Research Article - Biomedical Research (2017) Volume 28, Issue 6
Single low dose preoperative pregabalin induces satisfactory analgesia following laparoscopic cholecystectomy: a randomized double blinded placebo controlled study
Mansour Choubsaz1, Somayeh Mohammadi1 and Nasrin Amirifard2,*1Department of Anesthesiology, Critical Care and Pain Management, Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Department of Radiology, Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
- *Corresponding Author:
- Nasrin Amirifard
Department of Radiology, Imam Reza Hospital
Kermanshah University of Medical Sciences, Iran
Accepted date: October 26, 2016
Abstract
Background: Limited supportive evidence, regarding the administration of low dose pregabalin, aimed to reduce pain following laparoscopic cholecystectomy (LC). The analgesic efficacy of a single dose of 75 mg pregabalin versus placebo in patients undergoing elective laparoscopic cholecystectomy with general anesthesia was evaluated.
Methods: The study was conducted on 168 candidates of laparoscopic cholecystectomy. Participants were assigned to one treatment arm with a single dose of 75 mg pregabalin per OS 30 minutes before anesthesia induction (n=84), and one control arm with placebo (n=84). The primary outcome measure was pain intensity measured by visual analog scale at three different time intervals including 4, 6 and 24 hours following anesthesia induction. Patients were educated preoperatively and were asked to rate their pain intensity on ranges of 1-4 (minor), 4-7 (moderate) and 7-10 (severe).
Results: Pain scores did not change over the interval of 4 to 6 hours after surgery. Though, both groups experienced a significant reduction in postoperative pain from 6 to 24 hours following LC. For both 4 and 6 hours following surgery, control patients were 2.9 times (95% CI, [1.2-7.1], P=0.013) more likely to have severe pain as denoted by VAS (7-10). After 24 hours, none of the patients experienced severe pain, however, control patients had significantly higher odds of moderate pain (OR 6.5, 95%CI [3.0-13.5], P<0.001). SPSS, version 18, were used to analyses the date. Shapiro-Wilk test, independent t-test, Pearson Chi-square test and Binary logistic regression were used.
Conclusions: Our findings extend the knowledge of preemptive analgesia and may encourage the use of lower doses of pregabalin to achieve satisfactory short-term pain control in the case of laparoscopic cholecystectomy. Since acute pain after LC peaks within 6 hours of the procedure, the restricted (short term) analgesic effect of low dose pregabalin may be justified.
Keywords
Pregabalin, Postoperative Period, Analgesia, Cholecystectomy, Laparoscopic.
Introduction
There is extensive evidence supporting the use of perioperative analgesia in reducing pain following surgery since postoperative pain control improves both the success of the surgical intervention and patient comfort. Different medications including opioids, anticonvulsants, and nonsteroidal anti-inflammatory agents have been used with considerable variations regarding effectiveness and side effects. Proper analgesia is often achieved at the expense of increased adverse effects including emesis, respiratory depression, altered mental status, increased sedation and visual disturbance [1,2]. Therefore, there is an ongoing need to search for new medications with less adverse outcomes and better therapeutic effects.
Among the anti-anticonvulsant family of drugs, Pregabalin is a γ-amino butyric acid (GABA) analog which is widely used in treating diabetic neuropathy, post herpetic neuralgia, and fibromyalgia and as an adjunctive therapy for partial-onset seizures [3,4]. Recently, a number of meta-analysis studies have documented the short-term analgesic and opioid-sparing effects of pregabalin after various surgeries [5,6]. Meanwhile, laparoscopic cholecystectomy (LC) is a widely practiced surgery around the world, though post-operative pain control remains a challenge in this surgery. Various randomized trials have outlined considerable post-operative analgesic effects of pregabalin in reducing acute pain following laparoscopic cholecystectomy [7-9]. Yet, limited supportive evidence, regarding the administration of single low dose pregabalin, aimed to reduce pain after LC [10,11]. To the best of our knowledge, there are no studies in the literature addressing the possible analgesic effect of a single low dose (75 mg) pregabalin after LC.
There appears to be sufficient evidence at present to justify a prospective randomized controlled trial with the aim of evaluating single 75 mg pregabalin analgesic role in patients undergoing elective laparoscopic cholecystectomy with general anesthesia.
Materials and Methods
Study design
The present study was designed as a prospective randomized double blinded placebo controlled trial with the aim of evaluating the role of a single 75 mg pregabalin dose in reducing post-operative pain in patients undergoing elective LC. The trial protocol was registered on the official website of the Iranian registry of clinical trials and approved by the ethical committee of Kermanshah university of medical sciences (ID: IRCT201312041617N8). This parallel designed trial was conducted in Imam Reza hospital affiliated to Kermanshah University of medical sciences starting from January 2014 to January 2015. All participants provided their written consent after being informed about the study design and purpose. Participants were randomly allocated to one treatment arm with a single dose 75 mg pregabalin per OS 30 minutes before anesthesia induction (n=84), and one control arm with placebo (n=84).
Participants
All consenting candidates of elective LC with ASA class I or II, aged 20 to 80 years were considered eligible for inclusion. Exclusion criteria were documented a history of impaired liver or kidney functions, inflammatory bowel disease, chronic pain, a daily intake of analgesics/corticosteroids/opioids/sedatives, uncontrolled medical disease (diabetes mellitus and hypertension), a history of hypersensitivity or serious adverse reaction to pregabalin. Two-hundred individuals were screened of whom 180 met the inclusion/exclusion criteria and were randomly assigned to treatment or placebo groups using a previously developed allocation sequence in the Microsoft excel program.
Interventions
In the treatment group (pregabalin group), all patients received one single 75 mg pregabalin capsule (Lyrica, Germany) 30 minutes before anesthesia induction. In the placebo group, patients were administered with a placebo identical in shape and color. Staff nurses who were not involved in the study administered all medications with sips of water. The hospital pharmacy provided all medications which were identical. In both groups, anesthesia, and surgical procedures were standardized. Briefly, induction was attempted with intravenous propofol 2 mg/kg and fentanyl 1 mcg/kg. Atracurium (0.5 mg/kg IV) was administered to facilitate orotracheal intubation and anesthesia was maintained using isoflurane for 5 min in combination with air and oxygen 50% each. Intravenous neostigmine 0.04 mg/kg and atropine 0.01 mg/kg were used to reverse neuromuscular blockage at the end of the operation. Extubation was administered after recovery of adequate spontaneous ventilation and patients were transferred to post anesthesia care unit where additional recovery was monitored.
Outcome
The primary outcome measure was the intensity of postoperative pain at rest (static) measured using a 10 cm visual analog score (VAS), where none indicates pain and 10 correspond to the worst pain imaginable. Measurements in both groups were taken by one anesthesiology assistant at three different time intervals including 4, 6 and 24 hours following anesthesia induction. Patients were educated preoperatively and were asked to rate their pain intensity on ranges of 1-4 (minor), 4-7 (moderate) and 7-10 (severe). No secondary outcome was defined as a 75 mg dose of pregabalin was not assumed to cause adverse effects. The endpoint was 24 hours after the operation.
Sample size, randomization and sequence generation
Assuming a type I (α) error of 0.05, a power of 0.98 (1-β) and a medium effect size (d), a sample size of 84 patients in each arm was estimated based on the following equation;
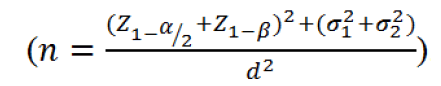
Considering possible drop outs, 90 patients were enrolled in each group. A statistician not otherwise involved in the study, provided the randomization process using the Microsoft excel program in which 180 random numbers were generated and fixed by the Rand function. Thereafter using the IF function, numbers larger than 0.5 were assigned to A (treatment group) and numbers smaller than 0.5 were assigned to B (control group). Allocation letters were printed and sent to pharmacy unit where sham or pregabalin capsules were placed in opaque envelopes considering the allocation letters and envelopes were sealed. The packages were transferred to surgery unit where blinded nurses administered the medication to each patient during the preoperative evaluations in a consecutive order. Post-operative pain assessment was performed by one anesthesiology assistant that was blinded to group allocation. Information for each patient was then gathered in the context of a previously designed data gathering form.
Statistical analysis
Data management and analysis were conducted using the Statistical Package for Social Sciences (SPSS, version 18, Chicago, Illinois). Normality was checked using the Shapiro- Wilk test and normally distributed continuous variables were summarized using mean and standard deviation (Mean ± SD) while summary statistics were the median and interquartile range (Median, [IQR]) for non-normally distributed variables. Categorical variables were summarized frequencies and proportions No (%). Comparison between case and control groups were made using the independent t-test regarding baseline continuous variables and Pearson Chi-square test for baseline categorical variables. Binary logistic regression analysis was employed to adjust for the confounding effect of baseline variables on postoperative pain. Upon initiation of inferential statistical testing, a two-sided p-value of less than 0.05 was considered statistically significant.
Results
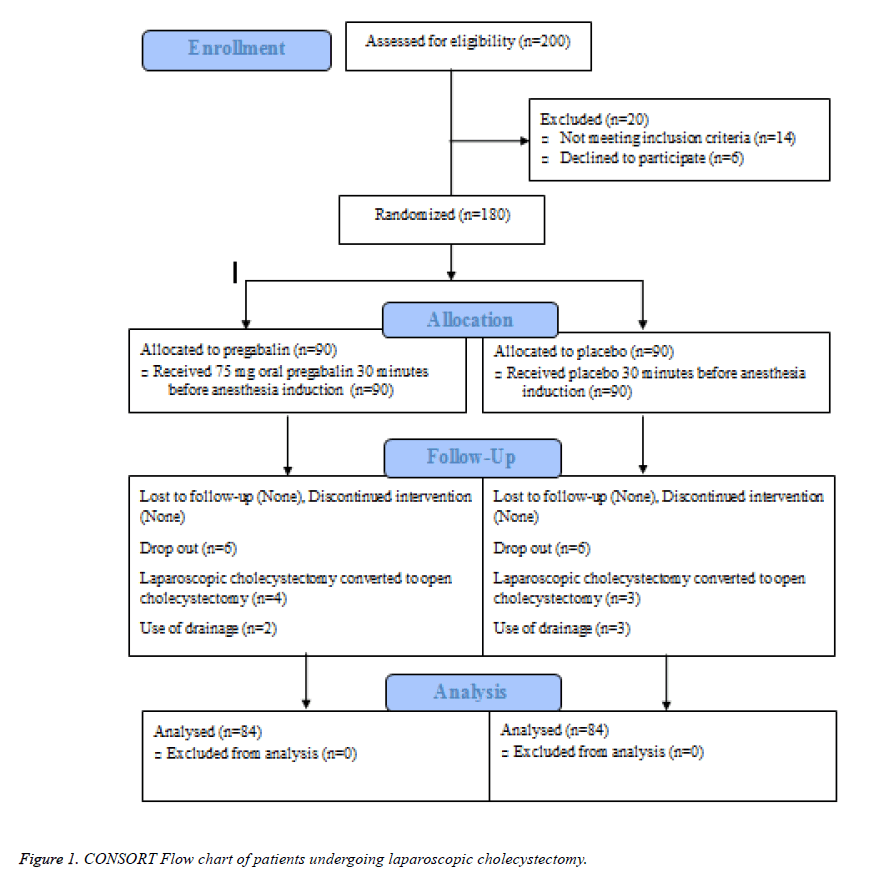
Figure 1 illustrates the flow of patients through the study. From January 2014 to January 2015, two hundred patients scheduled for laparoscopic cholecystectomy were screened for eligibility of whom 180 fulfilled the inclusion criteria and were randomly allocated to receive placebo (n=90) or pregabalin (n=90). Treatments were successfully administered to all patients in both groups, though four and three surgeries converted to open cholecystectomy in the pregabalin and placebo groups, respectively. In addition, two patients in the pregabalin group and three control subjects needed drainage, which were excluded resulting that in a total of 168 patients, 84 in each arm undergoing statistical analysis.
Participants were similarly distributed across the study groups considering age, gender, ASA class, weight, and height (Table 1). On a statistical perspective, however, patients assigned to pregabalin had significantly higher BMI compared to control individuals.
| Parameters | Pregabalin Group (n=84) | Control Group (n=84) | P value |
|---|---|---|---|
| Age, years | 42.2 ± 12.6 | 42.9 ± 11.5 | 0.72 |
| Gender | 0.70 | ||
| Male | 15 (17.4) | 17 (19.8) | |
| Female | 71 (82.6) | 69 (80.2) | |
| ASA Status | 0.82 | ||
| I | 75 (87.2) | 74 (86.0) | |
| II | 11 (12.8) | 12 (14.0) | |
| Weight, kg | 73.8 ± 10.3 | 71.4 ± 8.3 | 0.09 |
| Height, cm | 163.1 ± 7.4 | 163.9 ± 7.1 | 0.50 |
| BMI, cm | 27.9 ± 4.3 | 26.7 ± 2.5 | 0.02 |
| Data in table are Mean ± SD or Number (%) Statistical tests: χ2 test for gender and ASA status, independent t-test for age, weight, height and BMI |
|||
Table 1. Patients’ demographics and baseline data.
Pain scores remained stable over the interval of 4 to 6 hours after surgery. Though, both groups experienced a significant reduction in postoperative pain from 6 to 24 hours following LC. At either time intervals, patients receiving pregabalin were significantly less likely to rate their pain as severe, comparing to control subjects (Table 2). For both 4 and 6 hours following surgery, control patients were 2.9 times (95% CI, [1.2-7.1], P = 0.013) more likely to have severe pain as denoted by VAS (7-10). After 24 hours, none of the patients experienced severe pain, however, control patients had significantly higher odds of moderate pain (OR 6.5, 95%CI [3.0-13.5], P<0.001).
| Pain Scores | Pregabalin Group (n=84) | Control Group (n=84) | Total | P value |
|---|---|---|---|---|
| 4 hours | 0.013 | |||
| Minor-Moderate | 78 (90.7) | 66 (76.7) | 144 (83.7) | |
| Severe | 8 (9.3) | 20 (23.3) | 28 (16.3) | |
| Odds of Severe pain (95 CI) | 0.6 (0.5-0.8) | 1.9 (1.1-3.5) | ||
| OR (95 CI) | 2.9 (1.2-7.1) | |||
| 6 hours | 0.013 | |||
| Minor-Moderate | 78 (90.7) | 66 (76.7) | 144 (83.7) | |
| Severe | 8 (9.3) | 20 (23.3) | 28 (16.3) | |
| Odds of Severe pain (95 CI) | 0.6 (0.5-0.8) | 1.9 (1.1-3.5) | ||
| OR (95 CI) | 2.9 (1.2-7.1) | |||
| 24 hours | <0.001 | |||
| Minor | 74 (86.0) | 42 (48.8) | 116 (67.4) | |
| Moderate | 12 (14.0) | 44 (51.2) | 56 (32.6) | |
| Odds of Moderate pain (95 CI) | 0.3 (0.2-0.5) | 2.2 (1.7-2.9) | ||
| Odds Ratio (95 CI) | 6.5 (3.0-13.5) | |||
| Data in table are Mean ± SD or Number (%) | ||||
Table 2. Post-operative pain scores after 4, 6 and 24 hours of surgery.
Since pain scores were exactly the same for all patients at 4 and 6 hours of surgery, two binary logistic regression models were employed in order to control the possible confounding effect of demographics on post-operative pain at 6 and 24 hours intervals. None of the predictors significantly affected the odds of having severe pain at 6 hours except for the group membership, i.e. control subjects had significantly higher odds of severe pain after controlling age, gender, BMI and ASA status (AOR 3.0, 95% CI [1.2-7.5], P=0.02) (Table 3). Similarly, at 24-hour time point, control patients had 6.6 times higher adjusted odds of moderate pain (95%CI [3.1-14.3]) after controlling covariates listed above (P<0.001).
| Predictors | Estimates | |
|---|---|---|
| P value | OR (95% CI) | |
| Severe/Minor-Moderate Pain at 4 or 6 hours | ||
| Study Group (Control/Case) | 0.02* | 3.0 (1.2-7.5) |
| Age | 0.89 | 0.9 (0.9-1.0) |
| Gender (Male/Female) | 0.05 | 0.1 (0.0-0.9) |
| ASA Status (II/I) | 0.83 | 0.8 (0.2-3.4) |
| BMI | 0.63 | 0.9 (0.8-1.1) |
| Constant | 0.61 | (-) |
| Moderate/Minor Pain at 24 hours | ||
| Study Group (Control/Case) | <0.001* | 6.6 (3.1-14.3) |
| Age | 0.66 | 0.9 (0.9-1.0) |
| Gender (Male/Female) | 0.45 | 0.6 (0.2-1.7) |
| ASA Status (II/I) | 0.79 | 1.1 (0.3-3.9) |
| BMI | 0.83 | 1.0 (0.9-1.1) |
| Constant | 0.29 | (-) |
Table 3. Logistic regression analysis of control confounding effects of demographics on postoperative pain.
Discussion
In this randomized double-blinded trial, we attempted to evaluate the efficacy of single dose 75 mg preoperative pregabalin capsules for pain management in patients undergoing LC under general anesthesia. Our findings were in favor of a considerable effect size regarding pregabalin reducing post-operative pain at rest comparing to placebo. Such results extend the knowledge of preemptive analgesia and may encourage the use of lower doses of pregabalin to achieve satisfactory pain control in the case of laparoscopic surgeries. Such a low dose may not be applicable to other surgical methods since a recent meta-analysis of randomized trials declares a 225-300 mg/day pregabalin to be the lowest effective dose irrespective of surgery method [5]. Meanwhile, multiple trials have documented the beneficial use of pregabalin as an analgesic agent in various surgeries; however, methodological heterogeneity regarding dose differences, pain assessment intervals, procedures, and subjects has resulted in controversial findings [1,2].
To further compare our results with the existing literature, we only considered recent trials in the setting of LC surgeries. Various doses of pregabalin ranging from 50 to 600 mg administered in single or divided forms have been tested. Agrawal et al. evaluated the efficacy of a single preoperative dose of pregabalin (150 mg) for attenuating postoperative pain and showed that both static and dynamic postoperative pains along with postoperative patient-controlled fentanyl consumption were reduced in the pregabalin group [10]. Peng et al. showed that comparing to the placebo, pain scores were lower in the pregabalin 75 mg group only in the first 90 minutes after the surgery. In addition, Pregabalin 50 mg resulted in pain reduction at 30 and 45 minutes [11]. Similar findings were reported by Babalan et al. at therapeutic doses of 150 and 300 mg pregabalin [8]. Our findings both confirm and extend these results, though we did not gather information on opioid consumption nor did we study possible side effects since a 75 mg pregabalin dose was not expected to increase adverse effects. Meanwhile, Administration of 600 mg pregabalin per os, divided into two preoperative doses has been shown to effectively reduce postoperative pain in a study conducted by Sarakatsianou et al. At this dose, however, analgesic effects were associated with increased incidence of dizziness [9]. In the most recent study of preemptive pregabalin efficacy by LC, Bekawi et al. which documented a considerable effect of 150 mg pregabalin capsules administered 2 hours preoperatively, 12 hours postoperatively, and twice daily for 2 days [7].
From the findings of this study along with those of previous research, one may conclude that single preoperative dose of pregabalin (75-150 mg) offers satisfactory postoperative pain control with proper safety profile. Since acute pain after LC peaks within 6 hours of the procedure [12], the restricted analgesic effect of low dose pregabalin may be justified. Higher doses (300-600 mg) induce promising analgesia for longer periods, though side effects are considerable. It is, therefore, implied that determining preemptive pregabalin dose may be individualized for each patient based on expected pain duration and intensity with respect to intraoperative/ preoperative determinants.
One of the major limitations of this study was that pain scores were aggregated into three categories of minor, moderate and severe which prevented us from the further comparison of our findings with others. In addition, we did not include information about opioid consumption and possible side effects which are important outcome measures. To the best of our knowledge, this study was the first of its kind emphasizing the analgesic effect of one single 75 mg preoperative pregabalin in preventing postoperative pain following LC. Comparing to the existing studies, drop -outs were relatively small and a larger number of subjects were studied increasing the impact of these results. It is proposed that in future studies of LC patients, risk factors for high-intensity pain or long lasting pain may be studied in order to provide a comprehensive patient-specific pregabalin dose specification.
Acknowledgements
The authors would like to appreciate the contribution of deputy dean of School of Medicine and sponsorship of deputy chancellor of Kermanshah University of Medical Sciences. We also thank Khosro Setayeshi for assistance in surgical procedures and Mona Amiri as the anesthesiology assistant. We express our gratitude to Shadi Ghasemi for statistical supervision. This study has been registered with number IRCT201312041617N8 as a clinical trial.
References
- Eipe N, Penning J, Yazdi F, Mallick R, Turner L, Ahmadzai N, Ansari MT. Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain 2015; 156: 1284-1300.
- Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 2015; 114: 10-31.
- Zhang SS, Wu Z, Zhang LC, Zhang Z, Chen RP. Efficacy and safety of pregabalin for treating painful diabetic peripheral neuropathy: a meta-analysis. Acta Anaesthesiol Scand 2015; 59: 147-159.
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev 2009.
- Engelman E, Cateloy F. Efficacy and safety of perioperative pregabalin for post-operative pain: a meta-analysis of randomized-controlled trials. Acta Anaesthesiol Scand 2011; 55: 927-943.
- Yao Z, Shen C, Zhong Y. Perioperative Pregabalin for Acute Pain after Gynecological Surgery: A Meta-analysis. Clin Ther 2015; 37: 1128-1135.
- Bekawi MS, El Wakeel LM, Al Taher WM, Mageed WM. Clinical study evaluating pregabalin efficacy and tolerability for pain management in patients undergoing laparoscopic cholecystectomy. Clin J Pain 2014; 30: 944-952.
- Balaban F, Yagar S, Ozgok A, Koc M, Gullapoglu H. A randomized, placebo-controlled study of pregabalin for postoperative pain intensity after laparoscopic cholecystectomy. J Clin Anesth 2012; 24: 175-178.
- Sarakatsianou C, Theodorou E, Georgopoulou S, Stamatiou G, Tzovaras G. Effect of pre-emptive pregabalin on pain intensity and postoperative morphine consumption after laparoscopic cholecystectomy. Surg Endosc 2013; 27: 2504-2511.
- Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth 2008; 101: 700-704.
- Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, Chung F. Use of low-dose pregabalin in patients undergoing laparoscopic cholecystectomy. Br J Anaesth 2010; 105: 155-161.
- Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain 2001; 90: 261-269.