Research Article - Journal of Fisheries Research (2023) Volume 7, Issue 3
Survival of Tilapia guineensis Fingerlings Transported With Different Plant Extracts As Anaesthetics
Akinrotimi OA1*, Ikeogu CF2 and Anyaobu-Cookey IK31Department of Oceanography and Marine Research, African Regional Aquaculture Center/Nigerian Institute for Oceanography and Marine Research, Rivers State, Nigeria
2Department of Fisheries and Aquaculture Management, Nnamdi Azikiwe University, Awka Nigeria
3Department of Aquaculture, Nigerian Institute for Oceanography and Marine Research, Victoria Island, Lagos
- *Corresponding Author:
- Akinrotimi OA
Department of Oceanography and Marine Research
African Regional Aquaculture Center/Nigerian Institute for Oceanography and Marine Research
Rivers State, Nigeria
E-mail: ojoakinrotimi@gmail.com
Received: 06-Mar-2023, Manuscript No. aajfr-22- 91048; Editor assigned: 10-Mar-2023, PreQC No. aajfr-22-91048(PQ); Reviewed: 29-Mar-2023, QC No. aajfr-22-91048; Revised: 29-Apr-2023, Manuscript No. aajfr-23-91048(R); Published: 09-May-2023, DOI:10.35841/aajfr-7.3.141
Citation: Akinrotimi OA, Ikeogu, CF and Anyaobu-Cookey IK. Survival of Tilapia guineensis fingerlings transported with different plant extracts as anaesthetics. J Fish Res. 2023;7(3):141
Abstract
The survival of Tilapia guineensis fingerlings transported with different plant extracts such as clove, nut Meg and mustard seed as anaesthetics was carried out. A total of 450 specimens of T.guineensis fingerlings (mean length 6.99cm±1.07 SD and mean weight 11.88g±1.81SD) were sourced from NIOMR Brackish Water Fish Farm, Buguma, Rivers State, Nigeria at low tide. They were exposed in three replicates to different concentrations (0.00mg/L- control; 10.00; 20.00; 30.00 and 40.00 mg/L) of clove, nut Meg and mustard seed aqueous extracts. The exposed fish were later transported in open plastic tanks placed inside a bus from Buguma to Rivers State University, Port Harcourt over a distance of 60km. During this process, the survivals of the transported fish were monitored at time intervals of 0, 30, 60, and 90 minutes. The results of the study indicated that the survival of the fish increased significantly (P<0.05) with increasing concentration of the anaesthetics in the exposed fish. The lowest survival rate (10.0%) was recorded in the fish transported with no anesthetics, while 100% survivals were recorded in fish exposed to 40.0 mg/L of clove seed extracts. Comparative survival of in the fingerlings of T.guineensis transported with these plant extracts indicated that higher survival rates were recorded in fish transported with clove seed extracts when compared to nut Meg and mustard seed. In conclusion, this study suggests that application of clove seed extracts using 40.00 mg/L reduced the stress response in T.guineensis during transportation, thereby enhances their survival.
Keywords
Aquaculture, Fish, Transportation, Tilapia, Stress.
Introduction
Fish transportation is an important aspect of aquaculture practices, it involves movement of small or large quantity of fish over some distances to the waters in which they are to be stocked [1,2]. Fish are usually transported for numerous reasons such as: collection and movement of brood stock, fingerlings, juveniles and adult fish, to stocking sites or market and also for the purpose of introduction of fish species into a new culture environment [3,4]. Several authors have reported that fish transportation from one location to another can introduce stress, which negatively affects fish performance and consequently reduced its survival in the culture medium [5-7]. As a result, huge loses and severe mortality in newly stocked fish farms has been associated with acute stimulus such as handling and transport [8]. As part of the handling procedures in intensive fish farming, transportation times may vary considerably, depending on the distance covered. As a rule fingerlings and juveniles are usually transported from the hatchery to culture site. In this process, the fish should arrive at the farm in good physiological conditions to meet the criteria demanded by the farmer [9,10].
Anesthetics are being used to minimize the stress associated with aquaculture procedures. Anaesthetizing fish prior to transport can reduce metabolic rate, oxygen demand, reduce general activity, decrease effect of handling and mitigate the incidence of stress response [11-13]. In this study the main substance used is an aqueous extract of seed of the clove plant (Syzygium aromaticum), and nut meg Its major and active ingredient is eugenol (70-90%). Clove extracts is considered an appropriate anesthetic for fish because of its low cost, availability and safety to fish and humans [14]. In Nigeria, clove, mustard and nut Meg seeds are currently being used as an additive in food in many homes in both rural and urban areas.
Plant extracts are potential source of new and effective anesthetics for use in fish handling and transportation in intensive aquaculture [15]. With the recent awareness on safe aquaculture practices, to develop “green” anesthetics with low environmental and health risks, coupled with the prohibitive cost and scarcity of conventional anesthetics [16], there is the need therefore to develop a viable alternative anesthetics of plant origin which could be used in fish transportation. Many farmers reported incidents of mortalities when transporting some species of mullet, tilapia and some clariids [17,18]. This work will provide information on the application of local anesthetics, such as clove, mustard and nut Meg seed extracts and create awareness of the efficacy of these plant extracts that could be used as an alternative to the chemical anesthetics. The aims of the research therefore are to investigate the efficacy of clove, nutmeg and mustard seed extracts as anaesthetics in transportation of Tilapia guineensis.
Materials and Methods
Sources of Experimental Fish
A total of 450 specimens of T.guineensis fingerlings (mean length 6.99cm 1.07SD and mean weight 11.88g 1.81SD) were sourced from NIOMR Brackish Water Fish Farm, Buguma, Rivers State, Nigeria at low tide.
Acclimation of Experimental Fish
They were transferred in 50L jerry cans to the Fish Disease Laboratory at the Center and be acclimated for a period of seven days. During this period they were fed with ARAC feed (35.0% CP) at 3% body weight. The water in acclimation tanks was renewed every two days (Gabriel et al., 2004).
Preparation of Plant Extracts
Nutmeg (Myristica fragrans), Mustard seed (Brassica nigra) and dried buds of clove plant; (Syzigium aromaticum) were purchased from Choba Market in Obio Akpor Local Government Area of Rivers State. Plants authentication was done using the keys of Agbaje, [19]. These seeds were taken to the laboratory and ground into power using a kitchen blender (Model H2, Ken Wood, and Japan). The milled seeds were sieved using 0.1 micro nylon meshes to obtain the fine powder.
Experimental Design
The design of the experiment was Completely Randomized Design (CRD) having five treatments levels each with three replicates for each of the plant extracts. A total of 45 plastic basins of dimension (52x44x34 cm3) each were used for the experiments. The 45 basins were labelled based on the treatment levels and replicates. Each basin was stocked with 10 fish per tank.
Experimental Procedure
The powder was weighed into different concentrations (10.0, 20.0, 30.0, 40.0 and 50 mg/l) using a sensitive weighing balance. It was applied directly in three replicates into the water (10L) level in 20L experimental plastic aquaria. The mixtures were stirred vigorously to ensure homogenous mixture. The fish was weighed with 20 kg round top weighing scale (Model 1123HK, Digital Scales, Ltd, and Beijing, China). While the length of the fish was measured with transparent meter rule.
They were then be introduced into prepared experimental aquaria, containing five concentrations of each of the powdered plant seeds (10.00; 20.00; 30.00; 40.00 and 50.00 mg/l) at the rate of 10 fish per tank in triplicates. The Fish was then transported from the ARAC/NIOMR farm in Buguma to Rivers State University fish farm in department of Fisheries and Aquatic Environment, over a distance of 60 km.
Evaluation of Water Quality Parameters
Water quality parameters such as dissolved Oxygen, Ammonia, and Sulphide were determined by using Lammotee water quality analysis kit (Model AQ4, Chestown, Maryland, USA). Following the manufacturer’s instructions. The water pH was determined in situ in each of the aquarium with a pH meter (Hanna Products, Portugal). This was achieved by dipping the end of the electrode into the test solution and the mode button was selected and reading was taken. The temperature of the water was measured by placing the mercury in glass thermometer in the water and taking a reading after five minutes at 15 cm depth.
Statistical Analysis
The data obtained from the study was collated and analyzed using statistics software 8.0 for windows. Data was first tested for normality (Kolmogorov-Smirnov test) and homosesdasticity of variance (Bartetts test). When these conditions were satisfied, a two way analysis of variance (ANOVA) was employed to reveal significant differences in measured variables among control and experimental groups. When a difference is detected (P<0.05), Tuckey’s multiple comparison tests was applied to identify which treatment are significantly different.
Results
The water quality parameters in experimental tanks of T.guineensis exposed and transported with clove seed extracts are presented in Table 1. The results indicated a significant reduction (P<0.05) in the values of dissolved oxygen, in the water without anaesthetics (0.00 mg/l). Whereas, higher values of ammonia and sulphide were also recorded in the control. While other water quality parameters were within the same range with no significant different in relation to the concentration of the anaesthetics (P>0.05). Mean values for the water quality variables such as temperature, pH, dissolved oxygen (DO), ammonia and sulphide obtained during exposure of O.niloticus to nut meg and mustard seed extracts are presented in Table 2 and 3 respectively. Lower values of dissolved oxygen were observed in the control, when compared to other concentrations. Whereas, higher values of ammonia and sulphide were also recorded in the control. While temperature and pH values were within the same range with no significant different (P>0.05).
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Parameters | 0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| Temperature (°C) | 29.92 ± 1.13a | 29.94 ± 1.18a | 29.98 ± 1.27a | 29.92 ± 1.01a | 29.96 ± 0.12a |
| pH | 6.84 ± 0.21a | 6.82 ± 0.12a | 6.81 ± 0.18a | 6.83 ± 0.11a | 6.82 ± 0.12a |
| DO (mg/l) | 3.71 ± 0.12a | 5.69 ± 0.21b | 5.68 ± 0.14b | 5.71 ± 0.11b | 5.78 ± 0.11ab |
| Ammonia (mg/l) | 1.04 ± 0.01c | 0.82 ± 0.01b | 0.22 ± 0.02a | 0.02 ± 0.01a | 0.01 ± 0.01a |
| Sulphide | 0.05 ± 0.01b | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a |
Table 1: Water quality parameters in experimental tanks of t. guineensis fingerlings exposed to clove seed extracts (Mean ± SD).
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Parameters | 0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| Temperature (°C) | 29.92 ± 1.13a | 29.94 ± 1.18a | 29.98 ± 1.27a | 29.92 ± 1.01a | 29.96 ± 0.12a |
| pH | 6.83 ± 0.11a | 6.97 ± 0.22a | 6.91 ± 0.27a | 6.89 ± 0.31a | 6.74 ± 0.42a |
| DO (mg/l) | 3.74 ± 0.11a | 5.50 ± 0.21a | 5.41 ± 0.14a | 5.32 ± 0.11a | 5.09 ± 0.11a |
| Ammonia (mg/l) | 1.05 ± 0.02c | 1.29 ± 0.01b | 1.12 ± 0.02a | 0.95 ± 0.01a | 0.83 ± 0.01a |
| Sulphide | 0.05 ± 0.01b | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.02 ± 0.01a | 0.03 ± 0.01a |
Table 2: Water quality parameters in experimental tanks of T. guineensis fingerlings exposed to nut meg extracts (Mean ± SD)
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Parameters | 0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| Temperature (°C) | 29.91 ± 0.13a | 29.91 ± 0.41a | 29.95 ± 0.33a | 29.91 ± 0 .34a | 29.97 ± 0.33a |
| pH | 6.83 ± 0.11a | 6.97 ± 0.22a | 6.91 ± 0.27a | 6.89 ± 0.31a | 6.74 ± 0.42a |
| DO (mg/l) | 3.74 ± 0.11a | 5.50 ± 0.21a | 5.41 ± 0.14a | 5.12 ± 0.11a | 5.01 ± 0.17a |
| Ammonia (mg/l) | 1.05 ± 0.02c | 1.30 ± 0.01b | 1.22 ± 0.02a | 0.99 ± 0.01a | 0.88 ± 0.01a |
| Sulphide | 0.05 ± 0.01b | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.03 ± 0.01a | 0.03 ± 0.01a |
Table 3: Water quality parameters in experimental tanks of T. guineensis fingerlings exposed to mustard seed extracts (Mean ± SD)
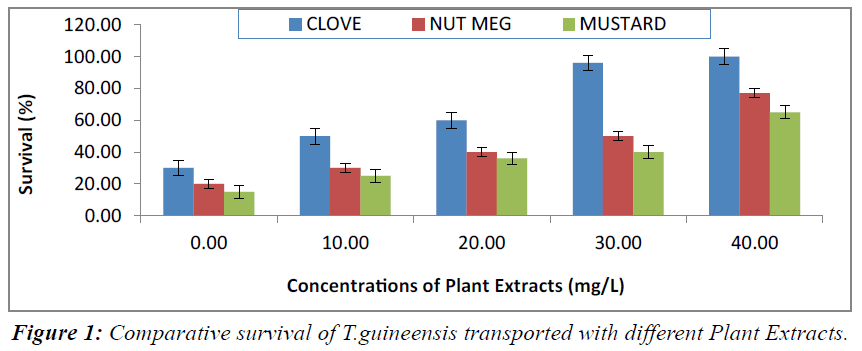
The percentage survival in the fingerlings of T.guineensis transported with clove seed extracts are presented in Table 4. Generally, the survival of the fish decreased significantly (P<0.05) as the transportation time increased, except at 40.0 mg/l concentration of the anaesthetics where 100.0% survival was recorded. The lowest survival rate (30.00%) was recorded in the fish with no anaesthetics (control), in 80 minutes, while the highest values of survival (100.0%) were observed in the fish transported with 40.00mg/l concentration of clove seed extracts The survival in the fingerlings of T.guineensis exposed and transported with nut Meg and mustard seed extracts are presented in Table 5 and 6 respectively. The survival of the fish decreased significantly (P<0.05) as the transportation time increased. The lowest survival rate (20.00%) was recorded in the fish with no anaesthetics (control), in 80 minutes, while the highest values of survival were observed in the fish transported with 40.00mg/l concentration of the plant extracts. The comparative assessment of different plant extracts used in transportation of T.guineensis is presented in Figure 1. At the end of the transportation period, the fish transported with clove seed extracts consistently recorded the highest survival in all concentrations of the plant extracts. This was followed by those transported with nutmeg, while those in mustard had the lowest survival.
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Time (mins) |
0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| 0.00 | 100.00 ± 0.01d | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00a |
| 20.00 | 80.00 ± 0.01c | 82.00 ± 0.04b | 89.00 ± 0.01b | 96.00 ± 0.00b | 100.00 ± 0.01a |
| 40.00 | 65.00 ± 0.02ab | 75.01 ± 0.02b | 81.00 ± 1.11a | 96.00 ± 0.00b | 100.00 ± 0.01a |
| 60.00 | 45.00 ± 0.01b | 60.00 ± 0.02b | 64.00 ± 0.01a | 96.00 ± 0.00a | 100.00 ± 0.01a |
| 80.00 | 30.00 ± 0.02a | 50.00 ± 1.01a | 60.00 ± 0.01a | 96.00 ± 0.01a | 100.00 ± 0.01a |
Table 4: Survival (%) in T. guineensis fingerlings transported with clove seed extracts
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Time (mins) |
0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| 0.00 | 100.00 ± 0.01d | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00a |
| 20.00 | 75.00 ± 0.01c | 77.00 ± 0.04b | 87.00 ± 0.01b | 92.00 ± 0.00b | 96.00 ± 0.01a |
| 40.00 | 50.00 ± 0.02ab | 58.01 ± 0.02b | 65.00 ± 1.11a | 78.00 ± 0.00b | 90.00 ± 0.01a |
| 60.00 | 35.00 ± 0.01b | 40.00 ± 0.02b | 54.00 ± 0.01a | 65.00 ± 0.00a | 82.00 ± 0.01a |
| 80.00 | 20.00 ± 0.02a | 30.00 ± 1.01a | 40.00 ± 0.01a | 50.00 ± 0.01a | 77.00 ± 0.01a |
Table 5: Survival (%) in T. guineensis Fingerlings Transported with Nut Meg Extracts
| Concentrations (mg/l) | |||||
|---|---|---|---|---|---|
| Time (mins) |
0.00 | 10.00 | 20.00 | 30.00 | 40.00 |
| 0.00 | 100.00 ± 0.01d | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00b | 100.00 ± 0.00a |
| 20.00 | 70.00 ± 0.01c | 71.00 ± 0.04b | 87.00 ± 0.01b | 90.00 ± 0.00b | 92.00 ± 0.01a |
| 40.00 | 40.00 ± 0.02ab | 52.01 ± 0.02b | 60.00 ± 1.11a | 75.00 ± 0.00b | 88.00 ± 0.01a |
| 60.00 | 25.00 ± 0.01b | 40.00 ± 0.02b | 50.00 ± 0.01a | 60.00 ± 0.00a | 80.00 ± 0.01a |
| 80.00 | 15.00 ± 0.02a | 25.00 ± 1.01a | 36.00 ± 0.01a | 40.00 ± 0.01a | 65.00 ± 0.01a |
Table 6: Survival (%) in T. guineensis Fingerlings Transported with Mustard Seed Extracts
Discussion
The water quality parameters in experimental tanks results indicated a significant reduction (P<0.05) in the values of dissolved oxygen, in the water without anaesthetics (0.00mg/l). Whereas, higher values of ammonia and sulphide were also recorded in the control. While other water quality parameters were within the same range with no significant different in relation to the concentration of the anaesthetics Similar result was observed in the transportation waters of largemouth bass (Micropterus salmoides) using clove [20,21]. This is because use of anaesthetics in fish transport minimizes its activity, and the excretion of ammonia through the gills. Hence these plants extracts maintain a relatively good water quality during fish transportation [22,23]. However, Wedemeyer [24] reported that typical oxygen consumption rates of spring Chinook salmon, Oncorhynchus tshawytscha smolts are 210 mg kg/1h/1 in untreated transportation tank water and 190 mg kg/1h/1 when 10 mg/L MS-222 is added. Kolarova, et al. [25] have also observed that 2-phenoxyethanol did not affect the oxygen consumption or ammonia production of goldfish, Carassius auratus during transport; on the other hand Lays, et al. [26] have showed that it suppressed oxygen consumption rates of guppy, Poecilia reticulata, in simulated transportation experiment. In simulated air transport of platy fish, Xiphophorus maculates, 2-phenoxyethanol and quinaldine sulphate were efficient in decreasing the excretion of CO2 and ammonia, MS-222 reduced ammonia but not CO2 production and metomidate had no effect on excretion of metabolic wastes [27]. Iversen, et al. [28] observed that the use of anesthetic (ethynelglycolmonophenylether) during simulated transportation of red porgy, Pagrus fry had no significant effect on CO2, NH3 and NH4 concentrations of exposure water. The results showed by Leji, et al. [29] experiment indicate wide variation in oxygen consumption between six species of fish in response to low concentrations of clove oil during extended exposure. These examples indicate that different anesthetics may differ in their efficiency and it may also indicate the presence of inter fishspecies variability in metabolic rates during exposure to low sedation concentration of anesthetics.
The need for some means of immobilizing in aquatic animals without harm to the subject has long been recognized. Documents show that basic and applied researches have focused on immobilization of fish as the most applicable aquatics subjected to aquaculture. Anesthesia as a method of immobilizing is now a common practice in fish. The use of anesthetics could possibly improve transport survival [30,31]. In this work, high survival was recorded in the fish exposed to different concentrations of clove, nut Meg and mustard seed extracts. This observation corroborated that of Adamek, et al. [32] in transportation of young carp (Cyprinus carpio), and that of Akinrotimi and Ukwe [33] in transportation of three sizes of Clarias gariepinus with clove extracts. This according to Akinrotimi, et al. [34] is due to light anaesthesia induced by the application of clove for the mitigation of stress associated with transportation, which allows the fish to breathe effectively, and maintain equilibrium during transportation, thereby enhances its survival. Apart from reduction in activity, low doses of anesthetics are also used to reduce metabolic rate of aquatics during transportation. This may reduce physiological stress, oxygen consumption and CO2 and ammonia production [35] and thus decrease mortalities during and after transportation.
Conclusion
In conclusion, results indicated that comparative survival in the fingerlings of T.guineensis transported with nut Meg mustard and clove seed extracts indicated that higher survival rate was recorded in fish transported with clove seed extracts when compared to mustard and nut Meg extracts. The water quality parameters in experimental tanks results indicated a significant reduction (P<0.05) in the values of dissolved oxygen, in the water without anaesthetics (0.00mg/l). Whereas, higher values of ammonia and sulphide were also recorded in the control. While, other water quality parameters were within the same range with no significant different in relation to the concentration of the anaesthetics. From the results obtained in this study, a concentration of 40.0 mg/l of clove seed extracts is ideal for transportation of T.guineensis in aquaculture with no mortality.
References
- Akinrotimi A, Ansa EJ, Owhonda KN, et al. Effects of transportation stress on haematological parameters of black chin tilapia, Sarotherodon melanotheron. J Animal & Veterinary Adv. 2007;6(7):841-5.
- Gabriel UU, Deekae SN, Akinrotimi OA, et al. Assessment of efficacy and egg hatchability in African catfish Clarias gariepinus exposed to anesthetic metomidate. ARC J Animal & Veterinary Sci. 2015a;1(1):24-31.
- Akinrotimi OA, Edun OM, Mebe ED. Effects of clove seeds as anaesthetic agents in two species of grey mullets Liza falcipinnis and Liza grandisqumis. J Aquatic Sci. 2013a;1(1):7-10.
- Akinrotimi OA, Gabriel UU, Edun OM. The efficacy of clove seed extracts as an anaesthetic agent and its effect on haematological parameters of African catfish (Clarias gariepinus). Int J Aquaculture & Fishery Sci. 2015b;1(2):42-7.
- Akinrotimi OA, Abu OMG, Aranyo AA. Environmental friendly aquaculture key to sustainable fish farming development in Nigeria. Continental J Fisheries & Aquatic Sci. 2011;5(2):17-31.
- Akinrotimi OA, Edun OM, Ibama JEW. The roles of brackish water aquaculture in fish supply and food security in some coastal communities of Rivers state, Nigeria. Int J Agr Sci & Food Tech. 2015c;1(1),12-9.
- Akinrotimi OA, Ansa EJ, Edun OM. Effectiveness of clove seed extracts as anaesthetics in transportation of Tilapia guineensis juveniles. J Aquaculture Eng & Fisheries Res. 2016;2(2);67-75.
- Akar AM. Effects of clove oil on the response of blue tilapia (Oreochromis aureus) by transportation stress. J Arabian Aquaculture Soc. 2011;6(1):77-86.
- Carneiro PC, Urbrinati EC, Martins ML. Transport with different concentration of benzocairne concentrations and its consequences on haematological parameters and gill parasite populations of matrixa. Acta Sci. 2002;24:555-56.
- Agokei OE, Adedisi AA. Tobacco as anaesthetics for fish handling procedures. J Medicinal Plants Res. 2010;4(14):1396-9.
- Akinrotimi OA, Okenwa U, Stephen MU, et al. Effects of Different Combinations of Two Spices: Clove and Nutmeg Seed Extracts on Antioxidants Levels in African Catfish (Clarias gariepinus). J Fisheries Sci. 2021;3(2):26-44.
- Gabriel UU, Akinrotimi OA. Management of Stress in fish for sustainable aquaculture development. Res. 2011;3(4):28-38.
- Barton BA. Stress in fish: A diversity of responses wish particular references to changes in circulating corticosteroids. Integrative Comparative Biology. 2002;42:517-28.
- Akinrotimi OA, Gabriel UU, Orokotan OO. Changes in Enzymes activities of Clarias gariepinus brood fish exposed to anaesthetics metomidate. Appl Ecol & Environmental Sci. 2013;1(3):37-40.
- Gabriel UU, Akinrotimi OA, Orlu EE. Haematological characteristics of the bloody cockle Anadara Senilis from Andoni Flats, Niger Delta, Nigeria. Sci World J. 2011b;6(1):1-4.
- Akinrotimi OA. Assessment of the efficacy of synthetic and natural anaesthetics on the African catfish Clarias gariepinus (Burchell, 1822). PhD Thesis. Department of Fisheries and Aquatic Environment, Rivers State University of Science technology Port Harcourt. 2014;260.
- Gabriel UU, Obomanu FG, Edori OS. Hematology, Plasma enzymes and organ indices of Clarias gariepinus after intramuscular injection with aqueous leaves extracts of Lepidagathis alopecuruides. Afr J Biochemical Res. 2009;3(9):312-6.
- Gabriel UU, Akinrotimi OA, Ariweriokuma VS. Changes in metabolic enzymes activities in selected organs and tissues of Clarias gariepinus exposed to cypermethrin. J Environmental Eng & Tech. 2012;1(2):13-9.
- Agbaje EO. Gastro intestinal effects of Syzigium aromaticum in animal model. Nigeria Quarterly J Hospital Med. 2008;18(3):137-41.
- Cooke SJ, Sunk CD, Ostrand KG, et al. Behavioral and physiological assessment of low concentrations clove oil anaesthetics for handling and transporting largemouth bass. Aquaculture. 2004;239:509-29.
- Cordova MS, Braun CB. The use of anaesthesia during evoked potential audiometry in gold fish (Carassius auratus). Brain Res. 2007;1153:78-83.
- Costas B, Argao C, Mancera JM, et al. High stocky density induces crowding stress and affects amino acid metabolism in senegalese sole (Solea senegalensis) Juveniles. Aquaculture Res. 2008;39:1-9.
- Gabriel UU, Akinrotimi OA, Eseimokuma F. Haematological responses of wild Nile tilapia Oreochromis niloticus after acclimation to captivity. Jordan J Bio Sci. 2011a;4(4):223-30.
- Wedemeyer GA. Some potentials and limits of the leucorit test as fish health assessment method. J Fish Biology. 1996;23:711-6.
- Kolarova M, Kolarova L, Perina A, et al. Animal products and selected human infections disease. Cech J Animal Sci. 2012;47:297-307.
- Lays N, Iversen MMT, Frantzen M, et al. Physiological stress responses in spotted wolfish (Anarhinchas minur) subjected to acute disturbance and progressive hypoxia. Aquaculture. 2017;295:126-33.
- Hur JW, Park IS, Chang YJ. Changes of haematological characteristics of cultured sweet fish (Plecoglossus altivelis) by anaesthetic transport. Ocean Polar Res. 2005;27:251-60.
- Iversen M, Finstad B, Mackinley RS, et al. The efficacy of metomidate, clove oil, Aqui-S and Benzoic(R) as anaesthetics in Atlantic salmon (Salmon Salar.) Smolts and their potential in stress reducing capacity. Aquaculture. 2003;221:549-66.
- Leji J, Babitha GS, Rejitha V, et al. Thyroid and osmoregulatory responses in tilapia (Oreochromis mossambicus) to the effluents of coconut husk netting. J Endocrinology & Reproduction. 2015;11:24-31.
- Gabriel UU, Auyanwu PE, Akinnotimi AO. Blood characteristics Associated with Black Chin-Tilapia, Sarotherodon melanotlenon. J Fish Int. 2007a;2(2):186-9.
- Gabriel UU, Anyanwu PE, Akinrotimi, OA. Effects of different acclimation period on haematological characteristics of brackish water tilapia Sarotherodon. J Fisheries Int. 2007b;2:115-10.
- Adamek Z, Tasic K, Paul K, et al. The effect of 2-phnenoxyethanol narcosis on blood of young carp. Veterinary Arc. 2016;63:245-50.
- Akinrotimi OA, Ukwe OIK. Assessment of mustard seed powder as anaesthetic agents in three sizes of Clarias gariepinus. J Agricultural Res Adv. 2019;1(2):38-45.
- Akinrotimi OA, Gabriel UU, Deckae SN. Anaesthetic efficacy of sodium bicarbonate and its effect on the blood parameters of African catfish, Ckaruas gariepinus. J Aquatic Sci. 2014a;29(IB):223-46.
- Akinrotimi OA, Gabriel UU, Dekae SN. Investigations on the potential of Indian almond free (Terminalia catapaa) leaf extracts as anesthetic agents in African catfish (Clarias gariepinus). J Aquatic Sci. 2014b;29(IB):223-31.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref