Research Article - Biomedical Research (2017) Volume 28, Issue 2
Protective effect of secologanin on neuronal cell damage induced in epilepsy
Ye-Fen Lu, Hui-Fen Huang, Jian-Jun Chen, Ting-Ting Zeng, Wei-Jing Chen, Lin-Lin Yu, Jie Rao, Wei-Yan Sun, Xiu-Mei Liu, Wei-wen Qiu*Department of Neurology, The Fifth Affiliated Hospital ,Wenzhou Medical University, Lishui City, Zhejiang, PR China
- *Corresponding Author:
- Wei-wen Qiu
Department of Neurology
The Fifth Affiliated Hospital
Wenzhou Medical University, Zhejiang, PR China
Accepted on July 1, 2016
Abstract
Present study investigates the protective effect of Secologanin (SLG) on neuronal cell damage induced by epilepsy. Epilepsy was induced by pilocarpine (350 mg/kg, Ip) and scopolamine (1 mg/kg, Subcutaneous) injection at an interval of 30 min. SLG was administered at a dose of 10 mg/kg and 20 mg/kg of body weight for the period of 7 day after the injection of pilocarpine. Effect of SLG on convulsions was monitored for 30 min at the end of protocol. Whereas, its neuroprotective effect was assessed by caspase activity, neurochemicals concentration (Dopamine and 5 HT) and oxidative stress parameters like Superoxide Dismutase (SOD); Catalase (CAT); Lipid Peroxidation (LPO) in brain tissues. Neuronal cell damage was assessed by histopathological study using NeuN staining. Result suggested that SLG treatment significantly increases (p<0.01) the latency of myoclonic jerking and absent of latency and duration generalized tonic seizer compared to negative control group. There was significant decrease (p<0.05) in the caspase activity in SLG treated group compared to negative control group. Treatment with SLG increases the neurochemical (Dopamine and 5 HT) level and ameliorates the oxidative stress parameters compared to negative control group. However histopathology study suggested that treatment with SLG improves the quantity of normal cell and decreased the injured neuronal cell. Present investigation concludes that SLG possess neuroprotective effect in epilepsy induced rat model and also postulates its possible mechanism, by managing the neurochemical balance and amelioration of oxidative stress.
Keywords
Secologanin, Epilepsy, Neuroprotection, Dopamine, 5 HT, Oxidative stress.
Introduction
Epilepsy is a severe neurological disease, affecting 1% of people globally [1]. Characteristic symptoms of epilepsy are sudden start of convulsion or loss of consciousness for short period of time, over excitement of sensory nerves. Convulsions in epilepsy arrive due to excessive firing of neurons or decreased threshold potential of the neuronal cells [2]. These changes in the neuronal cells are caused by neurochemical imbalance such as glutamate, 5 hydroxy triptamine, dopamine and GABA [3].
Reported study reveals that dopamine and 5 hydroxy triptamine plays a role in the pathogenesis of epilepsy. Literature suggested that altered release of glutamic neurotransmitter results in the neuronal cell death due to induction of apoptosis which is controlled by dopamine [4]. Whereas, dopamine agonists are the drug used in the management of epilepsy and neuronal damage in Parkinson’s disease and schizophrenia [5].
Alternative medicines gain interest for the management of epilepsy throughout the globe and many of herbal drugs shows potent anticonvulsant activity. Secologanin is an alkaloid isolated from Catharanthus roseus and its medicinal property was not explored till dated. Literature suggested that SLG is a precursor alkaloid of dopamine and 5 HT in the plant [6]. On the basis of this property present study evaluates the neuroprotective effect of SLG in Epilepsy induced cell damage.
Material and Methods
Animals
Healthy male wistar rats (200-250 g) were procured from Avian Disease Research Center, Sichuan Agricultural University, China. All the animals were kept under controlled condition as per the guidelines with pallet feed and water ad libitum. Investigation protocols of the present study approved by Institutional ethical committee.
Acute toxicity
Acute toxicity of secologanin was performed as per the OECD guideline (423). In this study overnight fasted male wistar rats were used for the dosing of SLG. Lethal dose of SLG was estimated and 1/10th and 1/20th of LD50 considered as effective dose [7].
Induction of epilepsy
In all the animals epilepsy was induced in all the rats by a single intraperitoneal injection of pilocarpine (4%, Merck) at a dose of 350 mg/kg body weight. Effect of peripheral acetylcholine was reduced by the injection of scopolamine at a dose of 1 mg/kg subcutaneously, 30 min prior to pilocarpine. Seizures were developed after 10 min. of pilocarpine injection. Seizures were seized with the Ip injection of diazepam at a dose 10 mg/kg after 90 min of study. Control group receives saline solution in place of pilocarpine, scopolamine and diazepam [8].
Administration of secologanin
All the animals were separated in to two major groups: control group (n=10) was treated with saline instead of pilocarpine and also received saline for 7 days. Whereas epilepsy induced group was further separated into 3 sub-groups i.e. Negative control group (n=10) received pilocarpine and saline for 7 days, SLG treated group received 10 mg/kg and 20 mg/kg (n=10, each group), Ip of SLG 30 min prior to pilocarpine. Effect of SLG on epilepsy induced neuronal damage was assessed at the end of protocol [9].
Monitoring of seizures
After the drug administration, every day rats were observed for the period of 30 min for myoclonic jerking and generalized tonic seizers’ latency and duration of it also [10].
Estimation of biochemical parameter
Brain tissue homogenate preparation: Rats were scarified and brain dissected out, washes it thoroughly with saline solution and divided into two halves. One half of brain of each rat was homogenized instantaneously in solution containing Tris-Hcl (50 mM, pH 7.4) and sucrose (300 mM). The tissue homogenate was centrifuged at 10000 RPM for 10 min at 4ºC and the supernatant was separated for the below given biochemical estimation. Another half of brain of each rat was used for histoplathological study.
Estimation of caspase activity: Caspase activity was estimated in rat brain. Tetra peptide substrates were incubated with brain tissues homogenate at 37ºC. These peptides were Trp-Glu-His-Asp (Ac-WEHD-AMC, 4 mM) for caspase 1 and Asp-Glu-Val-Asp (Ac-DEVD-AMC, 4 mM) for caspase 3. Homogenate again treated with inhibitors of caspase 1 and caspase 3 and incubated the given mixture with their particular substrate. Activity of caspase was estimated for 90 min using spectroflurimetery at 360 and 465 nm [11].
Estimation of dopamine and serotonin
Tissue extract was mixed with equal volumes of Isobutane (10%) and buffer (pH 10). Thereafter heptane (10%) was added in the given solution and then hydrochloric acid (0.1 N, 5 ml) added with vigorous shaking. The given solution was then centrifuge for the separation of organic mixture. Determination of dopamine and 5 HT was achieved by using separated aqueous phase.
Estimation of dopamine was achieved by adding 0.4 M EDTA and pH 6.9 buffer at a volume of 0.02 ml and 0.05 ml respectively in 0.02 ml of aqueous phase. Thereafter, for oxidation 0.1 M iodine solution prepared in ethanol (0.01 ml) added in the given solution. In the solution acetic acid was mix and heated at 100ºC for the period of 6 min. and the estimation spectra for excitation and emission for dopamine was done at 330-375 nm using spectroflurophotometer.
Estimation of 5 HT was achieved by using O-pthaldialdehyde method. In the aqueous phase of solution O-pthaldialdehyde (0.025 ml) was added and the mixture was heated at 100ºC for 10 min. Estimation of 5 HT spectra was achieved at 360 and 470 nm for excitation and emission respectively [12]..
Estimation of markers of oxidative stress
Superoxide Dismutase (SOD) was estimated in the brain tissue of rats by using riboflavin sensitized method. The alteration in absorbance was observed for 4 min at 460 nm [13].
Level of Lipid Peroxidation (LPO) was estimated the method given by Ohkawaka in the brain tissue of rats. The quantity of Malondialdehyde (MDA) was estimated at 532 nm [14].
Activity of Catalase (CAT) in the brain tissue was assessed on the ability of catalase to oxidize H2O2. The change in absorbance was recorded for 3 min at 1 min interval at 240 nm [15].
Histopathology study
NeuN staining was used for the estimation of neuronal cells in the regions (CA1 and CA3) as mentioned by TANG et al. Neuronal cells counted in the study having size more than 8 μm whereas cells with lower size considered as normal glial cells. Neuronal cells were also by characterized on the basis of morphology [9].
Results
Effect of secologanine on Convulsion
Effect of secologanine on latency myoclonic jerking, generalized tonic seizer and duration of generalized tonic seizer in pilocarpine induced epileptic rat mode as shown in Table 1. It was observed that SLG treatment significantly (p<0.01) increases the latency of myoclonic jerking and latency of generalized tonic seizer. There were generalized tonic seizers found absent in SLG treated group of rats. This increased latency of generalized tonic seizer proves the significant effect of SLG in the treatment of epilepsy.
| Sr. No. | Group | Latency myoclonic jerking (sec) | Latency generalized tonic seizer (sec) | Duration of generalized tonic seizer (sec) |
|---|---|---|---|---|
| 1 | Negative control | 39.4±3.2 | 46.2±3.1 | 20.3±3.1 |
| 2 | SLG 10 mg/kg | 217±11.3** | 205±5** | No generalized tonic seizer** |
| 3 | SLG 20 mg/kg | 294±15** | No generalized tonic seizer** | No generalized tonic seizer** |
Values are means ± SD (n=10); **p<0.01 (vs. Negative control group)
Table 1. Effect of secologanine on latency myoclonic jerking, generalized tonic seizer and duration of generalized tonic seizer.
Estimation of caspase activity
Activity of both the type of caspase found to be increases in the brain tissues of pilocarpine induced epileptic rats as shown in Table 2. It was observed that treatment with SLG decreases (p<0.05) the activity of caspase 1 significantly compared to negative control group in a dose dependant manner. Whereas SLG treatment was not able to alter the increased activity of caspase 3 in the brain tissues of pilocarpine induced epileptic rats.
| Sr. No. | Group | Caspase-1 (WEHDase activity) (RFU/min/mg of protein) |
Caspase-3 (DEVDase activity) (RFU/min/mg of protein) |
|---|---|---|---|
| 1 | Control | 25±7 | 4±2 |
| 2 | Negative control | 57±12@ | 12±7@ |
| 3 | SLG 10 mg/kg | 39±10* | 11±5@ |
| 4 | SLG 20 mg/kg | 30±8* | 11±4@ |
Values are means ± SD (n=10); @p < 0.01 (vs. Control group),*p<0.05 (vs. Negative control group)
Table 2. Effect of secologanin on the caspase activity in the brain tissues.
Estimation of dopamine and 5 HT
Dopamine and 5 HT level in the brain tissues were estimated as shown in Table 3. There were significant decrease in the level of dopamine and 5HT up to 485 and 86 pg/mg of wet tissue respectively in epileptic rats compared to controlled group. However treatment with SLG significantly (p<0.01) increases the dopamine and 5 HT level in the brain tissues compared to negative control group. SLG treatment ameliorates the level of dopamine and 5 HT in the brain tissues in dose dependent manner.
| Sr. No. | Group | Dopamine (pg/mg of wet tissue) |
5HT (pg/mg of wet tissue) |
|---|---|---|---|
| 1 | Control | 649.16±5.52 | 128.6±5.08 |
| 2 | Negative control | 485±5.21@ | 86.33±5.3@ |
| 3 | SLG 10 mg/kg | 632±7.09** | 97.5±4.13* |
| 4 | SLG 20 mg/kg | 737±5.77** | 130.1±8.5** |
Values are means ± SD (n=10); @p<0.01 (vs. control group),*p<0.05, **p<0.01 (vs. negative control group)
Table 3. Effect of secologanin on the Dopamine and 5 HT in the brain tissues.
Estimation of oxidative stress parameters
Effect of SLG on superoxide dismutase, lipid peroxidation, catalase in the brain tissue of pilocarpine induced epileptic rats as shown in Table 4. Pilocarpine induced epilepsy, results in significant (p<0.01) decrease in the SOD and increase (p<0.01) in the LPO and CAT level compared to control group of rats. However treatment with SLG significantly improved the SOD level in the brain tissues compared to negative control group of rats. LPO and CAT level were found to be significantly decreases (p<0.01) in the brain tissues of SLG treated group of rats compared to negative control group of rats. Moreover, study result also suggested that this improvement in the level of oxidative stress parameters was dose dependent.
| Sr. No. | Group | SOD (Unit/mg protein) |
LPO (nmol MDA/mg protein) |
CAT (µmol H2O2 consumed/min/mg protein) |
|---|---|---|---|---|
| 1 | Control | 15.3 ± 1.25 | 7.6 ± 0.3 | 42.15 ± 1.3 |
| 2 | Negative control | 4.2 ± 0.5@ | 13.2 ± 1.11@ | 69.2 ± 1.7@ |
| 3 | SLG 10 mg/kg | 9.8 ± 1.3** | 9.8 ± 0.7** | 53.1 ± 1.4** |
| 4 | SLG 20 mg/kg | 12.13 ± 1.2** | 7.9 ± 0.6** | 44.9 ± 1.1** |
Values are means ± SD (n=10); @p<0.01 (vs. control group),*p<0.05, **p<0.01 (vs. negative control group)
Table 4. Effect of secologanin on SOD, LPO and CAT in the brain tissue.
Histopathological analysis
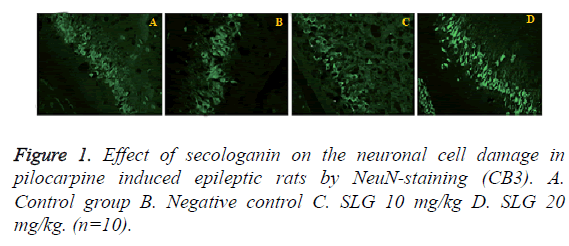
Histopathology study of brain tissue by using NeuN staining (CA3) was shown in Figure 1. TS of the brain tissues reveals sever neuronal cell damage in (CA3) the negative control group of rats. Whereas, TS of SLG treated group was showed comparative fewer damage of neuronal cell than that of negative control group. This observation suggested the neuroprotective activity of SLG in epilepsy induced neuronal damage rat model.
Discussion
Literature reveals that pathogenesis of epilepsy is related to altered neurochemical levels in the brain like glutamate, dopamine, 5 HT and GABA [16]. Altered level of biological amine progressed into neuronal cell damage through increased level of oxidative stress in the brain tissues [17]. The commonest animal model for epilepsy is pilocarpine and found to produce the neuronal damage.
Present investigation evaluates the neuroprotective effect of SLG in pilocarpine induced epileptic rat model. The result of the study demonstrated that treatment with SLG significantly increases the latency of myoclonic jerking in pilocarpine induced epileptic rats. Latency of generalized tonic seizer and its duration was absent in the SLG treated epileptic rats. Antiepileptic drugs were reported to reduce the myoclonic jerking and generalized epilepsy [18].
Imbalance between the level of neurochemical results in the development of epilepsy [16]. Result of this study also suggested that treatment with SLG significantly increases (p<0.01) the dopamine and 5 HT concentration in the brain tissues compared to pilocarpine induced epileptic rats. Reports suggested that inhibition of dopamine and 5 HT develops the oxidative stress by increasing the reactive oxygen species. However there was significant decrease in the level of oxidative stress parameters in SLG treated group compared to negative control group of rats. Thus treatment with SLG increases the dopamine and 5 HT level thereby reduced the oxidative stress in the brain tissues. On the basis of it was found that the number of neuronal cell damage decreases by reducing the caspase expression.
Conclusion
Present study concludes that secologanin protects the neurons in epilepsy induced neuronal damage on the basis of its modulatory effect on neurochemical and antioxidant.
Acknowledgement
Present study was financially supported by projects of Medical and Health Technology development program of Zhejiang Province of China (2015120636)Science and Technology Program of Lishui of China.
References
- Rapp PR, Bachevalier J. Cognitive development and aging. Fundamental Neuroscience. Academic Press USA (2nd edn.) 2003; 1167-1199.
- Balakrishna S, Pandhi P, Bhargava VK. Effects of nimodipine on the efficacy of commonly used antiepileptic drugs in rats. IndJ experbiol 1998;36:51-54.
- Kumar AS,Gandhimathi R. Effect of Guettardaspeciosa extracts on biogenic amines concentrations in rat brain after induction of seizure. Int J PharmPharmaceuticSci2009; 1: 237-243.
- Mckenzie GM, Soroko FF. Effects of apomorphine, amphetanine and L-dopa on maximal electroshock convulsion-a comparative study in the rat and mouse.J Pharm Pharmacol1972;24: 696-701.
- Bozzi Y, Vallone D, Borrelli E. Neuroprotective role of dopamine against hippocampal cell death. J Neurosci 2000; 20:8643-8649.
- Naudascher F,Doireau P, GuillotA,VielC,Thiersault M. Time-Course Studies on The Use of Secologanin byCatharanthusroseusCells Culturedin vitro. J Plant Physiol 1989; 134: 608-612.
- Xu L, Wu XY, Tang XH, Wang JH, Hong Z. Effect of Tenidap on expression of inwardly rectifying potassium channel 2.3 in hippocampus of chronic temporal lobe epileptic rats. Chin J Neurol 2010; 43: 464-468.
- Organization for economic co-operation and development, revised draft guidelines 423.Oecd Rev Doc 2000.
- Pahuja M,Mehla J,Reeta KH,Joshi S,Gupta YK. Root extract of Anacyclus pyrethrum ameliorates seizures, seizure-induced oxidative stress and cognitive impairment in experimental animals. Epilepsy Res2012; 98:157-165.
- Tang XH, Wu XY, Xu L, Fang Y, Wang J, Zhu G, Hong Z. Tenidap is neuroprotective in a pilocarpine rat model of temporal lobe epilepsy. Chin Med J 2013; 126: 1900-1905.
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Nicholson DW. A combinatorial approauch defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. JBiolChem 1997; 272: 17907-17911.
- Ray K, Hazra R, Guha D. central inhibitory effect of Moringaoleifera root extract: possible role of neurotransmitters. Ind J ExperBiol 2003; 41: 1279.
- Arutla S, Arra GS, Prabhakar CM. Pro-and anti-oxidant effects of some antileprotic drugsin vitroand their influence on super oxide dismutase activity. Arzneim-Forsch J Drug Res 1998; 48:10-24.
- Okhawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Ann Biochem 1979; 95:351-358.
- Beers RF, Sizer IW. Estimation of catalase. J BiolChem 1952; 195:133.
- Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. PharmacolBiochemBehav 1994; 49: 911-920.
- Puttachary S,Sharma S,Stark S,Thippeswamy T. Seizure-Induced Oxidative Stress in Temporal Lobe Epilepsy. BioMed Research International. 2015: 2015: 20.
- Pérez JF,Zebadúa PB,Marmolejo JM. Anticonvulsant effects of mefloquine on generalized tonic-clonic seizures induced by two acute models in rats. BMC Neurosci 2015; 16: 7.