Research Article - Biomedical Research (2018) Volume 29, Issue 10
The antioxidation and antiproliferation activity of flavonoids from Aquilaria agallocha and Aquilaria sinensis
Chi-Ming Liu1, Mei-Hui Perng2 and Chung-Yi Chen3*1School of Medicine, Yichun University, Yuanzhou District, Yichun, Jiangxi Province, PR China
2Department of Health Beauty, Fooyin University, Ta-Liao District, Kaohsiung City, Taiwan
3School of Medical and Health Sciences, Fooyin University, Ta-Liao District, Kaohsiung City, Taiwan
- *Corresponding Author:
- Chung-Yi Chen
School of Medical and Health Sciences
Fooyin University
Kaohsiung City, Taiwan
Accepted on April 10, 2018
DOI: 10.4066/biomedicalresearch.29-18-333
Visit for more related articles at Biomedical ResearchAbstract
Studies have shown that herbal extractions may be effective against various diseases like cancer and cardiovascular disease. In this study, we intended to evaluate the cytotoxicity and antioxidation activity of phytochemical constituents from the leaves of Aquilaria agallocha and the stems of Aquilaria sinensis. The chemical structures were identified by UV, IR, ESI-MS, 1H NMR and 13C NMR analyses. 5- hydroxy-7, 4'-dimethoxyflavone (1), 5, 3'-dihydroxy-7, 4'-dimethoxyflavone (2), 5-hydroxy-7, 3', 4'- trimethoxyflavone (3), 5, 7, 4′-trihydroxyflavone (4), and 3', 4', 5, 7-tetrahydroxyflavone (5) were isolated and further examined antioxidation and antiproliferation activity by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and MTT assay. According these results, 5-hydroxy-7, 3', 4'- trimethoxyflavone (3) had DPPH radical scavenging activity. Further, 5-hydroxy-7, 3', 4'- trimethoxyflavone (3) also showed significant positive anticancer activities (10-100 μM) against human skin cancer cell line A375 cells.
Keywords
Aquilaria sinensis, Thymelaeaceae, Aquilaria agallocha.
Introduction
The genus Aquilaria (Thymelaeaceae) is widely distributed in Asia. Aquilaria sinensis and Aquilaria agallocha belongs to Thymelaeaceae [1]. Aquilaria sinensis and Aquilaria agallocha are famous for the resinous wood and it is called agarwood. Agarwood is a well-known as incense in the oriental region such as in Taiwan, Thailand and China used as a sedative, analgesic, and digestive agent in traditional medicine. Flavonoid glycoside, volatile oil, benzenoid, sesquiterpenes and chromone derivatives were isolated and identified from the seed, leaves or stem of these plants [2-10]. Phytochemical constituents obtained from the agarwood including apigenin-7, 4’-dimethylether, genkwanin, quercetin, kaemperfol and 5, 6, 7, 8-tetrahydro-2-(2-phenylethtl) chromes with different pharmacological activities [8,11,12]. The plant Aquilaria sinensis and Aquilaria agallocha have several pharmacological activities and shows antioxidant, anti-diabetic, antiinflammatory, and antibacterial activities [2-6,11-14].
The free radicals are atoms with unpaired electrons. Free radicals can generate from our body by various endogenous systems leading to pathological states. Overproducing free radicals attack lipids, proteins, and DNA and make a lot of human diseases. Oxidative stress is a result of an imbalance between free radical production and antioxidant defenses. Oxidative stress results in many diseases including cancers, inflammatory diseases, gastric ulcers, hypertension and neurological disorders [15,16]. An antioxidant can protect cell against free radicals. Superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione S-transferase have antioxidant activity and they protect cell from oxidative stress. Antioxidants are found in the diet and natural products. Vitamin E, vitamin C (ascorbic acid), β- carotene and flavonoids are natural anti-oxidants [17-19]. Cancer is the leading cause of death in the world. Chronic inflammation is associated with the risk of developing cancer. Ultraviolet (UV) light can cause chronic inflammation and induce skin tumors. Skin cancer can be divided into melanoma and non-melanoma skin cancer depending on the cell type. There has been a significant increase in worldwide incidence of Non-Melanoma Skin Cancer (NMSC) [20]. However, melanoma skin cancer is much less common, but it is far more dangerous.
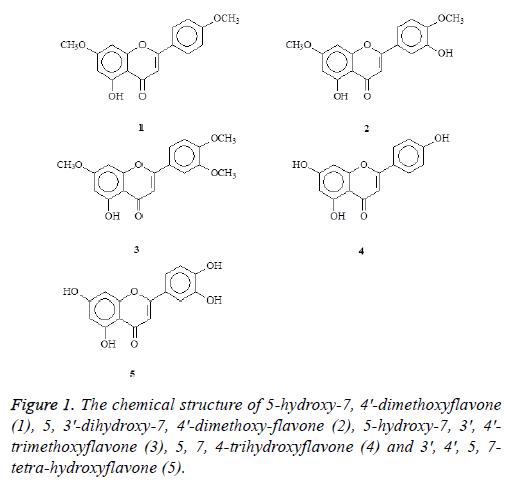
In the present study, we reported the isolation, structure identification and biological evaluation of five flavonoids including 5-hydroxy-7, 4'-dimethoxyflavone (1), 5, 3'- dihydroxy-7, 4'-dimethoxyflavone (2), 5-hydroxy-7, 3', 4'- trimethoxyflavone (3), 5, 7, 4-trihydroxyflavone (4), and 3', 4', 5, 7-tetrahydroxyflavone (5) (Figure 1) from the leaves of Aquilaria agallocha and the stems of Aquilaria sinensis. The structures of these compounds are shown in Figure 1. Previous studies have indicated that flavonoid possess anti-oxidation and anti-cancer activity [21]. However, a limited study on anticancer effects of agarwood was conducted. Recently, a study has shown that agarwood essential oil exhibited anticancer activity in breast cancer cell line MCF-7 [22]. In this study, we further isolated novel flavonoid compounds. All of these compounds were further examined the antiproliferation and antioxidation activities.
Materials and Methods
General procedures
UV spectra were obtained on a Jasco UV-240 spectrophotometer in MeCN. IR spectra were measured on a Hitachi 260-30 spectrophotometer (Hitachi, Tokyo, and JP). 1H-NMR (400/500 MHz) and 13C-NMR (100 MHz), HSQC, HMBC, COSY and NOESY spectra were obtained on a Varian (Unity Plus) NMR spectrometer (Varian, CA, USA). For each sample, 128 scans were recorded with the following settings: 0.187 Hz/point; spectra width, 14400 Hz; pulse width, 4.0 μs; relaxation delay, 2s. Low-resolution ESI-MS spectra were obtained on an API 3000 (Applied Biosystems, CA, USA) and high-resolution ESI-MS spectra on a Bruker Daltonics APEX II 30e spectrometer (Bruker, Bremen, Germany). Silica gel 60 (Merck, 70~230 mesh, 230~400 mesh) was used for column chromatography. Pre-coated silica gel plates (Merck, Kieselgel 60 F-254), 0.20 mm and 0.50 mm, were used for analytical TLC and preparative TLC, respectively, and visualized with 10% H2SO4.
Plant material
The stems of Aquilaria sinensis were collected from Guansi Township, Hsinchu County, Taiwan in May, 2007. Plant material was identified by Dr. Fu-Yuan Lu (Department of Forestry and Natural Resources College of Agriculture, National Chiayi University, Chiayi, Taiwan). A voucher specimen (A. sinensis) was deposited in the School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan. The specimen of A. agallocha was collected from Shanshang District, Tainan City, Taiwan in May, 2008. A voucher specimen was identified by Professor Fu-Yuan Lu (Department of Forestry and Natural Resources College of Agriculture, National Chiayi University) and was deposited in the School of Medical and Health Sciences, Fooyin University, Kaohsiung, Taiwan.
Extraction and isolation
The stems (2.3 kg) of Aquilaria sinensis were chipped and airdried and extracted repeatedly with MeOH (5 L × 2) at room temperature. The combined MeOH extracts (58.3 g) were then evaporated and further separated into 5 fractions by column chromatography on silica gel (4.5 kg, 70-230 mesh) with gradients of n-hexane/CH2Cl2/acetone/MeOH. Part of fraction 1 (32.2 g) was subjected to silica gel chromatography by eluting with n-hexane-acetone (60:1), enriched with acetone to furnish two further fractions (2-1-2-4). Fraction 2-2 (21.1 g) was further purified on a silica gel column using n-hexane/ acetone mixtures to obtain 5-hydroxy-7, 4′-dimethoxyflavone (1) (16.3 mg). Fraction 2-3 (6.2 g) was further purified on a silica gel column using n-hexane/acetone mixtures to yielded 5-hydroxy-7, 3′, 4′-trimethoxyflavone (3) (5.3 mg). Part of fraction 3 (24.9 g) was subjected to silica gel chromatography by eluting with n-hexane-acetone (40:1), enriched with acetone to furnish two further fractions (3-1-3-4). Fraction 3-2 (10.2 g) was subjected to silica gel chromatography, eluting with CH2Cl2-MeOH (100:1), and enriched gradually with MeOH, to obtain two fractions (3-2-1-3-2-2). Fractions 3-2-2 (3.5 g) were subjected to further silica gel column chromatography and purified by preparative TLC (thin layer chromatography) to yield 5, 3′-dihydroxy-7, 4′-dimethoxyflavone (2) (6.1 mg) and 5, 4′-dihydroxy-7, 3′-dimethoxyflavone (5.2 mg). The air-dried branches of Aquilaria agallocha (2.5 kg) were extracted with MeOH (50 L × 6) at room temperature and the MeOH extract (29.2 g) obtained after concentration under reduced pressure. This was chromatographed over silica gel (800 g, 70-230 mesh) using n-hexane/CH2Cl2/acetone/MeOH as eluent to produce 5 fractions. These (supporting material) were subjected to repeated Si gel CC and preparative TLC fraction 1 to produce 3 fractions (respectively: fraction1-1~1-3), fraction1-1 to afford 5-hydroxy-7, 4′-dimethoxyflavone (1) (12.3 mg); fraction 1-2 to produce 4 fractions (respectively: fraction 1-2-1,1-2-4), fraction 1-2-4 to afford 5, 3'- dihydroxy-7, 4'-dimethoxyflavone (2) (8.3 mg) and 5- hydroxy-7,3',4'-trimethoxyflavone (3) (6.8 mg). Fraction 4 to produce 2 fractions (respectively: fraction 4-1~4-2), fraction 4-1 to afford 5, 4'-dihydroxy-7-methoxyflavone, fraction 4-2 to afford 5, 7, 4′-trihydroxyflavone (4) (4.3 mg); fraction 5 to produce 2 fractions (respectively: fraction 5-1~5-2), fraction 5-2 to afford 3', 4', 5, 7-tetrahydroxyflavone (5) (5.1 mg).
Cell culture
The human skin cancer A375 cell line was derived purchased from the Bioresource Collection and Research Center (BCRC number: 60039, Hsinchu, Taiwan, ROC). The cells was cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% Fetal Bovine Serum, 1.5 g/L NaHCO3,10 μg/mL penicillin, 10 μg/mL streptomycin, 2 mM L-glutamine and 0.25 μg/mL fungizone at 37°C with 5% CO2 in a humidified incubator.
Determination of DPPH radical scavenging capacity
DPPH is an abbreviation for an organic chemical compound 2, 2-diphenyl-1-picrylhydrazyl. It is a dark-colored crystalline powder composed of stable free-radical molecules. The mechanism of its radical scavenging activity is the antioxidant transfer of an electron or a hydrogen atom to DPPH. DPPH were obtained from Sigma-Aldrich (GmbH, Sternheim, Germany). Various concentrations of the three compounds were added to 0.1 M of stable DPPH (60 μmole/L) solution. When DPPH reacts with hydrogen-donating antioxidant, it is reduced, resulting in a decrease in absorbance at 517 nm. The analyzed time interval was 10 min per point, up to 30 min by using UV-Vis spectrophotometer (Jasco V-530, Japan Servo Co., Ltd. and Tokyo, Japan). Vitamin C was used as a positive control. Measurements were taken in triplicate. The DPPH•radical scavenging capacity (%) was determined as: 1- ((Acontrol-Asample)/Acontrol) × 100.
Cell viability assay-MTT assay
The effects of compounds on cell growth were according to the MTT assay procedures. MTT were obtained from Sigma- Aldrich. MTT form impermeable crystals of a dark-blue formazan, thus resulting in accumulation within healthy cells. Briefly, cells were seeded in 96-well microplates at a density of 1 × 104 cells/well. The testing samples were dissolved in sterile DMSO to treat a working concentration. Each concentration was added to a microplate in three replicates and incubated under the same conditions as above for 24 h. After 24 h of incubation, the medium was replaced with 100 μl of fresh medium including 0.5 mg/mL MTT.
The microplate was cultured in a 37°C incubator filled with 5% CO2 for 2 h. Each precipitate was dissolved in 100 μl of DMSO to dissolve the purple formazan crystals. After the dishes were gently shaken for 10 min in the dark to ensure maximal dissolution of formazan crystals, the absorbance (A) values of the supernatant were measured at 595 nm (UV_vis, BioTek, Winooski, VT). Cell growth was calculated as: (Asample-Ablank)/(Acontrol-Ablank) × 100%. All experiments were repeated at least 3 times.
Statistical analysis
All experiments were carried out at three times and at least triplicate. The results were expressed as the average of the mean values ± standard deviation (SD). Analysis of the data was done with SigmaPlot software (Version 8.0, SPSS Scientific, and Chicago, IL, USA) and SigmaStat (Version 2.03, SPSS Scientific) run on an IBMcompatible computer.
Results
DPPH radical scavenging activity
DPPH has been widely used for free radical-scavenging activity of antioxidant compounds such as phenolic compounds or crude extracts of plants. DPPH radical was scavenged by antioxidants through the donation of hydrogen. In the present study, all compounds were flavonoids. 5-hydroxy-7, 3', 4'- trimethoxyflavone (3) was found to be effective scavengers against DPPH radical at the concentration of 10 mM compared with vitamin C. Vitamin C is as a positive control in this study. As shown in Table 1, 5-hydroxy-7, 3', 4'-trimethoxyflavone (3) had 20.68 ± 5.50% activity at the concentration of 10 mM.
| DPPH radical scavenging activity (%) | |
|---|---|
| 5-hydroxy-7, 4'-dimethoxyflavone | 5.23 ± 0.11 |
| 5, 3'-dihydroxy-7, 4'-dimethoxyflavone | 4.38 ± 0.33 |
| 5-hydroxy-7, 3', 4'-trimethoxyflavone | 20.68 ± 5.50 |
| 5, 7, 4-trihydroxyflavone | 3.74 ± 0.08 |
| 3', 4', 5, 7-tetrahydroxyflavone | 6.22 ± 1.21 |
| Vitamin C | 100.00 ± 9.87 |
Table 1. DPPH radical scavenging activity (%) of various compounds from Aquilaria agallocha and Aquilaria sinensis.
Anti-proliferative properties of compounds 3 on A375 cells
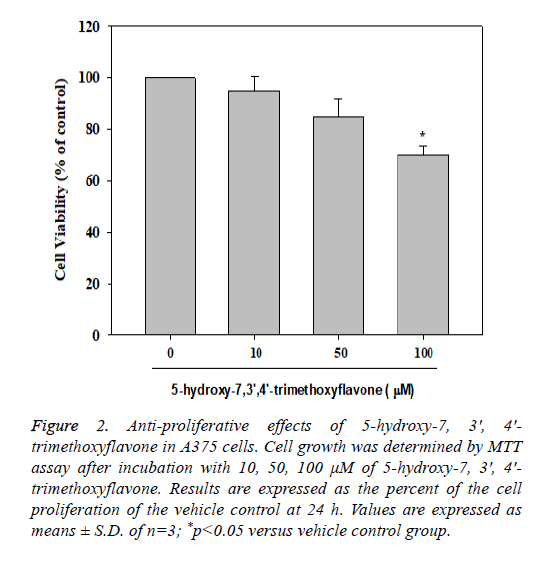
The MTT assay is a colorimetric assay used to measure cell viability. The MTT assay was used to investigate the antiproliferation activity of tested compounds in A375 cells after 24 h treatment. The cells were treated with 5-hydroxy-7, 3', 4'- trimethoxyflavone (3) at different concentrations (10, 50 and 100 μM). The compound had significant inhibition at the concentration of 100 μM in A375 cells. 5-hydroxy-7, 3', 4'- trimethoxyflavone (3) had 68.00 ± 1.33% of cell survival rate (Figure 2).
Figure 2: Anti-proliferative effects of 5-hydroxy-7, 3', 4'- trimethoxyflavone in A375 cells. Cell growth was determined by MTT assay after incubation with 10, 50, 100 μM of 5-hydroxy-7, 3', 4'- trimethoxyflavone. Results are expressed as the percent of the cell proliferation of the vehicle control at 24 h. Values are expressed as means ± S.D. of n=3; *p<0.05 versus vehicle control group.
Discussion
Oxidative stress can contribute to many diseases including inflammatory, cancers and aging. Reactive oxygen, nitrogen species and their metabolites play an important role in carcinogenesis. ROS can break DNA structure and DNAprotein cross link. An antioxidant can donate an electron to the free radical or remove the ROS/reactive nitrogen species. Synthetic and natural food antioxidants are usually used in food industry and medicine. Studies have shown that consumption of high antioxidant rich food and medical plants is useful for the prevented diseases. Many antioxidant compounds extracted from plant have been identified as free radical scavengers. Polyphenolic phytochemicals are bioactive secondary metabolites from a variety of plants. Flavonols, flavones, isoflavones, flavanones, procyanidins, and anthocyanins belong to flavonoids. Flavonoids are a group of nature compounds with phenolic structure and common characteristics C6-C3-C6 skeleton consisting of two benzene rings. Flavonoids possess anti-oxidative effects as free radical scavengers and metal ion cheaters properties associated with the phenolic hydroxyl groups attached to ring structures. Intensity of the antioxidant activity of a flavonoid strongly depends on its chemical structure. Flavonoids possess antioxidation, anti-cancer, and anti-inflammatory activities [23]. Many in vitro studies have shown flavonoids have chemoprevention and chemotherapy activities in many cancer types including breast, prostate, pancreas and colon cancer [24]. Melanoma is a highly aggressive skin cancer and often resistant to cancer therapy. Pigmentation is a complex biochemical process involving different signal pathways and enzymes.
In this study, we isolated five compounds. Among them, luteolin (3', 4', 5, 7-tetrahydroxyflavone) is a well kwon flavonoid. Previous study has indicated that luteolin inhibited melanin synthesis in B16 melanoma cells [25]. 2, 2- diphenyl-1-picrylhydrazyl radical (DPPH assay), ferric reducing antioxidant power (FRAP assay) and ABTS assay are in vitro antioxidation assays. Studies have shown that EGCG, catechins, luteolin, baicalein and kaempferol are flavonoids with antioxidation activity. These compounds display antimelanoma effect via inhibiting cell proliferation, invasion and inducing apoptosis with multiple pharmacological mechanisms [25-32]. Flavonoids exhibit anti-proliferative and apoptotic effects via HGF/SF-Met signaling, PI3K-AKT pathway, MAPK pathway or cell cycle regulation in melanogenesis. A study has shown that luteolin inhibited xanthine oxidase activity and reduced cellular ROS levels in B16 cells [33]. Taken these the results of previous studies together, the phenolic compounds may display anti-oxidative actions through different mechanisms inhibiting cell proliferation.
Aquilaria agallocha and Aquilaria sinensis are used to treat inflammation in folk medicine. The purpose of the study was to extract five new compounds of flavonoid and examine the antioxidation and antiproliferation activity. It was observed that 5-hydroxy-7, 3', 4'-trimethoxyflavone (3) was shown to scavenge directly the stable DPPH radical scavenging and antiproliferation activities. These findings indicate that 5- hydroxy-7, 3', 4'-trimethoxyflavone (3) may be useful in as free radical scavengers for preventing diseases.
Conclusions
Based on this study it can be concluded that 5-hydroxy-7, 3', 4'-trimethoxyflavone was found to be more sensitive to human skin cancer cell line A375 cell line with anti-oxidation. The detail pharmacological study and in vivo study need to be conducted in the future.
Acknowledgements
This work was financially supported by Fooyin University (AI-103045).
References
- Chen JY, Xiong, Y, Deng MH. Study on the physiological and biochemical characteristics of different types of leaves of Aquilaria sinensis. Zhong Yao Cai 2011; 34: 1664-1666.
- Canli K, Yetgin A, Akata I, Altuner EM. In vitro antimicrobial screening of Aquilaria agallocha roots. Afr J Tradit Complement Altern Med 2016; 13: 178-181.
- Huo HX, Gu YF, Sun H, Zhang YF, Liu WJ, Zhu ZX, Shi SP, Song YL, Jin HW, Zhao YF, Tu PF, Li J. Anti-inflammatory 2-(2-phenylethyl) chromone derivatives from Chinese agarwood. Fitoterapia 2017; 118: 49-55.
- Huo, HX, Zhu ZX, Pang DR, Li YT, Huang Z, Shi SP, Zheng J, Zhang Q, Zhao YF, Tu PF, Li J. Anti-neuro inflammatory sesquiterpenes from Chinese eaglewood. Fitoterapia 2015; 106: 115-121.
- Huo, HX, Zhu ZX, Song YL, Shi SP, Sun J, Sun H, Zhao YF, Zheng J, Ferreira D, Zjawiony JK, Tu PF, Li J. Antiinflammatory Dimeric 2-(2-Phenylethyl) chromones from the resinous wood of Aquilaria sinensis. J Nat Prod 2018; 81: 543–553.
- Wang SL, Hwang TL, Chung MI, Sung PJ, Shu CW, Cheng MJ, Chen JJ. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one derivative, and anti-inflammatory constituents from the stem barks of Aquilaria sinensis. Molecules 2015; 20: 20912-20925.
- Yang DL, Wang H, Guo ZK, Dong WH, Mei WL, Dai HF. A new 2-(2-phenylethyl) chromone derivative in Chinese agarwood 'Qi-Nan' from Aquilaria sinensis. J Asian Nat Prod Res 2014; 16: 770-776.
- Yang MX, Liang YG, Chen HR, Huang YF, Gong HG, Zhang TY, Ito Y. Isolation of flavonoids From wild Aquilaria sinensis leaves by an improved preparative high-speed counter-current chromatography apparatus. J Chromatogr Sci 2018; 56: 18-24.
- Ye W, He X, Wu H, Wang L, Zhang W, Fan Y, Li H, Liu T, Gao X. Identification and characterization of a novel sesquiterpene synthase from Aquilaria sinensis: An important gene for agarwood formation. Int J Biol Macromol 2018; 108: 884-892.
- Yuan H, Zhao J, Wang M, Khan SI, Zhai C, Xu Q, Huang J, Peng C, Xiong G, Wang, W, Khan IA. Benzophenone glycosides from the flower buds of Aquilaria sinensis. Fitoterapia 2017; 121: 170-174.
- Hara H, Ise Y, Morimoto N, Shimazawa M, Ichihashi K, Ohyama, M, Iinuma M. Laxative effect of agarwood leaves and its mechanism. Biosci Biotechnol Biochem 2008; 72: 335-345.
- Ito T, Kakino M, Tazawa S, Watarai T, Oyama M, Maruyama H, Araki Y, Hara H, Iinuma M. Quantification of polyphenols and pharmacological analysis of water and ethanol-based extracts of cultivated agarwood leaves. J Nutr Sci Vitaminol 2012; 58: 136-142.
- Alam J, Mujahi, M, Badruddeen, Jahan Y, Bagga P, Rahman MA. Hepatoprotective potential of ethanolic extract of Aquilaria agallocha leaves against paracetamol induced hepatotoxicity in SD rats. J Tradit Complement Med 2017; 7: 9-13.
- He ML, Qi SY, Hu LJ. Rapid in vitro propagation of medicinally important Aquilaria agallocha. J Zhejiang Univ Sci B 2005; 6: 849-852.
- Ginter E, Simko V, Panakova V. Antioxidants in health and disease. Bratisl Lek Listy 2014; 115: 603-606.
- Yang Y, Karakhanova S, Werner J, Bazhin AV. Reactive oxygen species in cancer biology and anticancer therapy. Curr Med Chem 2013; 20: 3677-3692.
- Madunic J, Madunic IV, Gajski G, Popic J, Garaj-Vrhovac V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett 2018; 413: 11-22.
- Huang D. Dietary antioxidants and health promotion. Antioxidants 2018; 7: 9.
- Barone D, Cito L, Tommonaro G, Abate AA, Penon D, De Prisco R, Penon A. Forte IM, Benedetti E, Cimini A, Indovina P, Nicolaus B, Pentimalli F, Giordano A. Anti-tumoral potential, antioxidant activity and carotenoid content of two Southern Italy tomato cultivars extracts: San Marzano and Corbarino. J Cell Physiol 2018; 233: 1266-1277.
- Califano J, Nance M. Malignant melanoma. Facial Plast Surg Clin North Am 2009; 17: 337-348.
- Dajas F, Abin-Carriquiry JA, Arredondo F, Blasina F, Echeverry C, Martinez M, Rivera F, Vaamonde L. Quercetin in brain diseases: Potential and limits. Neurochem Int 2015; 89: 140-148.
- Hashim YZ, Phirdaous A, Azura A. Screening of anticancer activity from agarwood essential oil. Pharmacognosy Res 2014; 6: 191-194.
- Li AL, Chen BJ, Li GH, Zhou MX, Li YR, Ren DM, Lou HX, Wang XN, Shen T. Physalis alkekengi L. var. franchetii (Mast.) Makino: An ethnomedical, phytochemical and pharmacological review. J Ethnopharmacol 2018; 210: 260-274.
- Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr 2012; 31: 206-238.
- An SM, Kim HJ, Kim JE, Boo YC. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother Res 2008; 22: 1200-1207.
- Nihal M, Ahsan H, Siddiqui IA, Mukhtar H, Ahmad N, Wood GS. (-)-Epigallocatechin-3-gallate (EGCG) sensitizes melanoma cells to interferon induced growth inhibition in a mouse model of human melanoma. Cell Cycle 2009; 8: 2057-2063.
- Liu-Smith F, Meyskens FL. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol Nutr Food Res 2016; 60: 1264-1274.
- Pal HC, Hunt KM, Diamond A, Elmets CA, Afaq F. Phytochemicals for the Management of Melanoma. Mini Rev Med Chem 2016; 16: 953-979.
- Iwashita K, Kobori M, Yamaki K, Tsushida T. Flavonoids inhibit cell growth and induce apoptosis in B16 melanoma 4A5 cells. Biosci Biotechnol Biochem 2000; 64: 1813-1820.
- Said A, Tundis R, Hawas UW, El-Kousy SM, Rashed K, Menichini F, Bonesi M, Huefner A, Loizzo MR, Menichinib F. In vitro antioxidant and antiproliferative activities of flavonoids from Ailanthus excelsa (Roxb.) (Simaroubaceae) leaves. Z Naturforsch C 2010; 65: 180-186.
- Kim YK, Kim YS, Choi SU, Ryu SY. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch Pharm Res 2004; 27: 44-47.
- Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol 2001; 61: 1205-1215.
- Choi MY, Song HS, Hur HS, Sim SS. Whitening activity of luteolin related to the inhibition of cAMP pathway in alpha-MSH-stimulated B16 melanoma cells. Arch Pharm Res 2008; 31: 1166-1171.