Research Article - Journal of Clinical Endocrinology Research (2018) Volume 1, Issue 1
Risk factors for lymph node recurrence in papillary thyroid carcinoma:experience with 2,000 patients operated on by a single surgeon.
Celso UM Friguglietti1*, Chin S. Lin2
1Head and Neck Surgeon, School of Medicine at University of Sao Paulo (USP), Av. Paulista 1159, Cj 1514, Sao Paulo, SP, Brazil–CEP 01311-200
2Physician Assistant at the Head and Neck Surgery Unit at the Cancer Institute of the State of Sao Paulo (ICESP), School of Medicine of the University of Sao Paulo (FMUSP), R Eca de Queiroz 415/73, Sao Paulo, SP, Brazil–CEP 04011-032
- *Corresponding Author:
- Celso UM Friguglietti
Head and Neck Surgeon,
Av. Paulista 1159, Cj 1514,
Sao Paulo, SP,
Brazil–CEP 01311-200
E-mail: frigugli@uol.com.br
Accepted date: June 14, 2018
Citation: Friguglietti CUM, Lin CS. Risk factors for lymph node recurrence in papillary thyroid carcinoma: experience with 2,000 patients operated on by a single surgeon. J Clin Endocrinol Res. 2018;1(1):6-16.
Abstract
Background: This study determined the predictive factors affecting lymph node recurrence in a large cohort with papillary thyroid carcinoma operated on by a single surgeon.
Methods: Retrospective, cross-sectional study including 2,112 patients with papillary thyroid carcinoma undergoing total thyroidectomy and therapeutic lymph node dissection.
Results: During a mean follow-up of 41 months, 63 patients experienced lymph node recurrence. On multivariate analysis, lymph node recurrence was associated with lymph node metastases (odds ratio [OR] 19.205 for metastasis to a single lymph node, p<0.001), use of radioiodine therapy (OR 3.344, p=0.012), and extrathyroidal extension (OR 2.167, p=0.011). The presence of two positive lymph nodes (OR 18.9, p<0.001) and a primary tumor size of 1.15 cm were predictors of lymph node recurrence.
Conclusions: In a large cohort with papillary thyroid carcinoma, the presence of two positive lymph nodes and a primary tumor size of 1.15 cm predicted lymph node recurrence.
Keywords
Thyroid neoplasms, thyroid cancer, metastasis, thyroidectomy, lymph node excision
Introduction
Papillary carcinoma comprises 80% of the malignancies of the thyroid. Despite showing a substantially increasing incidence, the mortality rates associated with this malignancy have remained largely unchanged. As a result, treatment of papillary thyroid cancer remains focused on the occurrence of local and regional recurrence [1].
Even though papillary thyroid cancer is considered to be a low aggressive tumor with excellent survival rates, occult lymph node metastases affect about 90% of the patients. Lymph node metastases are clinically palpable in 10% of the individuals and occult in up to 90% of the patients undergoing prophylactic lymph node dissection. In up to 30% of the patients with clinically occult lymph node disease, the metastases are detected by preoperative ultrasound [1-4]. The risk of disease recurrence over 30 years is about 30%, of which 70% occur as lymph node metastasis [2]. The presence of metastatic lymph node at diagnosis is an independent risk factor for recurrence [3,4]. Other clinical and pathological factors have been speculated as potentially increasing the risk of recurrence, including the histological variant of the tumor, the occurrence of extrathyroidal extension, and size and number of metastases [1-4]. However, the association between these variables and the risk of disease recurrence is still speculative.
Over the past 15 years, one of us (C.U.M.F.) has operated on more than 2,000 patients with papillary thyroid carcinoma. This is one of the largest series of papillary thyroid carcinoma surgically treated by a single surgeon, thus guaranteeing the adoption of uniform procedures. The patients belong to a mixed Brazilian population, ensuring biological variability to the cohort. This extensive experience offers a unique opportunity to evaluate the factors involved on lymph node recurrence in this type of neoplasm. The present study analyzed the risk factors for lymph node recurrence in papillary thyroid carcinoma, with emphasis on the characteristics of the primary tumor and lymph nodes involved at the time of the surgery.
Materials and Methods
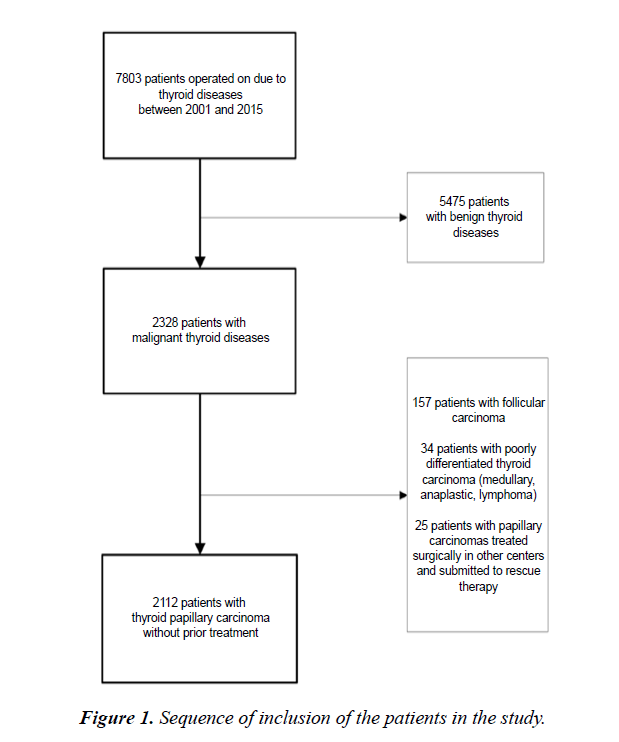
This was a retrospective, cross-sectional study of 2, 112 patients with papillary thyroid carcinoma who underwent surgical treatment between January 2001 and December 2015. A single surgeon performed all operations and followed up the patients. The flow diagram in Figure 1 shows the sequence of inclusion of the study patients. The study was conducted according to the Declaration of Helsinki and meets local ethical guidelines, including adherence to legal requirements for studies involving human subjects.
The inclusion criteria were a diagnosis of papillary thyroid carcinoma confirmed on histopathology and a regular follow-up for at least 16 months. The exclusion criteria were a prior treatment for thyroid cancer and incomplete or questionable information recorded in the histopathology report or during follow-up.
Total thyroidectomy was the standard procedure in all cases. We performed therapeutic central compartment neck dissection when the presence of central or lateral metastases was confirmed preoperatively by ultrasonography and fine-needle aspiration biopsy (FNAB). During surgery, unilateral (level VI) or bilateral (levels VI and VII) central neck compartment dissection was systematically performed upon identification of lymph nodes with the following features: round, cystic, hard on palpation (suggesting calcification), large (>4 mm), and which would appear as suspicious on postoperative ultrasonography. We only performed therapeutic dissection of the lateral neck compartment when lymph node metastases (levels II to V) were confirmed by imaging and cytopathology.
During the entire study, the surgical team comprised a single surgeon, five assistants, and two surgical technicians. Between 2001 and 2012, all procedures were carried out in four different hospitals in Sao Paulo, Brazil (Oswaldo Cruz, Santa Catarina, 9 de Julho, and Santa Rita). Two pathology laboratories (CICAP and Ferdinando Costa) performed all frozen section and final histopathology exams. After 2012, all procedures were centralized at Hospital Santa Catarina and Laboratório Ferdinando Costa.
The diagnosis of papillary carcinoma and its variants was defined according to the World Health Organization classification [5]. The information collected included the size of the primary tumor, presence of multifocal disease or extrathyroidal extension, and number of resected and positive lymph nodes reported on the final histopathology. No information was available in regards to the bilaterality of the neoplastic foci, lymph nodes sizes, or the presence of extranodal extension. We defined as microscopic lymph nodes those lymph nodes found on histopathology of patients undergoing total thyroidectomy without neck compartment dissection. This definition was based on the size of the lymph nodes and the fact that they were not identified by the surgeon and, consequently, were not systematically dissected. In contrast, we defined as macroscopic lymph nodes those lymph nodes resected by therapeutic dissection.
Ablative or therapeutic radioiodine therapy was recommended to patients with risk factors for disease recurrence: (a) clinical factors – male gender, age >45 years, increased antithyroglobulin antibody levels (which could interfere with the patient's followup); (b) pathological factors – tumors with a diameter >2 cm, multicentric, of an aggressive variant and with extrathyroidal extension, as well as presence of lymph node metastases; and (c) surgical factors – tumors close to the recurrent nerve or in the upper thyroid pole and with a small resection margin, due to uncertainty about the extension (radicality) of the surgery. The radioiodine dose administered ranged from 100 to 250 mCi, depending on the indication (remnant ablation or adjuvant therapy); after 2013, doses of 30 mCi were administered for ablation in patients considered as low risk based on intraoperatory characteristics. Radioiodine therapy was followed by whole body scanning (WBS). Cases with abnormal uptake on WBS were evaluated with ultrasonography of the neck and computed tomography (CT) of the lungs in search of potential metastatic lesions.
All cases were followed up by both the surgeon and an endocrinologist. Evaluation of TSH suppression and detection of recurrence were done with measurement of levels of TSH, free T4, and antithyroglobulin antibodies every 3 months during the first year and every 6 months until the fifth year, in addition to cervical ultrasonography every 6 months. Until 2012, routine WBS surveillance was performed 12 months after surgery.
The analysis included only patients who had started treatment until December 2015. This was done to ensure a minimum follow-up of 16 months and because after this period, we changed our indication for radioiodine therapy after publication of the recommendations of the 2015 ATA consensus, which were then adopted by the surgeon.
After the initial treatment, the occurrence of lymph node recurrence was established as the finding of suspicious lymph nodes on imaging tests (ultrasound, CT, positron emission tomography [PET], or WBS), later confirmed by FNAB and thyroglobulin washout. All patients with recurrence underwent systematic dissection of the affected compartment (“berrypicking” was not performed in any of the patients).
The analysis included data on the patients' gender and age, disease management, surgical extension, histological variant and size of the primary tumor, presence of multifocal disease and extracapsular extension, involvement of lymph nodes, number of affected lymph nodes, disease staging, presence of autoimmunity, and treatment with radioiodine therapy. All information entered in the database were collected by the surgeon.
Statistical analysis
All statistical analyses were performed with SPSS, version 17 (IBM, Chicago, IL, USA). Classificatory variables are descriptively presented in contingency tables containing absolute (n) and relative (%) frequencies. Associations between variables were evaluated with the chi-square test or likelihood ratio test. The normality of quantitative variables was assessed with the Kolmogorov-Smirnov test. Quantitative variables are presented descriptively in tables as mean and standard deviation or median and interquartile range values. The groups and recurrence rates were compared with Student's t test or Mann-Whitney test when the criteria for parametric analysis were not met. We used receiver operator characteristic (ROC) curves to establish cut-off values for the variables "size of the primary tumor" and "number of positive lymph nodes," taking into account the sensitivity and specificity of each variable. Significant variables in the univariate analysis were included in a multiple logistic regression model with stepwise variable selection. P values < 0.05 were considered statistically significant, with the exception of the univariate analysis in the regression model, in which only variables with p<0.03 were included in the multiple regression model [6].
Results
All 2,112 patients were initially treated with total thyroidectomy. Of these, 182 required therapeutic dissection of the central compartment (TT+CentDis; 71 unilateral and 111 bilateral) and 50 underwent therapeutic dissection of the central and lateral compartments (TT+CentLatDis; 44 unilateral and six bilateral).
Of the 1,880 patients undergoing total thyroidectomy alone (TT alone), 367 (19.5%) presented lymph nodes on the final histopathology that were not identified during surgery. A total of 299 (81.5%) of these lymph nodes had no metastasis (cN0 and pN0), whereas 68 (3.6%) of them were metastatic (cN0 and pN1, considered microscopic lymph nodes). Of the 182 patients undergoing therapeutic dissection of the central compartment, 73 (40.1%) presented metastatic disease (cN+ and pN1, considered macroscopic lymph nodes) and 109 (59.9%) were negative for metastases (cN+ and pN0). In contrast, all 50 patients undergoing therapeutic dissection of the central and lateral compartments presented central and lateral metastases (Table 1). In all, 1,571 patients (74.38%) received radioiodine therapy for treatment of residual disease (Table 2).
Table 1: Frequency distribution of lymph node recurrence according to surgical procedure and number of affected lymph nodes. (N).
| Without recurrence | With recurrence | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total thyroidectomy alone | |||||
| pNX | 1513 | 1482 | 97.9 | 31 | 2.0 |
| pN0 | 299 | 297 | 99.3 | 2 | 0.6 |
| pN1 | 68 | 61 | 89.7 | 7 | 10.2 |
| Total thyroidectomy and central resection |
|||||
| pN0 | 73 | 73 | 100 | 0 | 0 |
| pN1 | 109 | 90 | 82.5 | 19 | 17.4 |
| Total thyroidectomy and central and lateral dissection |
|||||
| pN1 | 50 | 46 | 92.0 | 4 | 8.0 |
| Total | 2112 | 2049 | 97.0 | 63 | 3.0 |
P<0.001 for all analyses (likelihood ratio test).
Table 2: Characteristics of the patients on univariate analysis.
| Variable | Without recurrence | With recurrence | Total | P |
|---|---|---|---|---|
| 2049 (96.8%) | 63 (3.0%) | 2112 (100%) | ||
| Age (years) | 43.5 ± 12.0 | 37.3 ± 11.4 | 43.3 ± 12.1 | <0.001* |
| Gender | 0.746** | |||
| Female | 1628 (97.1%) | 49 (2.9%) | 1677 | |
| Male | 421 (96.8%) | 14 (3.2%) | 435 | |
| Procedure | <0.001** | |||
| Total thyroidectomy alone | 1840 (97.9%) | 40 (2.1%) | 1880 | |
| Total thyroidectomy with central compartment dissection and therapeutic lateral compartment dissection | 46 (92%) | 4 (8%) | 50 | |
| Total thyroidectomy with therapeutic dissection of the central compartment | 163 (89.6%) | 19 (10.4%) | 182 | |
| Multifocal diseaseity | 0.002** | |||
| Single tumor | 1553 (97.7%) | 37 (2.3%) | 1590 | |
| Multiple tumor | 496 (95%) | 26 (5%) | 522 | |
| Extrathyroidal extension | <0.001** | |||
| No | 1810 (97.7%) | 42 (2.3%) | 1852 | |
| Yes | 239 (91.9%) | 21 (8.1%) | 260 | |
| TNM - T | <0.001*** | |||
| 1 | 1680 (97.7%) | 39 (2.3%) | 1719 | |
| 2 | 119 (98.3%) | 2 (1.7%) | 121 | |
| 3 | 247 (92.2%) | 21 (7.8%) | 268 | |
| 4 | 3 (75%) | 1 (25%) | 4 | |
| TNM - N | <0.001** | |||
| 0 | 368 (99.5%) | 2 (0.5%) | 370 | |
| 1 | 198 (86.8%) | 30 (13.2%) | 228 | |
| X | 1483 (98%) | 31 (2%) | 1514 | |
| Staging | 0.113*** | |||
| Stage I | 1847 (97.1%) | 55 (2.9%) | 1902 | |
| Stage II | 40 (100%) | 0 (0%) | 40 | |
| Stage III | 136 (96.5%) | 5 (3.5%) | 141 | |
| Stage IV | 26 (89.7%) | 3 (10.3%) | 29 | |
| Radioiodine therapy | 0.001** | |||
| No | 536 (99.1%) | 5 (0.9%) | 541 | |
| Yes | 1513 (96.3%) | 58 (3.7%) | 1571 | |
| Antithyroglobulin antibody | 0.765** | |||
| Absent | 1562 (97.1%) | 47 (2.9%) | 1609 | |
| Present | 487 (96.8%) | 16 (3.2%) | 503 |
*Student’s t test; **Pearson’s chi-square test; ***Likelihood ratio. The results are presented as absolute frequencies (relative - %) with the exception of age, which is represented as mean ± standard deviation.
Overall, 63 patients experienced lymph node recurrence during a mean follow-up of 41 months (10–198 months). The recurrences occurred in the central compartment in 19 (30.2%) cases, in the lateral compartment in 33 (52.4%) cases, and in both central and lateral compartments in 11 cases (17.5%). Five patients presented a second recurrence. We are unaware of any death directly related to the carcinoma in this surgical cohort.
As shown in Table 2, the following variables were significantly associated with lymph node recurrence on univariate analysis: (a) age: patients presenting recurrence were on average 6 years younger than those not experiencing this complication (p<0.001); (b) management: patients undergoing therapeutic dissection (therefore, presenting macrometastases) recurred more frequently (p<0.001); (c) presence of multifocal disease (p=0.002); (d) extrathyroidal extension (p=0.001); (e) TNM-N classification: the larger the size of the primary tumor (T) and metastatic lymph node (N), the higher the recurrence rate (p<0.001); and (f) radioiodine therapy: the use of radioiodine therapy was associated with an increased risk of recurrence (p=0.001). The variables sex, stage, and positive antithyroglobulin antibody levels showed no significant association with lymph node recurrence in this analysis.
In regards to other histological data, we found an increased risk of lymph node recurrence related to the diffuse sclerosing variant of papillary carcinoma, tumors with the largest diameter ≥ 1.2 cm (p<0.001), finding of metastasis in two or more resected lymph nodes (p<0.001), and a ratio of 0.66 between positive to dissected lymph nodes (p<0.001) (Table 3).
Table 3. Univariate analysis based on the histological classification and size of the resected tumor, amount of resected lymph nodes with metastases, and ratio between the number of lymph nodes with metastasis eto the number of total lymph nodes.
| Variable | RECURRENCE | P | |
|---|---|---|---|
| No | Yes | ||
| 2049 (96.8%) | 63 (3.0%) | ||
| Histology | 0.019* | ||
| Tall cells | 22 (100%) | 0 (0%) | |
| Classic | 1072 (96.2%) | 42 (3.8%) | |
| Diffuse sclerosing | 28 (90.3%) | 3 (9.7%) | |
| Trabecular | 5 (100%) | 0 (0%) | |
| Follicular variant | 861 (98%) | 18 (2%) | |
| Hurthle | 61 (100%) | 0 (0%) | |
| Size of the primary tumor (continuous variable) | 0.8 (0.5-1.3) | 1.2 (0.8-1.7) | <0.001** |
| Size of the primary tumor (categorical variable) | <0.001*** | ||
| <1.2 | 1045 (97.7%) | 25 (2.3%) | |
| ≥ 1.2 | 635 (94.6%) | 36 (5.4%) | |
| Number of positive lymph nodes (continuous variable) | 0 (0-1) | 5 (2-10) | <0.001** |
| Positive lymph nodes (categorical variable) | <0.001*** | ||
| <2 | 434 (98.9%) | 5 (1.1%) | |
| ≥ 2 | 133 (83.1%) | 27 (16.9%) | |
| Number of resected lymph nodes | 3 (2 - 8) | 7.5 (3-15.75) | 0.001** |
| Ratio between positive lymph nodes over resected lymph nodes=0.66 | 0 (0-22.22) | 66.67 (43.08-100) | <0.001** |
*Likelihood ratio; **Mann-Whitney test; ***chi-square test. The results are represented as absolute frequencies (relative - %), with the exception of size (continuous variable), number of resected lymph nodes, and ratio of positive lymph nodes to resected lymph nodes, which are represented as mean (interquartile range).
In the multivariate analysis, lymph node recurrence was associated with the presence of lymph node metastases (odds ratio [OR] of 19.205 for metastasis to a single lymph node, p<0.001), use of radioiodine therapy (OR 3.344, p=0.012), and extrathyroidal extension (OR 2.167, p=0.011). On the other hand, the greater the age, the lower the risk of recurrence (OR 0.96, p<0.001) (Table 4). As for the size of the metastasis and the surgical procedure, the risk of lymph node recurrence was present in the group TT alone pN1 with microscopic lymph nodes (OR 8.27, p=0.014) and greater in the group TT+CentDis pN1 with macroscopic lymph nodes (OR 11.9, p=0.003). In regards to the number of metastatic lymph nodes at diagnosis, the risk was higher when two or more positive lymph nodes were resected (OR 18.9, p<0.001) (Table 4).
Table 4: Multiple logistic regression for lymph node recurrence.
| Variable | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Age (years) | 0.96 | 0.938 | 0.982 | <0.001 |
| Extrathyroidal extension | 2.167 | 1.192 | 3.943 | 0.011 |
| N | <0.001 | |||
| 0 | Reference | |||
| 1 | 19.205 | 4.487 | 82.189 | <0.001 |
| X | 4.221 | 1.002 | 17.777 | 0.05 |
| Radioiodine therapy | 3.344 | 1.311 | 8.529 | 0.012 |
| Positive lymph nodes | 1.173 | 1.06 | 1.298 | 0.002 |
| ≥2 positive lymph nodes | 18.933 | 6.481 | 55.303 | <0.001 |
| Surgical procedure | 0.001 | |||
| Total thyroidectomy with central and lateral resection dissection pN1 (TT+CentLatDis pN1) | 1.597 | 0.165 | 15.435 | 0.686 |
| Total thyroidectomy with central resection dissection pN1 (TTCentDis pN1) | 11.904 | 2.383 | 59.465 | 0.003 |
| Total thyroidectomy with central resection dissection pN0 (TT+CentDis pN0) | * | * | * | 0.997 |
| Total thyroidectomy pN1 (TT alone pN1) | 8.272 | 1.532 | 44.651 | 0.014 |
| Total thyroidectomy pN0 (TT alone pN0) | Reference | |||
*Without recurrence.
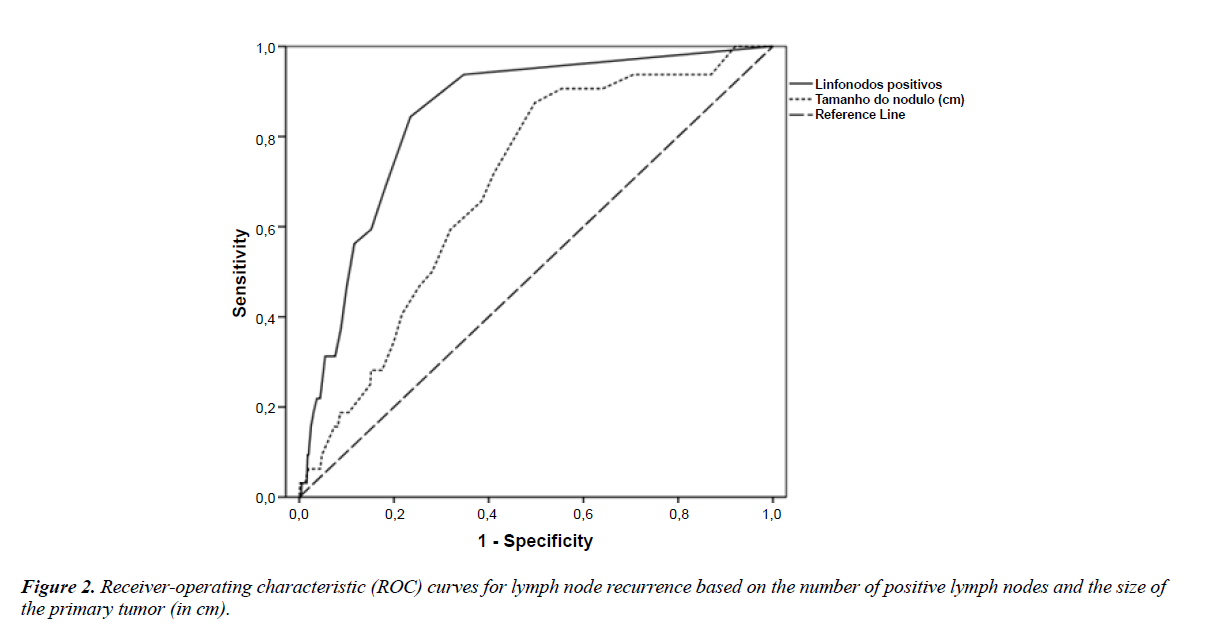
Predictive values for recurrence using the variables "number of positive lymph nodes" and "size of the primary tumor" were analyzed using ROC curves. The presence of two positive lymph nodes (sensitivity of 0.69 and specificity of 0.82) and a primary tumor size of 1.15 cm (sensitivity of 0.66 and specificity of 0.62) emerged as predictors of lymph node recurrence (Figure 2).
Of the 260 patients with extrathyroidal extension, 236 were identified as having minimal extension on final histopathology, whereas 24 were observed to have gross extension due to the finding during surgery of invasion of adjacent structures and need of partial resection (shaving) or total resection. The tumor invaded the inferior laryngeal nerves in 14 patients, the prethyroid muscles in 11 the trachea in four, and the esophagus in two of them. As for lymph node recurrence analyzed under this criterion, we found 20 patients (8.4%) with minimal extension and one patient (4.1%) with gross extension.
Table 1 presents information on lymph node recurrence in relation to the extent of the initial surgical procedure and lymph node staging, and Table 5 presents the results of the location of lymph node recurrence according to the extent of the initial treatment and lymph node staging. In total, 30 patients required reoperation of the central compartment. The type of procedure performed initially was TT alone pNX in 22, TT alone pN1 in four, TT+CentDis pN1 in three, and TT+CentLatDis pN1 in one. Three patients undergoing TT+CentDis pN1 required reoperation of the lateral compartment.
Table 5: Location of lymph node recurrence according to the surgical procedure and N.
| Type of procedure | Location of lymph node recurrence | |||||
|---|---|---|---|---|---|---|
| Central | Lateral | Central + Lateral | ||||
| Total thyroidectomy | ||||||
| pNX (n=31) | 12 (38.7%) | 9 (29%) |
|
|||
| pN0 (n=2) | 0 |
|
0 | |||
| pN1 (n=7) | 0 | 3 (42.9%) | 4 (57.1%) | |||
| pN0 (n=0) | ||||||
| pN1 (n=19) | 1 (5.3%) | 16 (84.2%) | 2 (10.5%) | |||
| Total thyroidectomy with central and lateral dissection | ||||||
| (n=4) | 1 (25%) | 3 (75%) | 0 | |||
| Total (n=63) | 14 | 33 | 16 | |||
The mean stimulated thyroglobulin level at radioiodine therapy according to groups were 0.1 μg/L (TT pN0), 0.8 μg/L (TT pNX), 1.83 μg/L (TT pN1), 0.28 μg/L (TT+CentDis pN0), 0.1 μg/L (TT+CentDis pN1), and 1.39 μg/L (TT+CentLatDis pN1). In patients presenting with recurrence, the corresponding levels after the initial treatment per group were 0.4 μg/L (TT pN0), 14.48 μg/L (TT pNX), 3.95 μg/L (TT pN1), 53 μg/L (TT+CentDis pN1), and 11.78 n μg/L (TT+CentLatDis pN1).
Discussion
Most papillary thyroid carcinomas follow an indolent course, grow slowly, show a tendency toward multifocality, and present frequent regional micrometastases without a substantial clinical impact on survival. Preoperative evaluation with cervical ultrasonography of affected patients is fundamental to evaluate suspicious lymphadenopathy (present in 20 to 30% of the patients), which can affect the surgical plan [7,8]. Lymph node metastasis usually occurs at level VI (central compartment) and subsequently at levels II, III, and IV (lateral compartment), although tumors located in the upper thyroid pole can metastasize directly to level II (“skip”) [9].
The surgical treatment is the most important step in the multidisciplinary management of papillary thyroid carcinoma, and the quality of the surgery is directly dependent on the surgeon's experience in identifying how radical the resection should be and in preventing complications [10-13]. To the best of our knowledge, this study examines one of the largest case series conducted in a private clinic by a single experienced highvolume surgeon with an average of 140 papillary carcinomas operated per year over 15 years [14,15]. This ensures uniformity in the surgical procedures and in eventually unplanned intraoperative decisions, and guarantees radical treatments with removal of the primary disease, possible extrathyroidal extension, and significant lymph node metastasis with a direct impact on both the clinical evolution and complications rates [10-13], considering that metastatic residual disease is the main cause of persistence and recurrence [16-18].
Because the cohort in this study was derived from a private clinic, blood tests and ultrasonographic evaluations conducted before and after surgery were performed in different laboratories, preventing proper comparison between the results. However, we observed that the patients in groups TT pNX, TT pN1, and TT+CentDis pN1 who presented recurrence, already had increased levels of stimulated thyroglobulin, probably due to residual disease that was not eliminated with the radioiodine treatment, and ended up recurring. Considering that, thyroglobulin measurements were not taken into consideration in this analysis, thus preventing the assessment of biochemical risk, as recommended in the 2015 ATA consensus [1]. All information related to the patients’ treatment and follow-up were collected and entered into the database by the surgeon in charge of the operations to ensure standardized recording.
Current consensuses recommend total thyroidectomy as the treatment of choice for tumors >1 cm, due to better survival and lower risk of recurrence. However, recent studies have shown similar outcomes with lobectomy and total thyroidectomy [1,19-23]. We chose to perform total thyroidectomy regardless of the size of the primary tumor, since the resection of the entire parenchyma can offer precise information about the occurrence of multicentric disease and reduce the risk of local recurrence, in addition to facilitating the use of radioiodine therapy and follow-up with measurement of thyroglobulin levels despite the occurrence of hypothyroidism and low risk of complications in the hands of experienced surgeons [24]. In 2007, the surgeon in charge of the operations in the present study published an analysis of the permanent complications in 778 patients undergoing total thyroidectomy due to thyroid microcarcinoma and reported the occurrence of 1.54% of permanent hypoparathyroidism and 0.25% of paralysis of the recurrent laryngeal nerve. When total thyroidectomy was associated with therapeutic central neck compartment dissection, the rates increased to 9.02% of permanent hypoparathyroidism and 2.77% of recurrent laryngeal nerve paralysis [25].
We opted for therapeutic dissection of the central neck compartment in the presence of central or lateral metastases confirmed before surgery or detected by the surgeon during the operation. Lymph nodes with suspicious features – enlarged, round, with calcification, or apparently normal but increased in size – which could interfere with the local postoperative ultrasonographic evaluation – were resected therapeutically en bloc along with the thyroid; this approach could include normal lymph nodes which were increased in size due to the presence of autoimmunity [26]. The surgeon decided whether to dissect or not these lymph nodes based only on their macroscopic appearance and without performing frozen section analysis. The extension of the central neck dissection followed the parameters and limits recommended by the consensuses of the ATA and the American Head and Neck Society [27-29]. Lateral dissection (level II to V) and bilateral central dissection were performed in all patients with a preoperative diagnosis of metastasis to the lateral neck compartment [1].
The history of neck treatment in patients with thyroid cancer has three lines of thought: the Japanese, which traditionally recommends prophylactic dissection without radioiodine therapy and is accompanied by a high percentage of occult metastases; the American, which proposes therapeutic dissection followed by radioiodine therapy in high-risk patients and is accompanied by a low rate of lymph node recurrence; and the European, which favors prophylactic dissection for proper disease staging and assessment of the actual need of radioiodine therapy. In a 2003 study in patients with papillary thyroid microcarcinoma, Wada et al. compared 24 patients with palpable lymph node metastases treated with therapeutic dissection with 235 patients without palpable metastases treated with prophylactic dissection [30]. The rate of lymph node recurrence was minimal in patients treated with prophylactic dissection and equivalent to that found in the group of patients not undergoing dissection [30]. Sequentially, Cranshaw et al. demonstrated that the presence of macrometastases is related to lymph node recurrence after prophylactic dissection, whereas the occurrence of micrometastases has no clinical significance [31]. Such findings generated substantial discussion in regards to the application of this prophylactic approach, which was considered excessive by some [31]. In 2009, Bonnet et al. proposed prophylactic lymph node dissection of papillary carcinomas smaller than 2 cm in order to allow for staging of the central neck compartment and selection of patients who should receive radioiodine therapy [32]. These authors concluded that the recommendation of radioiodine therapy changed in 30% of the pN1 patients, which stimulated other European centers to popularize the prophylactic technique [32]. Although the information obtained by prophylactic dissection allows proper staging of the central compartment, occult microscopic metastases are frequently encountered during the procedure, which changes the TNM staging from I to III in patients older than 45 years [33-37].
The problem of recurrence lies in the fact that most lymph node metastases remain quiescent and only a few develop and become apparent. Additionally, many patients present occult metastases that are not detected before or during surgery. Considering that, it is difficult to identify if metastatic disease that becomes apparent after surgery constitutes a recurrent or persistent disease. According to studies by Wada et al. [30] and Cranshaw et al. [31], the initial size of the lymph node metastasis is crucial in defining whether the metastasis will or not develop: the smaller the micrometastasis, the more time it will take to grow or remain stable. Therefore, the clinical meaning of small or microscopic lymph node metastases is often minimal or absent. There is no consensus in the literature or definition of size to classify the lesions as macrometastases or micrometastases, but the best proposal may be the one by Randolph et al., who defined as micrometastasis those lesions with up to 0.2 cm, as small nodal metastasis those between 0.2 and <1.0 cm, as intermediate sized nodal metastases those between 1 and 3 cm, and as large nodal metastases those with >3 cm [38]. These authors concluded that despite the presence of micrometastases and small nodal metastases (pN1micro) being associated with a low risk of recurrence (<5%), there was an increased use of radioiodine therapy [38]. On the other hand, the demonstration of negative lymph nodes by prophylactic dissection decreases the use of radioiodine therapy [34]. Leboulleux et al. have demonstrated that the presence of microscopic extracapsular extension in metastatic lymph nodes increases by up to 24% the risk of recurrence [39]. These criteria have contributed to the ATA 2015 system stratifying the risk of recurrence: (a) low – pN1 without extranodal extension and less than three affected lymph nodes (risk of 2%), pN1 with less than five affected lymph nodes (risk of 5%), and pN1 with affected lymph nodes and all with no more than 0.2 cm in diameter (risk of 5%); (b) intermediate – pN1 with more than five affected lymph nodes (risk of 20%); (c) high – pN1 with affected lymph nodes greater than 3 cm (risk of 30%) and pN1 with extranodal extension (risk of 40%) [1].
The improvement of imaging methods with progressively higher resolution, such as ultrasound, increasingly improves the identification of occult metastasis and increases the percentage of therapeutic dissection approaches. Currently, physicians in Japan, Germany, France, and South Korea favor prophylactic dissections, while those in Britain, America, Italy, and Latin American countries prefer therapeutic dissection [22,40-42]. It should be noted that the recommendation by consensuses of prophylactic dissection of all patients is risky since it increases the risk of transient hypoparathyroidism when performed by experienced surgeon’s at large centers, and of all complications, when performed by inexperienced surgeons in small centers. The authors consider therapeutic dissections to be a more rational approach, instead of prophylactic ones, and recommend that the small number of the patients experiencing recurrence (3% in the present series) should be reoperated on by an experienced surgeon.
The recommendation for the type of dissection also depends on the intraoperative evaluation of the central neck compartment by the surgeon; however, this approach is considered controversial, subjective, and with questionable efficacy. In this study, we performed central therapeutic dissection in 232 patients, but the lymph nodes were negative (pN0) in 73 of them, yielding a false positivity rate of 31.46%. On the other hand, lymph nodes were found on pathologic examination in 599 patients; in 367 of them, the lymph nodes were missed by the surgeon due to their size, yielding a false negative rate of 61.26%. These high rates of false positivity and negativity could argue for prophylactic dissection aiming at a thorough removal of all lymph nodes present in the area to confirm the presence of metastasis. However, this approach would increase the risk of complications in all patients, particularly the occurrence of transient hypoparathyroidism [43,44]. In addition to that, a meta-analysis published by Zeitoune et al. was unable to confirm that prophylactic central dissection reduces the risk of lymph node recurrence [45]. A prospective study is required to compare the prophylactic and therapeutic techniques, but unfortunately, the number of patients required for such study renders it impractical [46].
All surgical specimens in the present study were evaluated with both frozen section and paraffin. The analysis included pathological information cited in the final paraffin report, including the histological variant, size of the largest tumor, multifocal disease, extracapsular extension (without quantification), presence of dissected lymph nodes, and presence of affected lymph nodes. The results showed that the size of the primary tumor and the presence of extracapsular extension (T3 and T4), multifocal disease, a histological diagnosis of diffuse sclerosing variant, and lymph node involvement emerged as significant factors for lymph node recurrence, as described in the literature [47-49]. Unfortunately, we lack information about the size of the lymph nodes and presence of extranodal extension, but we considered as microscopic lymph nodes those lymph nodes found close to the thyroid without a formal dissection procedure (group TT alone pN1). The group with micrometastases (TT alone pN1) had a significant recurrence rate (10.2%), which was superior to the rate observed in the group without information about the central compartment (TT alone pNx; 2%), and the group without metastasis (TT alone pN0; 0.6%). These rates are higher than those reported by Randolph et al. [38] and Haugen et al. [1], but lower than that observed in the group with macrometastasis in our study (TT+CentDis pN1; 17.4%) (p<0.001). Similarly, Bardet et al. have demonstrated a significantly higher risk of recurrence in macroscopic pN1 (49%) compared with microscopic pN1 (24%), which was superior to pN0 (12%) and pNx (6%) in a study with a smaller number of patients comprising 128 (42%) pNx, 84 (28%) pN0, 44 (14%) microscopic pN1, and 49 (16%) macroscopic pN1 [50]. In addition to the size of the lymph nodes, some authors consider the number of positive lymph nodes in the central compartment as a risk for metastasis in the lateral compartment [51,52], as well as a risk for recurrence in the central compartment itself, as shown by Leboulleux et al. (risk of 21% with more than 10 positive lymph nodes and 3% when less than five) [53] and Sugitani et al. (risk of 19% when more than five positive and 8% when fewer than five) [54]. Schneider et al. described that a ratio of positive to dissected lymph nodes >0.86 was a risk factor for recurrence in 16 of 63 patients previously submitted to central therapeutic dissection (OR 19.5, p<0.1) [55]. These authors concluded that the ratio of positive to dissected lymph nodes is a superior tool to predict recurrence when compared with the number of positive lymph nodes alone, and that due to a variability from patient to patient in regards to the occurrence of metastases (mainly in the central compartment), the ratio could reflect a complete or incomplete resection of the entire compartment [55]. Lee et al. treated 211 patients with prophylactic central dissection and found a significant risk of recurrence when the ratio was >0.26 (OR 11.63, p=0.003) [56]. There is an important difference in ratios based on the type of dissection recommended (prophylactic or therapeutic). Both are associated with a risk of recurrence but the most important feature, in the opinion of the authors, is the characteristic of each lymph node metastasis. In this study, the presence of two positive lymph nodes and a ratio of 0.66 significantly increased the risk of lymph node recurrence in the univariate analysis, identifying those patients deserving a more frequent follow-up. In patients not undergoing dissection and with a risk of recurrence, a more frequent follow-up would be safe, since recurred lymph nodes remain stable for several years, although those with annual growth >3 mm have decreased survival [57].
It is difficult to assess the efficacy of radioiodine therapy in the treatment of micrometastases and minimal occult disease. Sawka et al. were unable to identify in a systematic review a significant benefit of radioiodine therapy in decreasing recurrence and mortality in patients with early-stage papillary cancer [58]. Radioiodine therapy was recommended to 74.38% of the patients in the present series based on the presence of classic risk factors; strangely, there was a significantly increased rate of recurrence in the treated group (3.7%) compared with the untreated one (0.9%). To conclude from this finding that radioiodine therapy is harmful would be scary and absurd. The authors believe this finding to be the result of a selection bias due to the inclusion of both a large number of patients and patients with any risk factor; it would be as though the recurrence of all patients were related to total thyroidectomy. This finding was then disregarded.
The surgeon in charge of the operations in this study opted for therapeutic dissection, thereby the risk of complications occurred only in patients with recurrence (3% of the patients in the present series) who required new dissection. Overall, a new dissection was performed in the central compartment in 22 patients initially categorized as group TT alone pNX, four in the group TT alone pN1, three in the group TT+CentDis pN1, and one in the group TT+CentLatDis pN1, whereas a new dissection of the lateral compartment was performed in three patients in the TT+CentLatDis pN1 group. The complication rates remained unchanged in these reoperations, which is similar to findings in the literature [59-61].
Tuttle et al. recently proposed a new AJCC/TNM staging system, which increased the patient’s age to 55 years and removed from stage III the occurrence of minimal extrathyroidal extension and lymph node metastasis without changing the risk of recurrence classification [62]. Based on the results of the present study, the authors propose a modification to the current risk classification of the ATA which recommends that patients with at least two positive lymph nodes should be upgraded to a high-risk category in order to receive a more strict follow-up with periodic cervical ultrasonography.
The findings of our study are strengthened by the fact that the surgical cohort comprised a mixed population and, therefore, had great biological variability. Another important characteristic was the inclusion of a cohort derived from a real-world setting. Due to that, the findings observed have a greater potential of generalization. Potential limitations of the study include the retrospective design and possible heterogeneity of the results of pathology and diagnostic tests, considering that they were performed in different centers. However, this reflects the clinical practice reality of most head and neck surgeons.
Conclusion
In this study’s cohort comprising patients with papillary thyroid carcinoma undergoing total thyroidectomy and therapeutic dissection, the risk of lymph node recurrence was greater in the presence of younger age, multifocal disease, extracapsular extension, increased size of the primary tumor, lymph node metastasis (especially with two or more positive lymph nodes), a 0.66 ratio of positive to dissected lymph nodes, and radioiodine therapy. The presence of macrometastases was associated with a higher percentage of recurrence than that of micrometastases. In the multivariate analysis, the risk of lymph node recurrence was approximately two times higher in the presence of extrathyroidal extension, eight times greater with the occurrence of micrometastases and 11 times greater with macrometastases, and almost 19 times greater with the finding of two positive lymph nodes. When assessing positive lymph nodes and the size of the primary tumor as predictors of lymph node recurrence using ROC curves (similar to an examination for diagnostic purposes), we found the cut-off values of two positive lymph nodes (sensitivity of 0.69 and specificity of 0.82) and a primary tumor greater than 1.15 cm (sensitivity of 0.66 and specificity of 0.62). Considering these results, the authors propose a modification to the ATA risk classification, in which patients should be upgraded from low to high risk when more than two positive lymph nodes are found in the central neck compartment on pathological analysis.
Declaration of Interest
The authors report no conflict of interest.
Author Contributions
C.U.M.F. operated on all the patients included in the study, designed the study, and was in charge of the manuscript preparation. C.S.L. assisted in most of the surgeries and was in charge of the statistical analysis. Both authors revised and approved the final version of the manuscript.
Acknowledgments
The study was conducted without grant support. Milena Braga- Basaria, M.D. (Voxmed Medical Communications) assisted in the preparation of the manuscript.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26(1): 1-133.
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994; 97(5): 418-28.
- Kouvaraki MA, Lee JE, Shapiro SE, et al. Preventable reoperations for persistent and recurrent papillary thyroid carcinoma. Surgery. 2004; 136(6): 1183-91.
- Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009; 19(12): 1373-80.
- De Lellis RA, Lloyd RV, Heitz PU, et al. World Health Organization Classification of Tumours. In Pathology and Genetics of Tumours of Endocrine Organs. IARC Press, Lyon, France, 2004.
- Rosner, B. Fundamentals of Biostatistics. Duxbury Press, 4th edition, New York, NY, p. 682, 1994.
- O'Connell K, Yen TW, Quiroz F, et al. The utility of routine preoperative cervical ultrasonography in patients undergoing thyroidectomy for differentiated thyroid cancer. Surgery. 2013; 154(4): 697-701.
- Leboulleux S, Girard E, Rose M, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007; 92(9): 3590-4.
- Park JH, Lee YS, Kim BW, et al. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012; 36(4): 743-7.
- Sosa JA, Bowman HM, Tielsch JM, et al. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998; 228(3): 320-30.
- Loyo M, Tufano RP, Gourin CG. National trends in thyroid surgery and the effect of volume on short-term outcomes. Laryngoscope. 2013; 123(8): 2056-63.
- Gourin CG, Tufano RP, Forastiere AA, et al. Volume-based trends in thyroid surgery. Arch Otolaryngol Head Neck Surg. 2010; 136(12): 1191-8.
- Stavrakis AI, Ituarte PH, Ko CY, et al. Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery. 2007; 142(6): 887-99.
- Kandil E, Noureldine SI, Abbas A, et al. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery. 2013; 154(6): 1346-52.
- Duclos A, Peix JL, Colin C, et al. Influence of experience on performance of individual surgeons in thyroid surgery: prospective cross sectional multicentre study. BMJ. 2012; 344: d8041.
- Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993; 114(6): 1050-7.
- Shah MD, Hall FT, Eski SJ, et al. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope. 2003; 113(12): 2102-7.
- Wang TS, Dubner S, Sznyter LA, et al. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg. 2004; 130(1): 110-3.
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19(11): 1167-214.
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007; 246(3): 375-81.
- Nixon IJ, Ganly I, Patel SG, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012; 151(4): 571-9.
- Pitoia F, Ward L, Wohllk N, et al. Recommendations of the Latin American Thyroid Society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2009; 53(7): 884-7.
- Matsuzu K, Sugino K, Masudo K, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014; 38(1): 68-79.
- Wang TS, Cheung K, Farrokhyar F, et al. A meta-analysis of the effect of prophylactic central compartment neck dissection on locoregional recurrence rates in patients with papillary thyroid cancer. Ann Surg Oncol. 2013; 20(11): 3477-83.
- Friguglietti CU, Kulcsar MA. [Thyroid microcarcinoma: experience and management in private clinic]. Arq Bras Endocrinol Metabol. 2007; 51(5): 774-82.
- Lai V, Yen TW, Rose BT, et al. The effect of thyroiditis on the yield of central compartment lymph nodes in patients with papillary thyroid cancer. Ann Surg Oncol. 2015; 22(13): 4181-6.
- Agrawal N, Evasovich MR, Kandil E, et al. Indications and extent of central neck dissection for papillary thyroid cancer: An American Head and Neck Society Consensus Statement. Head Neck. 2017; 39(7): 1269-1279.
- Orloff LA, Kuppersmith RB. American Thyroid Association's central neck dissection terminology and classification for thyroid cancer consensus statement. Otolaryngol Head Neck Surg. 2010; 142(1): 4-5.
- Carty SE, Cooper DS, Doherty GM, et al. Consensus statement on the te