Research Article - Biomedical Research (2017) Volume 28, Issue 12
The effect of wulongdan on neuroinflammation factors and the expression of P2X7 receptor in the SAH rats
Chuanwu Zhu1#, Fang Yang2# and Rongyan Jiang3*
1TCM School of Southern Medical University, Guangzhou, Guangdong Province, PR China
2Department of pharmacy, Nanfang Hospital, Southern Medical University, PR China
3Department of Cardiology, Bozhou People's Hospital, Bozhou, Anhui Province, PR China
#These authors contributed equally to the work
- *Corresponding Author:
- Rongyan Jiang
Department of Cardiology
Bozhou People's Hospital
Anhui Province, PR China
Accepted on April 25, 2017
Abstract
Objective: To investigate the effect of Wulongdan on the expression of P2X7 receptor (P2X7R) in the cerebral cortex and on neuroinflammation after Subarachnoid Hemorrhage (SAH) in rats.
Methods: A reliable SAH model was established by double injections of blood into cisterna magna in rats. Fifty-four adult Sprague-Dawley rats weighing 220 plus or minus 20 g were divided into three groups by randomized sorting method: Sham, SAH and Wulongdan (n=18 each). The levels of Tumor Necrosis Factor alpha (TNF-α) and Interleukin-1β (IL-1β) were measured by ELISA analysis. The expression of P2X7R in the cerebral cortex was tested by immunohistochemical and Western blot methods.
Results: Compared with those in the Sham group, the levels of TNF-α and IL-1β in the cerebral cortex were significantly up-regulated at each time point after SAH (P<0.05), with the peak occurring at 24 h. Compared with SAH group, the expressions of TNF-α, IL-1β and P2X7R were down-regulated in Wulongdan-treatment group (P<0.05).
Conclusion: Wulongdan could attenuate neuroinflammation in the cerebral cortex after SAH in rats, which may be associated with the down-regulation of P2X7R protein.
Keywords
Wulongdan, Subarachnoid hemorrhage, Neuroinflammation factor, P2X7R.
Introduction
Subarachnoid Hemorrhage (SAH) is caused by intracranial aneurysm rupture that leads to the sudden entry of blood into the subarachnoid space. Though a common cerebrovascular disease, SAH has a higher disability rate and mortality than other similar diseases [1]. Many fundamental researches have been conducted over SAH [2-6].
After the sudden entry of blood into the subarachnoid space, the disintegration of blood cells will trigger the cascade release of a large amount of immune-inflammatory mediators, such as 5-HTP, histamine, bradykinin, Interleukin-1 (IL-1), IL-6, and Tumor Necrosis Factor (TNF). Studies show that the inflammatory response mediated by cytokines such as IL and TNF is involved in the pathophysiological processes of secondary brain injury [7].
The concept of purinergic receptor was first proposed by Burnstock in 1972 to collectively refer to adenosine receptors and ATP receptors in the cell membrane [8]. Harden et al. later demonstrated the presence of ATP receptors on the molecular level in 1995 [9]. Extracellular nucleotide receptors, known as P2 receptors, consist of two types, P2X and P2Y. P2X receptors are ligand-gated ion channels, and 7 P2X receptors (P2X1-7) have been cloned, whose pharmacological actions have been clarified. P2X receptors are widely present in the human body, including various cells of the nervous system. P2X7 receptor (P2X7R) is composed of 595 amino acid residues, including the two-pass transmembrane proteins at the N-terminal region, C-terminal region, intracellular domain and conserved extracellular loop. The homology of P2X7R to other members at the N-terminal region is as high as 35-40%, and its sequence is highly conservative. The C-terminal region of P2X7R which consists of 200 amino acid residues is longer than any other P2X receptors and is not homologous to other P2X receptors. This feature serves as the molecular basis for the unique physiological function of P2X7R compared with other P2X receptors. As a result, P2X7R is distinct from other P2X receptors in terms of electrophysiological features and lysis channels.
ATP is the only natural agonist of P2X7R, which is highly selective on bivalent cations. Low-concentration P2X7R agonist can mediate the influx of Na+ and Ca2+ and the efflux of K+, activating phospholipases A2 and D as well as the Mitogen-Activated Protein Kinase (MAPK)/ nuclear factor kappa B (BF-ĸB) signaling pathways; moreover, it can induce the generation and release of IL-1β, IL-6 and TNF [10]. P2X7R-mediated K+ efflux is an important signal for inflammasome assembly, and it can activate the calcineurin-nuclear factor of activated T-cell signal transduction pathway, such as cyclooxygenase-2 and inducible nitric oxide synthase. However, the repeated or prolonged stimuli with P2X7R agonist can lead to the formation of non-selective membrane channels that permit the entry of molecules as large as 800 Da, leading to cell death [11]. Therefore, P2X7R is also known as the death receptor.
P2X7R is widely expressed in the neurons and on the surface of the astrocytes and microglial cells of the central nervous system, and plays a role in neurotransmitter release and gliocyte activation. Being regulatory of several signaling pathways related to pathology, P2X7R is considered the new target for the treatment of nervous system diseases such as ischemic stroke, traumatic brain injury, Alzheimer's disease, spinal cord trauma and neuropathic pain [12-15]. P2X7R knockout and the use of P2X7R antagonist can significantly improve the pathological changes and symptoms in vitro or in animal experiments. However, the role played by P2X7R after SAH is rarely known, and the effect of P2X7R on neuronal apoptosis, neuroinflammation and pathophysiological processes following SAH requires further investigation.
Cerebral protective and anti-inflammatory effects of Wulongdan have been demonstrated recently [16]. This study discussed the cerebral protective effect of Wulongdan after SAH in rats through its regulation of inflammation. The mechanism of Wulongdan improving the neuroinflammatory damage in SAH rats was investigated so as to shed new light on the fundamental research about the treatment of SAH.
Methods
Laboratory animals and grouping
Fifty-four adult Sprague-Dawley rats weighing 220 plus or minus 20 g were divided into three groups by randomized sorting method: Sham, SAH and Wulongdan, with 18 rats in each group. The rats were provided by Laboratory Animal Center of Nanfang Hospital, Southern Medical University (license No.: scxk (Guangzhou) 2011-0015). The rats were acclimatized for a week in a Specific Pathogen-Free (SPF) environment.
Drug preparation
Wulongdan was prepared by Nanfang Hospital, Southern Medical University and the main ingredients were Polygonum multiflorum, earthworm, Salvia miltiorrhiza, Astragalus mongholicus and Acori Graminei Rhizoma. 1 g of extract was equivalent to 2.67 g of crude drug. The concentration of the extract was 1 g/ml. The treatment group was given crude drug by gastric irrigation at a dose of 8.8 g/kg/d twice daily, for 5 days in total.
Reagents
TNF-α and IL-1β ELISA kits were purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. Rabbit anti-P2X7 polyclonal antibody and rabbit anti-β-actin monoclonal antibody were purchased from Abcam (USA). Immunohistochemistry, DAB color development and BCA protein assay kits were purchased from Wuhan Boster Biological Technology Co., Ltd.
Grouping and construction of SAH models
Intraperitoneal injection was performed with 10% chloral hydrate at a dose of 3 ml/kg. The anesthetized rats were immobilized to the operating table in a supine position. The skin of the neck and the right femoral artery was prepared and disinfected. Blunt dissection of the right femoral artery was performed and 0.3 ml of blood sample was collected. Then the rats were transferred to a prone position. Under stereotaxic guidance a needle was inserted vertically to a depth of about 1 mm. The atlantooccipital membrane was punctured and 0.1 ml of the cerebrospinal fluid was drawn. The blood was slowly injected into the cisterna magna using a microinjection pump. After that, the needle was withdrawn and the puncture was sealed with bone wax. The incision was sutured and the skin was disinfected again after surgery.
ELISA detection
The levels of TNF-α and IL-1β in the cerebral cortex of each group were detected by ELISA according to the manufacturer’s instructions.
Immunohistochemical detection
The fresh brain tissues were fixed and embedded in paraffin. The sections were conventionally dewaxed and dehydrated. Antigen retrieval was performed by putting the sections to 0.01 mol/L citrate for microwave treatment for 1 min. The endogenous peroxidase was removed by treatment with 3% hydrogen peroxide for 15 min. The sections were sealed with 5% BSA for 30 min. The sections were dried and incubated with rabbit anti-P2X7 antibody dropwise at 4°C overnight. On the next day the section was washed with 0.01 mol/L PBS (PH=7.2) for three times and incubated with goat anti-rabbit HRP-labeled secondary antibody. After incubation at 37°C for 30 min, the section was further incubated with SABC dilute buffer for 40 min, followed by DAB reaction for 5 min. Then the reaction was terminated, and the section was dehydrated, transparentized and sealed with neutral balsam
Statistical analysis
Statistical analyses were performed using SPSS13.0 software. The results were expressed as mean ± standard deviation. Difference among groups was analysed by One-way ANOVA. P<0.05 indicated significant difference.
Results
Levels of IL-1β and TNF –α in cerebral cortex
As shown in Tables 1 and 2, the levels of IL-1β and TNF-α in cerebral cortex in the Sham group at each time point were maintained at the baseline levels, indicating normal physiological activities. The levels of IL-1β and TNF-α at 6 h, 24 h and 48 h in the SAH group increased significantly compared with the Sham group (P<0.05). At 6 h, 24 h and 48 h, the levels in Wulongdan group and SAH group declined dramatically compared with the Sham group (P<0.05).
| Group | 6 h | 24 h | 48 h |
|---|---|---|---|
| Sham | 15.38 ± 1.07 | 16.27 ± 1.97 | 15.43 ± 2.63 |
| SAH | 55.43 ± 5.16* | 78.29 ± 6.24* | 65.39 ± 4.94* |
| Wulongdan | 41.65 ± 3.26# | 54.64 ± 5.25# | 46.30 ± 4.67# |
| Note: *P<0.05, the SAH group compared with the Sham group; #P<0.05, the Wulongdan group compared with the SAH group. | |||
Table 1. Contents of IL-1β in the cerebral cortex in each group (x ± s, ng/L).
| Group | 6 h | 24 h | 48 h |
|---|---|---|---|
| Sham group | 35.12 ± 2.16 | 38.25 ± 2.74 | 36.14 ± 2.25 |
| SAH group | 59.43 ± 4.92* | 87.13 ± 5.54* | 88.27 ± 4.47* |
| Wulongdan group | 49.58 ± 2.98# | 51.38 ± 3.45# | 49.15 ± 4.21# |
| Note: *P<0.05, the SAH group compared with the Sham group; #P<0.05, the Wulongdan group compared with the SAH group. | |||
Table 2. Content of TNF-α of cerebral cortex in each group (x ± s, ng/L).
Localization of P2X7R in the cerebral cortex
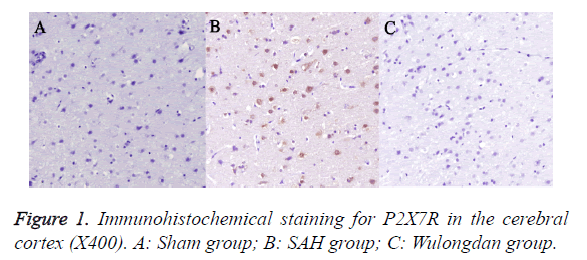
As shown in Figure 1, immunohistochemical staining indicated a large amount of P2X7R-positive cells in the cerebral cortex in the SAH group. The plasma membrane was stained deep brown; the positive cells, deep brown and round, existed in a large quantity. P2X7R in the cerebral cortex in Wulongdan group was weakly expressed; the P2X7R-positive cells were lightly stained and existed in a smaller quantity.
Levels of P2X7R in the cerebral cortex
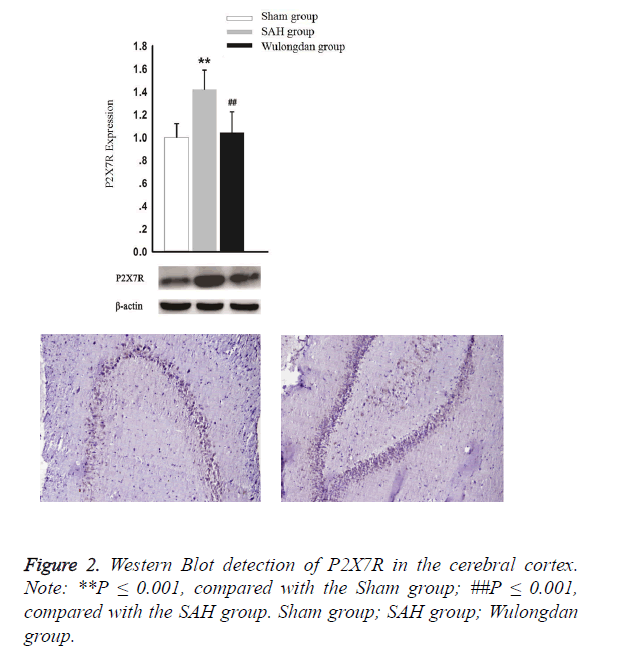
As shown in Figure 2, according to Western Blot detection, the expression of P2X7R in the SAH group was significantly higher than that of the Sham group and Wulongdan group; however, the expression of P2X7R was comparable between the Sham group and Wulongdan group.
Discussion
Dumont et al. believed that the inflammatory cytokines in the cerebrospinal fluid after SAH are closely associated with the changes in cerebral blood flow [17]. The increase in the expression of IL-1β and TNF-α after SAH may lead to neuronal death, thus aggravating brain damage after SAH. Feng et al. indicated the increase in the level of IL-1β after SAH in rats and that the extent of variation was directly proportional to the severity of cerebral edema. IL-1β may be involved in cerebral edema after SAH [7]. Ji et al. [18] also suggested that inflammation is involved in post-SAH brain damage. Therefore, reducing the release of IL-1β and TNF –α after SAH is crucial for relieving the brain damage [19]. IL-1β as a proinflammatory cytokine is involved in the proliferation and differentiation of T-cells and B-cells and it contributes greatly to enhancing the activity of the neuroinflammation factors. As the level of IL-1β increases in the presence of brain damage, ischemia and hypoxia, both neurons and non-neurons will be damaged. TNF-α can induce the release of arachidonic acid metabolites and the generation of lipid peroxide and oxygen free radicals, which leads to severe damage of cell membrane and alteration of cellular functions [20].
We built the SAH model in rats by double injection of blood into the cisterna magna. The levels of IL-1β and TNF-α in the cerebral cortex were detected using ELISA within 72 h after SAH. The severity of neuroinflammation and dynamic changes of the inflammatory cytokines in the early stage of SAH were observed. It was found that the levels of IL-1β and TNF-α increased significantly at 6 h after SAH, with the peak occurring at 24 h. Similar variation pattern was found with P2X7R.
P2X7R, an adenosine receptor, is involved in a variety of pathophysiological processes of nervous system diseases such as cerebral ischemia-reperfusion injury, spinal cord injury and epilepsy [21]. Xu et al. reported that P2X7R played a part in many nervous system diseases such as cerebral ischemia-reperfusion injury, spinal cord injury and epilepsy after immunohistochemical detection and Western Blot. P2X7R is persistently upregulated in the early stage of brain damage of rats after SAH, which leads to the release of a large amount of inflammatory cytokines [22].
Wulongdan is the clinical empirical formula invented by famous TCM physician Prof. Zang Kungtang. The fourteen ingredients of the formula include Astragalus mongholicus, Kudzu's root, Polygonum multiflorum Thunb, Ligusticum wallichii, Salvia miltiorrhiza, earthworm, scorpion, Bombyx batryticatus, leech, sun-dried ginseng, Acori graminei Rhizoma, Fructus ligustri lucidi, Rhizoma atractylodis Macrocephalae and Rhizoma atractylodis Macrocephalae. The combined use of these ingredients can result in the effects of benefiting qi for activating blood circulation, invigorating the kidney and filling the marrow, and dispelling blood stasis and clearing the collaterals. This formula is commonly used to treat ischemic encephalopathy with high efficacy. The existing studies have shown that Wulongdan can improve the sequelae after cerebral infarction via different pathways. However, the effect of Wulongdan on post-SAH brain dysfunction and the working mechanism remain unclear.
We found that Wulongdan relieved post-SAH brain damage by reducing the release of IL-1β and TNF-α, thus protecting the brain against further damage. The tentative mechanism may be that Wulongdan inhibits the expression of P2X7R after SAH and reduces the release of obnoxious IL-1β and TNF-α, thus relieving cerebral vasospasm.
Conclusion
Wulongdan can decrease the expression of P2X7 and the release of inflammatory cytokines (TNF-α and IL-1β) after SAH, which prevents further damage to the cerebral cells caused by the inflammatory response. This may be the mechanism of the cerebral protective effect of Wulongdan in SAH rats. However, considering the complex pharmacological ingredients in TCM prescription, the specific protective mechanism of Wulongdan against cerebral vasospasm after SAH requires further investigation.
References
- Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol 2011; 10: 349-356.
- Dorsch N. A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir 2011; 110: 5-6.
- Naidech AM, Drescher J, Trmul P. Acute physiological derangement is associated with early radiographic cerebral infarction after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2006; 77: 1340-1344.
- Macdonald RL, Higashida RT, Keller E. Clazosentan ,an endothelin receptor antagonist, in patients with aneurismal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10: 618-625.
- Macdonald RL, Higashida RT, Keller E. Randomized trial of clazosentan in patients with aneurismal subarachnoid haemorrhage undergoing endovascular coiling. Stroke 2012; 43: 1463-1469.
- Connolly ES, Rabinstein AA, Carhuapoma JR. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke 2012; 43: 1711-1737.
- Feng L, Li ZL, Ji PZ, Yan BC. The content of IL-1ß in rat brain after subarachnoid hemorrhage and its relation to secondary brain edema. Chinese J Prim Med Pharm 2013; 20: 197-199.
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle ations from different vertebrate species. Br J Pharmacol 1972; 46: 234-242.
- Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype-associated signaling responses and structure. Annu Rev Pharmacol Toxicol 1995; 35: 541-579.
- Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J 2010; 24: 337-345.
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol 2006; 78: 327-346.
- Bai HY, Li AP. P2X (7) receptors in cerebral ischemia. Neurosci Bull 2013; 29: 390-398.
- Trang T, Beggs S, Salter MW. ATP receptors gate microglia signaling in neuropathic pain. Exp Neurol 2012; 234: 354-361.
- Yu Q, Guo Z, Liu X. Block of P2X7 receptors could partly reverse the delayed neuronal death in area CA1 of the hippocampus after transient global cerebral ischemia. Purinergic Signal 2013; 9: 663-675.
- Takenouchi T, Sekiyama K, Sekigawa A, Fujita M, Waragai M. P2X7 receptor signaling pathway as a therapeutic target for neurodegenerative diseases. Arch Immunol Ther Exp (Warsz) 2010; 58: 91-96.
- Peng K, Zang KT, Liu CM. Leukocyte rheological change in rats with focal cerebral ischemia and effect of Wulongdan. J Chinese Microcirc 2003; 7: 292-293.
- Dumont AS, Dumont RJ, Chow MM. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 2003; 53: 123-133.
- Ji C1, Chen G. Signaling pathway in early brain injury after subarachnoid hemorrhage: news update. Acta Neurochir 2016; 121: 123-126.
- Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Mod Mech 2012; 5: 823-833.
- Zhang GS, Lin C, Zhang QJ. Study of mechanism and effect of piperine on delayed cerebral vasospasm following experimental subarachnoid hemorrhage in rabbits. Chinese J Neurosurg 2006; 22: 373-376.
- Xiao Z. Research progress of intracerebral P2X7R. J Epileptol Electroneurophysiol 2011; 10: 110-126.
- Xu XY, Chen H, Chen P, Wang K, Gao J, Sun LQ. Effect of estrogen on P2X7R and neuroinflammation after subarachnoid hemorrhage. Chinese J Immunol 2015; 11: 1472-1475.