for IBS-D diarrhea management, enterosgel is one of the safe and successful drugs [8]. The low FODMAP diet and probiotics downregulate pathobionts and upregulate beneficial bacteria (lactobacilli, bifidobacteria) to relieve mild IBS symptoms. Also, Fecal microbiota transplantation can also decrease symptoms [9]. The synbiotic can also reduce constipation and stomach pain [10]. The effect of mild moxibustion (MM) on IBS-D is not confirmed. Long-term MM data were unavailable. MM can treat IBS-D better than a placebo, but we need Larger group trials, including IBS-C, IBS-M, and IBS-U patients, to evaluate MM's mechanism of action and efficacy [11].
Geraniol: Geraniol is anti-inflammatory, anti-microbial, and gut-flora-beneficial. The study assessed Rome III-compliant patients. IBS patients had an anti-inflammatory loop between the gut microbiota and low-grade gastrointestinal inflammation. Biointestil, a patented low-absorbable geraniol. Geraniol exhibited a significantly higher IBS response rate than placebo due to the IBS mixed subtype. The gut microbiota was assessed. The first investigation of geraniol’s GM and IBS efficacy As a first-line IBS treatment, peppermint oil causes heartburn, dry mouth, and belching. geraniol's antispasmodic effects and GM variations may explain certain clinical outcomes. In the past, geraniol reduced AMPAR activation, desensitization, and inactivation, protecting neurons. Many rapid excitatory neurotransmissions use AMPARs. Geraniol may increase GM and alleviate IBS symptoms, notably in IBS-M [12]. Saccharomyces cerevisiae I-3856 for IBS-C can be helpful for moderate stomach pain. S. cerevisiae reduces stomach pain and increases quality of life [13].
Non-pharmacological Interventions: yoga can treat psychological distress-related IBS symptoms. The primary target is IBS improvement, while secondary is to increase QOL and to decrease stress, fatigue, depression, anxiety, etc. Thus, yoga aids IBS. Yoga exhibited clear results, but more research on the mechanism of action [14]. nurse-assisted gut-directed hypnotherapy for IBS patients. In secondary or tertiary care, nurse-administered gut-directed hypnotherapy was tested individually or in groups for irritable bowel syndrome (IBS) patients. individual hypnosis and group hypnosis. Both forms of hypnotherapy improved IBS, extracolonic, psychosocial, and quality of life, with no significant differences between groups. Group hypnosis improved symptoms and had permanent therapeutic effects after one year. Individual hypnotherapy improved anxiety and depression levels more, but both groups reduced IBS Severity Scoring System scores similarly. A 6-month follow-up confirmed positive results [15].
The FODMAP-Lowering Diet: FODMAP-lowering diet to Otilonium Bromide (OB) for IBS. FODMAP-lowering diets improve primary care IBS symptoms better than otilonium bromide. The FODMAP-lowering diet may be the first-line treatment for IBS in primary care [16].
Benson Relaxation Technique: Benson relaxation technique can improve the quality of life and reduce symptoms in children with IBS without medication. In clinical contexts, this relaxation technique is recommended as an additional intervention [17].
Transcutaneous Electrical Acustimulation: The procedure of transcutaneous electrical acustimulation in IBS-C patients can also be useful. TEA reduces rectal sensation, speeds colon transit time, and improves the quality of life for IBS-C patients. Studies suggest that enhanced vagal activity may control autonomic systems. These findings illuminate future colonic motility and abdominal pain medication for IBS-C patients whose therapeutic options are limited [18].
Psyllium Husk: Psyllium husk relieved IBS symptoms better than a placebo. Psyllium significantly outperformed placebo in the median interquartile range (IQR) of the total IBS-SSS score after four weeks. Psyllium husk reduces IBS symptoms more than a placebo, making it a pediatric IBS treatment. study found that psyllium husk is safe and effective for short-term pediatric IBS treatment. Including psyllium husk in children's IBS treatment is beneficial [19].
Alternative Therapies: Researchers examined how successfully Zataria and Trachyspermum (ZT) treated IBS symptoms. ZT can relieve IBS symptoms like pain, bloating, constipation, and bowel movements. But ZT also sometimes alleviates IBS symptoms like pain, bloating, constipation, and bowel movements. So ZT's efficacy as a therapeutic needs further study because of this, we can use complementary and alternative therapies (CAM) like herbal medicines these therapies can help IBS patients [20].
ROSE-010: ROSE-010 can be useful in managing irritable bowel syndrome. It has been investigated that ROSE-010 is a glucagon-like peptide-1 agonist and is beneficial in treating IBS by easing pain during attacks. All Age patients can take it also, BMI has no effect on ROSE-010 treatment, although it was discovered that women experienced more pain relief than men. In addition, it is noticed that compared to IBS with predominant diarrhea and unspecified IBS, IBS-C, and IBS-M had the best pain relief. Based on these results, we hypothesize that ROSE-010 will be more successful in treating IBS pain in female participants than in male participants, with 100 and 300 mg doses being the most beneficial. The side effect of higher doses is nausea [21].
Dietary Modifications
Low-FODMAP Diet: This involves reducing certain fermentable carbohydrates to alleviate IBS symptoms. Research suggests that it can significantly improve symptoms in many IBS patients [22]. Gluten-Free Diet: While not specifically designed for IBS, some individuals report symptom relief with a gluten-free diet. However, studies have shown mixed results [23]. Advantages like Dietary approaches like the low-FODMAP diet can be tailored to an individual's specific triggers, leading to symptom reduction. Dietary changes are non-invasive and can be implemented without the need for medication. But also, there are some disadvantages, like that some diets can be highly restrictive, making them difficult to follow and potentially leading to nutrient deficiencies. Identifying trigger foods and maintaining dietary changes can be challenging and require professional guidance.
Pharmacological Treatments
Antispasmodic medications: These drugs aim to relieve abdominal pain and cramping by reducing gut muscle spasms. They can provide relief for some individuals [24]. Antidepressants: Tricyclic antidepressants and Selective Serotonin Reuptake Inhibitors (SSRIs) can help manage pain and regulate bowel habits, especially in individuals with IBS-D (diarrhea-predominant) or IBS-M (mixed subtype). and Laxatives and Antidiarrheals: Depending on the predominant symptom, laxatives can alleviate constipation, and antidiarrheals can help manage diarrhea [25]. There are some advantages, like that meditation, e.g., antispasmodics and laxatives, can provide quick relief from specific symptoms, and medications can target specific symptoms, such as pain or diarrhea, effectively. But there are some medication side effects ranging from mild to severe, and some medications may lose effectiveness over time or provide only temporary relief.
Psychological Therapies
Cognitive Behavioral Therapy (CBT): CBT has shown promise in reducing IBS symptoms by addressing the interaction between psychological factors and gut function [26]. Hypnotherapy: Research suggests that hypnotherapy can provide relief from pain, bloating, and overall symptom severity by influencing the gut-brain connection by reducing stress, altering perceptions and responses to gut sensations, potentially rebalancing the autonomic nervous system, and enhancing coping mechanisms [27]. There are some advantages, like how CBT addresses the psychological and emotional aspects of IBS, helps patients manage stress and anxiety, and the skills learned in CBT can provide longterm benefits in managing symptoms. But there are some disadvantages, like that CBT requires multiple sessions over time, a commitment to practicing new coping strategies, and Access to trained therapists, and the cost of therapy can be barriers for some individuals.
Lifestyle Changes
Stress Management: Stress can exacerbate IBS symptoms. Techniques like yoga, meditation, and mindfulness may help reduce stress and improve symptoms by These practices reduce stress and improve well-being by promoting relaxation, emotional regulation, and enhanced focus, making them effective tools for managing various conditions [28]. Regular Physical Activity: Physical activity can promote regular bowel habits and alleviate symptoms, especially constipation [29].
It's important to note that the effectiveness of these interventions can vary widely among individuals. Personalized treatment plans are often necessary, and a multidisciplinary approach involving healthcare professionals from different specialties is recommended. Moreover, keeping a symptom diary and working closely with a healthcare provider can help identify triggers and tailor interventions to individual needs.
Conclusions
In conclusion, this systematic review of the effectiveness of different interventions for managing symptoms of Irritable Bowel Syndrome (IBS) has provided valuable insights into the available treatments. The study analyzed various interventions, including dietary modifications, medications, psychological therapies, and probiotics. The findings suggest that a multimodal approach combining dietary changes, such as the low-FODMAP diet, with psychological therapies like cognitive-behavioral therapy has shown promising results in alleviating IBS symptoms. Additionally, certain probiotics have demonstrated a potential benefit in reducing symptom severity and improving overall gut health. However, it is essential to acknowledge the heterogeneity in study designs and the limited number of high-quality randomized controlled trials. Further research is warranted to strengthen the evidence base and explore the long-term effectiveness of these interventions. Overall, this systematic review serves as a valuable resource for healthcare practitioners in guiding evidence-based treatment decisions for individuals with IBS, and it highlights the need for continued research to optimize symptom management and enhance the quality of life for those affected by this chronic gastrointestinal condition.
References
- De Palma G, Collins SM, Bercik P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut microbes. 2014;5(3):419-29.
- Aziz I, Palsson OS, Törnblom H, et al. The prevalence and impact of overlapping Rome IV-diagnosed functional gastrointestinal disorders on somatization, quality of life, and healthcare utilization: a cross-sectional general population study in three countries. Am J Gastroenterol. 2018;113(1):86-96.
- Kearney DJ, Brown-Chang J. Complementary and alternative medicine for IBS in adults: mind–body interventions. Nat Rev Gastroenterol Hepatol. 2008;5(11):624-36.
- Moayyedi P, Quigley EM, Lacy BE, et al. The effect of fiber supplementation on irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1367-74.
- Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol. 2018;113:1-8.
- Lackner JM, Jaccard J, Krasner SS, et al. How does cognitive behavior therapy for irritable bowel syndrome work? A mediational analysis of a randomized clinical trial. Gastroenterol. 2007;133(2):433-44.
- Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109(10):1547-61.
- Howell CA, Kemppinen A, Allgar V, et al. Double-blinded randomised placebo controlled trial of enterosgel (polymethylsiloxane polyhydrate) for the treatment of IBS with diarrhoea (IBS-D). Gut. 2022;71(12):2430-8.
- El-Salhy M, Mazzawi T, Hausken T, et al. The fecal microbiota transplantation response differs between patients with severe and moderate irritable bowel symptoms. Scand J Gastroenterol. 2022;57(9):1036-45.
- Oh JH, Jang YS, Kang D, et al. Efficacy of a Synbiotic Containing Lactobacillus paracasei DKGF1 and Opuntia humifusa in Elderly Patients with Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Gut Liver. 2023;17(1):100.
- Wang Z, Xu M, Shi Z, et al. Mild moxibustion for Irritable Bowel Syndrome with Diarrhea (IBS-D): A randomized controlled trial. J Ethnopharmacol. 2022;289:115064.
- Ricci C, Rizzello F, Valerii MC, et al. Geraniol treatment for irritable bowel syndrome: A double-blind randomized clinical trial. Nutrients. 2022;14(19):4208.
- Mourey F, Decherf A, Jeanne JF, et al. Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation. World J Gastroenterol. 2022;28(22):2509.
- D’Silva A, Marshall DA, Vallance JK, et al. Meditation and Yoga for Irritable Bowel Syndrome (MY-IBS Study): A Randomized Clinical Trial. Am J Gastroenterol. 2022:10-4309.
- Lövdahl J, Törnblom H, Ringström G, et al. Randomised clinical trial: individual versus group hypnotherapy for irritable bowel syndrome. Aliment Pharmacol Ther. 2022;55(12):1501-11.
- Carbone F, Van den Houte K, et al. Diet or medication in primary care patients with IBS: the DOMINO study-a randomised trial supported by the Belgian Health Care Knowledge Centre (KCE Trials Programme) and the Rome Foundation Research Institute. Gut. 2022;71(11):2226-32.
- Ebrahimloee S, Masoumpoor A, Nasiri M, et al. The effect of Benson relaxation technique on the severity of symptoms and quality of life in children with irritable bowel syndrome (IBS): a quasi-experimental study. BMC Gastroenterol. 2022;22(1):1-7.
- Huang Z, Lin Z, Lin C, et al. Transcutaneous electrical acustimulation improves irritable bowel syndrome with constipation by accelerating colon transit and reducing rectal sensation using autonomic mechanisms. Am J Gastroenterol. 2022;117(9):1491-501.
- Menon J, Thapa BR, Kumari R, et al. Efficacy of oral psyllium in pediatric irritable bowel syndrome: a double-blind randomized control trial. J Pediatr Gastroenterol Nutr. 2023;76(1):14-9.
- Jamalizadeh H, Ahmadi B, Shariffar F, et al. Clinical evaluation of the effect of Zataria multiflora Boiss and Trachyspermum copticum (L.) on the patients with irritable bowel syndrome. Explore. 2022;18(3):342-6.
- Touny AA, Kenny E, Månsson M, et al. Pain relief and pain intensity response to GLP-1 receptor agonist ROSE-010 in irritable bowel syndrome; clinical study cross-analysis with respect to patient characteristics. Scand J Gastroenterol. 2022;57(7):783-91.
- Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterol. 2014;146(1):67-75.
- Elli L, Tomba C, Branchi F, et al. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients. 2016 Feb 8;8(2):84.
- Ford AC, Lacy BE, Harris LA, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. J Am Gastroenterol. 2019;114(1):21-39.
- Black CJ, Thakur ER, Houghton LA, et al. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2020;69:1441-1451.
- Lackner JM, Mesmer C, Morley S, et al. Psychological treatments for irritable bowel syndrome: a systematic review and meta-analysis. J Consult Clin Psychol. 2004;72(6):1100.
- Peters SL, Yao CK, Philpott H, et al. Randomised clinical trial: the efficacy of gut-directed hypnotherapy is similar to that of the low FODMAP diet for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(5):447-59.
- Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678-1688.
- Johannesson E, Simrén M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. J Am Gastroenterol. 2011;106(5):915-22.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref