Research Article - Biomedical Research (2017) Volume 28, Issue 19
Protective effects of cystamine on apoptosis of hippocampal neurons in rats with cerebral ischemia
Meirong Zhu, Li Sun, Yichao Zhang, Shaohua Fan, Yichen Zhang and Yun Li*
Department of Critical Care Medicine, Jinan Central Hospital Affiliated Shandong University, Jinan, PR China
- *Corresponding Author:
- Yun Li
Department of Critical Care Medicine
Jinan Central Hospital Affiliated Shandong University
PR China
Accepted date: September 07, 2017
Abstract
The aim of this study was to investigate the effect of Cystamine (CYS) on the hippocampal CA1 region in rats with whole cerebral ischemia. A total of 104 adult male Sprague-Dawley rats were prepared using the modified Pulsinelli method for a global Cerebral Ischemia (GI) model, and then randomly divided into a sham group (S, n=8), a GI group (GI, n=48), and a GI+CYS group (GI+CYS, n=48). The latter two groups were further divided into several subgroups according to the reperfusion time (6 h/12 h/1 d/3 d/5 d/7 d). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed to observe apoptosis in the CA1 region, and immunohistochemistry was used to detect the protein expressions of Brain-Derived Neurotrophic Factor (BDNF), Tyrosine kinase B (TrkB), and caspase-3. Compared with those in group S, the TUNEL-positive cells in the hippocampal CA1 region of group GI increased over time, but those in group GI+CYS were significantly decreased compared with those in group GI. Compared with group S, BDNF and TrkB proteins in group GI were upregulated, and reached a peak on d 1 (D1). Group GI+CYS exhibited a greater upregulation in these proteins than those group GI, and reached a peak on D1. The difference between these two groups was statistically significant. Compared with group S, caspase-3 protein in Group GI was upregulated and peaked on D3, but was lower in Group GI+CYS than in Group GI. The peak difference from that on D1 was statistically significant. CYS can inhibit apoptosis and upregulate BDNF and TrkB, thus exhibiting protective effects toward neurons subjected to ischemia-reperfusion in the hippocampal CA1 region.
Keywords
Global cerebral ischemia and reperfusion, Cystamine, BDNF, TrkB, Apoptosis, Caspase-3
Introduction
Global cerebral ischemia commonly occurs in a variety of clinical conditions, such as shock, cardiac arrest, intraoperative hypobaric and hypoxic states, traumatic brain injury, status asthmaticus, or CO poisoning [1], and ischemia-induced brain damage leads to apoptosis-like delayed neuronal death in selectively vulnerable regions, which could result in further irreversible damage. Previous studies have demonstrated that neurons in the CA1 area of the hippocampus are particularly sensitive to ischemic damage [2], due to increased apoptotic cell death [3].
In addition to the process of cell death initiation after cerebral ischemia, a parallel protective process is also observed in neurons. When cerebral ischemia occurs, the nerve cells around the ischemic area can produce Brain-Derived Neurotrophic Factor (BDNF), which acts as an endogenous neuroprotective agent, enabling resistance to cerebral ischemic damage and permitting cells to survive. BDNF can generate signals through its cognate Tyrosine receptor kinase B (TrkB) to regulate synaptic health and glutamatergic activities [4]. Among the protective mechanisms following nerve cell damage, BDNF and its receptors (TrkB and p75) regulate dendritic and axonal growth during the development and maintenance of the mature nervous system [5], and participate in the process of neurogenesis in the hippocampus [6]. Cystamine (CYS) is a small molecule amine salt containing a disulfide bond. As a specific inhibitor of tissue transglutaminase (tTG), it prevents oxidative stress and inhibits nerve cell degeneration. Recent animal and in vitro experiments targeting degenerative neural diseases such as Parkinson's, Huntington's, and Alzheimer's disease have shown that CYS can upregulate BDNF and TrkB inside the brain, and inhibit neuronal apoptosis, thus providing new ideas for research on the prevention of apoptosis in whole cerebral ischemia [7]. In a mouse focal stroke model, data have provided evidence for the utility of CYS for therapies acting principally through the BDNF/TrkB pathway [8]. However, the anti-apoptotic mechanisms and specific neuronal pathways affect remain unclear, especially with regard to the association with cell damage in global cerebral ischemia, and various factors that can regulate ischemic expression have not been studied. This study established a rat model of global cerebral ischemia, applied CYS, and then evaluated changes in the expression of BDNF and TrkB, as well as the effect of these changes on caspase-3 expression and neural cell apoptosis, aiming to investigate possible neuroprotective mechanisms of CYS in global cerebral ischemia.
Materials and Methods
Animals
A total of 104 healthy, Specific-Pathogen-Free (SPF), adult male Sprague-Dawley rats, aged 3-4 months, weighing 280-320 g, were provided by the Experimental Animal Center, Shandong University of Traditional Chinese Medicine.
Animal grouping
The 104 rats were randomly divided into a sham group (S, n=8), a global cerebral ischemia group (GI, n=48), and a global cerebral ischemia with CYS treatment group (GI+CYS, n=48); each of the latter two groups were further divided into 6 subgroups according to the reperfusion time (6 h/12 h/1 d/3 d/5 d/7 d; n=8 in each subgroup). This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shandong University.
Animal model preparation
The modified Pulsinelli [9] 4-vessel occlusion method was used to prepare the rat global cerebral ischemia model (success rate 75%; animals that died were replaced). All animals were fed to the desired body weight (about 1-2 w), and adapted to the new environment to reduce stress responses. After being fasted for 24 h before surgery while free to drink water, each rat was anesthetized using 10% chloral hydrate (0.3-0.35 ml/g) and fixed in a prone position. A 2 cm incision was placed in the posterior neck midline to expose the bilateral foramen alaris of the first cervical vertebra; a pair of bipolar coagulation needles was then inserted and the bilateral vertebral arteries were electrocoagulated until complete closure. Following wound suture, the rats were returned to caged feeding and drinking. The rats were anesthetized 24 h later using the same method, fixed in supine position, and a 2 cm incision was placed in the anterior neck midline to expose the bilateral common carotid arteries. Needle electrodes were then inserted under the scalp to monitor the Electroencephalogram (EEG) dynamically; a noninvasive arterial microclip was then used to close the bilateral common carotid arteries; the appearance of resting EEG waves indicated successful preparation of the global cerebral ischemia model. The arterial clip was opened 10 min later to restore the blood supply, followed by wound closure. The animals in group S only underwent cauterization of the bilateral common vertebral arteries, without closure of the bilateral common carotid arteries; the remaining steps were the same.
Medication
To achieve optimal levels of CYS (467383-25G; Sigma- Aldrich Co.) in the model, we referred to the literature [10-12] as well as to pharmacokinetic data, and dissolved CYS in sterile water for injection (50 g/L, w/v). The rats in the treatment group immediately received an intraperitoneal injection of CYS (0.15 mg/g/d) after removal of the arterial clip; the injection was administered only once within 24 h after ischemia, but was performed daily at corresponding time points after 24 h; group S was given normal saline using the same method. The rats in each group were decapitated at different post-ischemia-reperfusion time points (6 h, 12 h, 1 d, 3 d, 5 d, and 7 d) to obtain the brains. Group S was intraperitoneally injected with 1 mL of saline, and then killed on d 3 (D3).
Cerebral perfusion fixation and sampling
Rats were anesthetized at corresponding time points with 10% chloral hydrate, and then cut open along the midline of the chest; a large-bore needle connected to a perfusion tube was then inserted into the left ventricle at the apex, and then inserted into the reentry ascending aorta, followed by fixation using a large hemostat; 200 ml of 0.1 M heparinized Phosphate Buffer Solution (PBS) (at 4°C) was used to quickly flush the residual blood from the brain, and then 400 ml of 0.1 M PBS with 4% paraformaldehyde (at 4°C) was used to perfuse the brain for 30 min before fixation; the brain tissue was then rapidly separated (being careful to maintain the integrity of brain tissue), followed by cutting into anterior, middle, and posterior sections, with 1 mm before and 4 mm after the optic chiasm as the boundaries; after ensuring recovery of the complete hippocampus, the middle segment was placed into 0.1-M PBS with 4% paraformaldehyde for 24 h of fixation, followed by dehydration in 20% and 30% sucrose solution in turn, until the brain sank to the bottom. Continuous coronal retrograde slicing was then performed from 2.2 mm behind the optic chiasm at -20°C, followed by slice staining.
Immunohistochemistry
Immunohistochemistry was used to detect BDNF (polyclonal antibody, Wuhan Boster Co.), TrkB (polyclonal antibody, Wuhan), and caspase-3 proteins (polyclonal antibody, Fuzhou Maxim Bioengineering Co. Ltd.). The procedure was as follows: frozen sections (10-μm thick) were held at room temperature for 30 min, followed by 20-min fixation in 4°C acetone, PBS rinsing (3 × 5 min), and 30 min immersion in 3% H2O2 (10 ml of 30% H2O2/100 ml of methanol) at room temperature to eliminate endogenous peroxidase activity. After distilled water rinsing (2 × 5 min) and 5 min PBS immersion, antigen retrieval was performed using 0.1 M sodium citrate buffer (pH 6.0) with 15 min microwave heating (for TrkB and caspase-3) or 5-10 min incubation with trypsin at 37°C after drying of the specimens (for BDNF). After PBS rinsing (3 × 5 min) and drying of the specimens, normal goat serum working solution was used for blocking and incubation at room temperature for 30 min. The serum was then discarded, followed by incubation with BDNF, TrkB, and caspase-3 antibodies (1:200) at 37°C for 120 min, respectively, with PBS replacing the primary antibody as the negative control. After PBS rinsing (3 × 5 min), biotin-labeled secondary antibodies were added for 30-min incubation at 37°C, followed by PBS rinsing (3 × 5 min), 30-min incubation with horseradish peroxidase-labeled streptavidin working fluid at 37°C, PBS rinsing (3 × 5 min), Diaminobenzidine (DAB) coloration under microscopy, thorough water rinsing, restaining, dehydration, hyalinization, and slice mounting in turn.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL)
The frozen sections (10 μm thick) were held at room temperature for 30 min, followed by 20 min fixation in 4°C acetone, PBS rinsing (3 × 5 min), and 30 min immersion in 3% H2O2 (10 ml of 30% H2O2/100 ml of methanol) at room temperature to eliminate endogenous peroxidase activity. After rinsing with distilled water (3 × 5 min) and PBS immersion for 5 min, the slices were dried and pepsin was added dropwise for 20 min incubation at room temperature. After PBS rinsing (3 × 5 min) and drying of the specimens, 50 μl of TUNEL reaction mixture was added by drops (50 μl of enzyme solution: 450 μl of marking fluid) for 60 min incubation at 37°C, followed by PBS rinsing (3 × 5 min), drying of the specimens, addition of 50 μl of conversion liquid with peroxidase for 30 min incubation at 37°C, PBS rinsing (3 × 5 min), DAB coloration, restaining, dehydration, hyalinization, and slice mounting in turn. All assay kits were purchased from Rohe.
Image analysis and statistics
BDNF, TrkB, and caspase-3-positive reactions located on the membrane or in the cytoplasm appeared as brownish-yellow or brown particles. TUNEL-positive reactions in the nucleus appeared as brownish-yellow or brown particles. All slices were examined for positive cells in the hippocampal CA1 region under 400X magnification. Three equal, nonoverlapping fields in the hippocampal CA1 region were randomly selected for counting, to determine the average number of positive cells in the slice. All data were expressed as mean ± standard deviation (͞x± s), and SPSS 11.5 software was used for the statistical analysis; t-tests, and analysis of variance were performed, with P<0.05 considered statistically significant.
Results
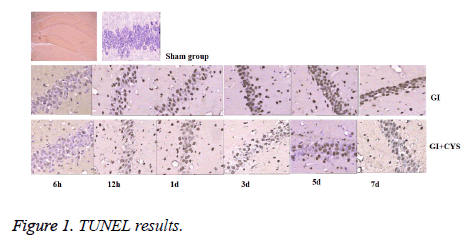
Detection of apoptosis-positive cells
Compared with group S, subgroups D1, 3, 5, and 7 in group G showed increased TUNEL-positive apoptotic cells in the CA1 region (P<0.05); compared with group G, subgroups D1, 3, 5, and 7 in group G+C showed decreased TUNEL-positive apoptotic cells in the CA1 region (P<0.05, Table 1 and Figure 1).
| Group | 6 h | 12 h | 1 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Sham (n=8) | 0.20 ± 0.24 | |||||
| GI (n=48) | 0.38 ± 0.52 | 1.38 ± 0.92 | 6.88 ± 1.96* | 16.13 ± 1.73* | 17.88 ± 1.56* | 22.25 ± 1.67* |
| G+C (n=48) | 0.25 ± 0.46 | 1.13 ± 0.83 | 2.05 ± 0.77∆ | 8.63 ± 1.37∆ | 14.35 ± 1.22∆ | 18.45 ± 1.73∆ |
| Note: Compare with group S, *P<0.05, compare with group G, ∆P<0.05. | ||||||
Table 1: TUNEL Positive cells in hippocampal CA1 region of different groups (͞x ± s, cell/HP) (40 × 10).
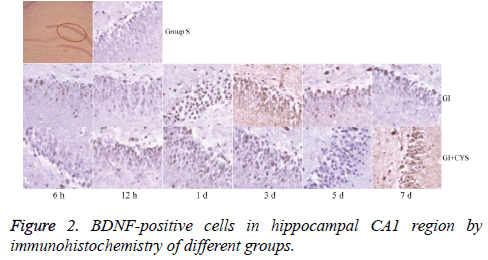
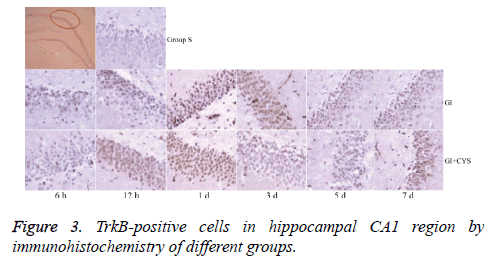
Average counts of BDNF and TrkB-positive cells
Compared with group S, subgroups h 12 (H12) and D1 and 3 in group G showed statistically significant increases in BDNF-and TrkB-positive cells in the CA1 region (P<0.01); compared with group G, subgroups D1, 3, 5, and 7 in group G+C showed statistically significant increases in BDNF- and TrkB-positive cells (P<0.01, Tables 2 and 3 and Figures 2 and 3).
| Groups | 6 h | 12 h | 1 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Sham (n=8) | 0.41 ± 0.31 | - | - | - | - | - |
| GI (n=48) | 1.01 ± 0.32 | 3.21 ± 0.89* | 8.56 ± 3.03* | 3.21 ± 0.72* | 0.81 ± 0.31 | 0.51 ± 0.32 |
| G+C (n=48) | 2.11 ± 0.32 | 3.91 ± 0.72 | 12.21 ± 1.04∆ | 10.33 ± 3.03∆ | 8.52 ± 5.04∆ | 5.34 ± 4.03∆ |
| Note: Compare with group S, *P<0.05, compare with group G, ∆P<0.05. | ||||||
Table 2: BDNF-positive cells in hippocampal CA1 region by immunohistochemistry of different groups (͞x ± s, Cell/HP) (40 × 10).
| Group | 6 h | 12 h | 1 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Sham (n=8) | 0.33 ± 0.78 | - | - | - | - | - |
| GI (n=48) | 0.93 ± 0.79 | 2.12 ± 0.82* | 9.10 ± 2.03* | 5.21 ± 2.03* | 1.26 ± 0.53 | 0.57 ± 0.24 |
| G+C (n=48) | 1.32 ± 0.76 | 2.82 ± 0.83 | 12.24 ± 3.04∆ | 9.28 ± 3.03∆ | 6.33 ± 2.04∆ | 4.26 ± 1.03∆ |
| Note: Compare with group S, *P<0.05, compare with group G, ∆P<0.05. | ||||||
Table 3: TrkB-positive cells in hippocampal CA1 region by immunohistochemistry of different groups (͞x ± s, Cell/HP) (40 × 10).
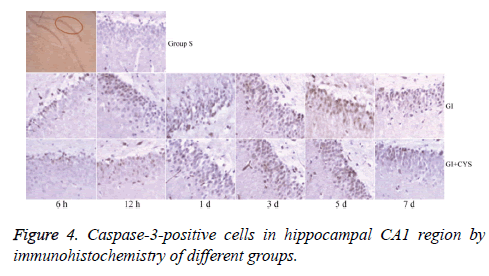
Average counts of caspase-3-positive cells
Compared with group S, subgroups H6, H12, and D1, 3, 5, and 7 in group G showed statistically significant increases in caspase-3-positive cells in the CA1 region (P<0.01); compared with group G, subgroups D1, 3, 5, and 7 in group G+C showed statistically significant decreases in caspase-3-positive cells (P<0.05, Table 4 and Figure 4).
| Group | 6 h | 12 h | 1 d | 3 d | 5 d | 7 d |
|---|---|---|---|---|---|---|
| Sham (n=8) | 0.33 ± 0.79 | - | - | - | - | - |
| GI (n=48) | 0.73 ± 0.78 | 1.21 ± 0.86* | 3.76 ± 0.99* | 23.83 ± 2.33* | 21.23 ± 1.97* | 10.07 ± 1.54* |
| G+C (n=48) | 0.31 ± 0.79 | 0.89 ± 0.80 | 1.93 ± 0.83∆ | 18.65 ± 2.01∆ | 15.61 ± 1.26∆ | 6.57 ± 1.12∆ |
| Note: Compare with group S, *P<0.05, compare with group G, ∆P<0.05. | ||||||
Table 4: Caspase-3-positive cells in hippocampal CA1 region by immunohistochemistry of different groups (͞x ± s, Cell/HP) (40 × 10).
Discussion
Studies have shown that in addition to the process of cell death initiation after cerebral ischemia (apoptosis), a parallel protective process is also observed in neurons, and both are regulated by gene expression [13]. Among the protective mechanisms observed following injury, the nerve cells around the ischemic lesion can generate BDNF, which acts as an endogenous neuroprotective agent, to resist ischemic damage and enable cell survival. Numerous studies have indicated that BDNF in the damaged central nervous system can block injury-induced neuronal degeneration and promote the development of uninjured neurons to reconstruct damaged neural circuits under certain conditions [14]. BDNF is a macromolecular protein and crosses the blood-brain barrier with difficulty; therefore, the clinical effects and cost-efficacy of exogenous BDNF are not ideal. Certain drug(s) that can increase the expression of endogenous BDNF and its receptors can be used to develop neuroprotective drugs, with potential application to the clinical treatment of ischemic disease.
CYS is a small molecule amine salt containing a disulfide bond; the main metabolic pathway is: CYS → cysteamine → hypotaurine → taurine [15]. CYS used in this experiment was purchased from Sigma-Aldrich Co., molecular weight: 250.36, with the formula as follows:
Studies have shown that CYS can undergo disulfide bond interchange in vitro with tTG [16,17], glutamate cysteine ligase [18], guanylate cyclase [19], glutathione S-transferase [20], and caspases [21], and then form disulfide compounds to inactivate these enzymes; therefore, CYS can exhibit its protective effects on the nervous system by inhibiting tTG and oxidation, inactivating caspase, or mitigating apoptosis. Based on the above effects, CYS can reportedly target chronic neurodegenerative diseases such as Alzheimer's (AD), Huntington's (HD), and Parkinson’s Disease (PD), as well as antipsychotic-induced neuropathological damage.
Dedeoglu found that CYS used in a transgenic HD mouse model can delay the loss of brain weight, brain atrophy, and striatal atrophy, and can greatly decrease the aggregation of mutation bodies, thus significantly increasing the survival rate, improving body weight and mobility, and delaying the development of nerve lesions [22]. CYS accomplishes these effects by inhibiting the increase in brain tissue tTG in the HD mouse model. CYS can form a disulfide complex by reacting with the cysteine residue of tTG, thus inhibiting its reaction with its substrate. Borrell-Pages et al. [23], while studying the therapeutic effects of CYS in mice and primate HD models, found that tTG couples with BDNF in the Golgi region, thus regulating its secretion; the secretion of BDNF can be blocked by the overexpression of tTG. tTG can reportedly inhibit or release various hormones and neurotransmitters, such as insulin, serotonin, or dopamine; several new studies have found that tTG is overexpressed in the HD animal model and human brain, and CYS can promote the secretion of BDNF by suppressing the overexpression of tTG, thus delaying neurodegeneration in HD and improving motor function and survival. Pillai used animal and cell experiments to confirm that [7] CYS can inhibit long-term haloperidol (HAL)-induced reduction of BDNF/TrkB protein in the frontal cortex of mice. An enzyme-linked immunosorbent assay was used to determine that CYS treatment for 7 d can significantly increase the BDNF level in the frontal cortex of mice. Western blot analysis also found that the phosphorylation of TrkB was increased 30 min after the injection of CYS; this increase lasted for at least 3 h, thereby reducing HAL-induced neuropathology. Studies have shown that CYS also inhibits apoptosis. Lesort et al. [21] reported that CYS can inhibit caspase-3 activity in a dose-dependent manner, acting as an anti-competitive inhibitor at low concentrations, but as a non-competitive inhibitor at high concentrations; therefore, it can combine with both caspase-3 and caspase-3-substrate complex. Caspases have a single cysteine-activation site; it is thought that CYS and the caspase cysteine-activation site undergo disulfide bond interchange, thus forming a complex that inhibits caspase activity.
Research on CYS has mainly been limited to its role in chronic degenerative neuropathy [24,25]. This study used CYS to treat mice with whole cerebral ischemia, aiming to observe neuronal apoptosis in the hippocampal CA1 region and changes in expression of BDNF, TrkB, and caspase-3, and to explore potential CYS neuroprotective effects and mechanisms in acute ischemic nerve injury. As the use of CYS in treating whole cerebral ischemia has not been reported, doses selected in this study are still in the investigational stage. This study examined the role of CYS in treating chronic degenerative nerve disease, demonstrated that it is non-toxic, and that a small dose (150 mg/kg daily) has neuroprotective effects. Previous studies reported that CYS can be administered orally, intraperitoneally, and intramuscularly. As the purpose of this study was to observe the short-term treatment effects of CYS in acute ischemic neuronal damage, intraperitoneal injection was selected [7,11,12]. The results showed that CYS administered after whole cerebral ischemia can decrease TUNEL-positive cells in the hippocampal region, upregulates BDNF and TrkB, and significantly downregulates caspase-3. Therefore, it can be concluded that the use of CYS in the acute stage of whole cerebral ischemia can reduce the damage caused by ischemia and hypoxia, thus exhibiting its neuroprotective effects. The role of CYS in upregulating BDNF and TrkB is closely related to its inhibition of caspase-3 expression.
A limitation of this study is that the mechanism by which CYS upregulates BDNF and TrkB was not clarified; moreover, the conclusion that CYS inhibits the overexpression of tTG, thus upregulating the secretion of BDNF, requires verification. In addition, as a treatment for hypoxia and ischemia-reperfusion injury, the optimal dosage and administration time, dosage form, and side effects of CYS require further investigation. CYS has only been used in animal models and in vitro cell experiments, and its side effects in humans are unknown. Furthermore, the neuroprotective role of CYS requires research targeting its effects on animal models of learning, memory, and behavior.
Conclusion
This study demonstrated that the use of CYS in the acute stage of whole cerebral ischemia can exhibit anti-apoptotic and protective effects against neuronal damage, and may have potential for use in clinical treatment of ischemic neurological disease.
Acknowledgements
This work was funded by Entrepreneurship Projects for Students Studying Abroad (first batch in 2009), Jinan, Shandong.
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- Dong Q, Lin X, Shen L, Feng Y. The protective effect of herbal polysaccharides on ischemia-reperfusion injury. Int J Biol Macromol 2016; 92: 431-440.
- Shao S, Xu M, Zhou J, Ge X, Chen G, Guo L, Luo L, Li K, Zhu Z, Zhang F. Atorvastatin attenuates ischemia/reperfusion-induced hippocampal neurons injury via Akt-nNOS-JNK signaling pathway. Cell Mol Neurobiol 2016.
- Park JH, Kim SE, Jin JJ, Choi HS, Kim CJ, Ko IG. Pentoxifylline alleviates perinatal hypoxic-ischemia-induced short-term memory impairment by suppressing apoptosis in the hippocampus of rat pups. Int Neurourol J 2016; 20: 107-113.
- Stucky A, Bakshi KP, Friedman E, Wang HY. Prenatal cocaine exposure upregulates BDNF-TrkB signaling. PLoS One 2016; 11: 0160585.
- Gonzalez A, Moya-Alvarado G, Gonzalez-Billaut C, Bronfman FC. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor (BDNF). Cytoskeleton (Hoboken) 2016; 73: 612-628.
- Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem 2016; 138: 204-221.
- Pillai A, Veeranan-Karmegam R, Dhandapani KM, Mahadik SP. Cystamine prevents haloperidol-induced decrease of BDNF/TrkB signaling in mouse frontal cortex. J Neurochem 2008; 107: 941-951.
- Li PC, Jiao Y, Ding J, Chen YC, Cui Y, Qian C, Yang XY, Ju SH, Yao HH, Teng GJ. Cystamine improves functional recovery via axon remodeling and neuroprotection after stroke in mice. CNS Neurosci Ther 2015; 21: 231-240.
- Pulsinelli WA, Levy DE, Duffy TE. Cerebral blood flow in the four-vessel occlusion rat model. Stroke 1983; 14: 832-834.
- Sun L, Xu S, Zhou M, Wang C, Wu Y, Chan P. Effects of cysteamine on MPTP-induced dopaminergic neurodegeneration in mice. Brain Res 2010; 1335: 74-82.
- Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med 2002; 8: 143-149.
- Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, Saint-Pierre M, Brownell AL. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntingtons disease. J Neurol Sci 2005; 231: 57-66.
- Peng K, Tan D, He M, Guo D, Huang J, Wang X, Liu C, Zheng X. Studies on cerebral protection of digoxin against hypoxic-ischemic brain damage in neonatal rats. Neuroreport 2016; 27: 906-915.
- Schmidt RH, Nickerson JM, Boatright JH. Exercise as gene therapy: BDNF and DNA damage repair. Asia Pac J Ophthalmol (Phila) 2016; 5: 309-311.
- Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJ. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J Neurochem 2005; 94: 1087-1101.
- Hwang IK, Yoo KY, Yi SS, Kim IY, Hwang HS, Lee KY, Choi SM, Lee IS, Yoon YS, Kim SY, Won MH, Seong JK. Expression of tissue-type transglutaminase (tTG) and the effect of tTG inhibitor on the hippocampal CA1 region after transient ischemia in gerbils. Brain Res 2009; 1263: 134-142.
- Lorand L, Conrad SM. Transglutaminase. Mol Cell Biochem 1984; 58: 9-35.
- Griffith OW, Larsson A, Meister A. Inhibition of γ-glutamylcysteine synthetase by cystamine: An approach to the therapy of 5-oxoprolinuria (pyroglutamic aciduria). Biochem Biophys Res Commun 1977; 79: 919-925.
- Brandwein HJ, Lewicki JA, Murad F. Reversible inactivation of guanylate cyclase by mixed disulfide formation. J Biol Chem 1981; 256: 2958-2962.
- Nishihara T, Maeda H, Okamoto K, Oshida T, Mizoguchi T, Terada T. Inactivation of human placenta glutathione S-transferase by SH/SS exchange reactions with biological disulfides. Biochem Biophys Res Commun 1991; 174: 580-585.
- Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem 2003; 278: 3825-3830.
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntingtons disease. J Neurosci 2002; 22: 8942-8950.
- Borrell-Pages M, Canals JM, Cordelières FP, Parker JA, Pineda JR, Grange G, Bryson EA, Guillermier M, Hirsch E, Hantraye P, Cheetham ME, Néri C, Alberch J, Brouillet E, Saudou F, Humbert S. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest 2006; 116: 1410-1424.
- Minarini A, Milelli A, Tumiatti V, Rosini M, Simoni E, Bolognesi ML, Andrisano V, Bartolini M, Motori E, Angeloni C, Hrelia S. Cystamine-tacrine dimer: a new multi-target-directed ligand as potential therapeutic agent for Alzheimers disease treatment. Neuropharmacology 2012; 62: 997-1003.
- Gibrat C, Cicchetti F. Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 380-389.