Editorial - Journal of Chemical Technology and Applications (2017) Volume 1, Issue 1
Practical reduction of manganese oxide
- *Corresponding Author:
- Fikri Erdem Şeşen
Metallurgical and Materials Engineering Department, Istanbul Technical University, Istanbul, Turkey
Tel: 00905334172455
E-mail: sesen@itu.edu.tr
Accepted date: August 16, 2017
Citation: Şeşen FE. Practical reduction of manganese oxide. J Chem Tech App. 2017;1(1):1-2.
DOI: 10.35841/chemical-technology.1.1.26-27
Visit for more related articles at Journal of Chemical Technology and ApplicationsAbstract
Manganese is an important metal used in steel industry. It is abundant in steel as an alloying element. Additionally, it is used as a deoxidiser in steel production. In steel industry, manganese metal is used as an intermediate product of ferromanganese. Ferromanganese is generally produced by reduction of oxidised manganese. Reduction is in the form of either metalothermic reduction or carbothermic reduction. Practically, metallographic reduction is performed with silicon or aluminium which form more stable oxides than magnesium. Carbothermic reduction means reduction with carbon. All of the reduction reactions are highly endothermic and a high amount of thermal energy is required for the accomplishment of these reactions [1, 2]. The most abundant forms of the manganese oxides are MnO2, Mn2O3, Mn3O4 and MnO. These compounds dissociate during heating.
Editorial
Manganese is an important metal used in steel industry. It is abundant in steel as an alloying element. Additionally, it is used as a deoxidiser in steel production. In steel industry, manganese metal is used as an intermediate product of ferromanganese. Ferromanganese is generally produced by reduction of oxidised manganese. Reduction is in the form of either metalothermic reduction or carbothermic reduction. Practically, metallographic reduction is performed with silicon or aluminium which form more stable oxides than magnesium. Carbothermic reduction means reduction with carbon. All of the reduction reactions are highly endothermic and a high amount of thermal energy is required for the accomplishment of these reactions [1,2]. The most abundant forms of the manganese oxides are MnO2, Mn2O3, Mn3O4 and MnO. These compounds dissociate during heating.
2MnO2 = Mn2O3+1/2O2
3Mn2O3 = 2Mn3O4+1/2O2
Mn3O4= 3MnO+1/2O2
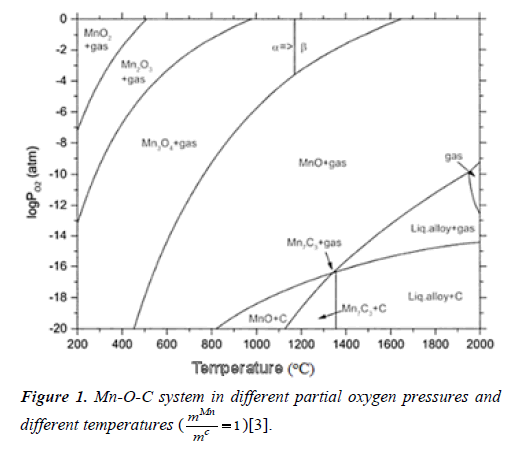
Therefore, different oxide phases are formed dependent on
the temperature and partial oxygen pressure. Mn-O-C system
is given in Figure 1 in different partial oxygen pressures and
different temperatures for 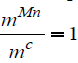 .
.
Reduction of manganese oxides is considered in two steps. The first step is the reduction of oxygen-rich oxides to MnO and the second one is the reduction of Mn to metallic manganese. Reduction starts with the transformation of MnO2 into Mn2O3 and Mn2O3 into Mn3O4 at temperatures over 450°C, then these two phases are reduced by either carbon or carbon monoxide in the system of Mn-C-O. The reduction reactions of manganese oxides and the standard free energies of formation of these chemical reactions are given in Table 1 [3-8].
| Reactions | ΔGo. kJ/mol | T (°C) |
|---|---|---|
| Reduction reactions of oxygen-rich oxides to MnO | ||
| 3Mn2O3 + C = 2Mn3O4 + CO | ΔGo = - 0.25 ? 0.17T | 25-1100 |
| 3Mn2O3 + CO = 2Mn3O4 + CO2 | ΔGo = -170.71 ? 0.004T | 25-1100 |
| Mn3O4 + C = 3MnO + CO | ΔGo = 110.96 ? 0.21T | 25-1244 |
| ΔGo = 84.35 ? 0.20T | 1244-1700 | |
| M3O4 + CO = 3MnO + CO2 | ΔGo = 110.96 ? 0.21T | 25-1244 |
| ΔGo = 84.35 ? 0.20T | 1244-1700 | |
| Reduction reaction of MnO with carbon monoxide | ||
| MnO + CO = Mn + CO2 | ΔGo = 102.38 + 0.01T | 25-1227 |
| ΔGo = 116.73 + 0.01T | 1227-1727 | |
| Boudouard reaction | ||
| CO2 + C = 2CO | ΔGo = 170.82 ? 0.18T | 25-1727 |
| Reduction reactions of MnO with carbon or iron carbide | ||
| MnO + C = Mn + CO | ΔGo = 287.6Â ? 0.16T | 25-1227 |
| MnO + 10/7C = 1/7Mn7C3 + CO | ΔGo = 284.22 ? 0.18T | 717-1087 |
| ΔGo = 282.01 ? 0.18T | 1087-1137 | |
| ΔGo = 280.22 ? 0.18T | 1137-1244 | |
| ΔGo = 280.35? 0.18T | 1244-1700 | |
| MnO + 10/7Fe3C = 1/7Mn7C3 + 30/7Fe + CO | ΔGo = 246.09 ? 0.15T | 717-840 |
| ΔGo = 269.42 ? 0.17T | 840-1087 | |
| ΔGo = 267.42 ? 0.17T | 1087-1137 | |
| ΔGo = 265.42 ? 0.17T | 1137-1244 | |
Table 1: Reduction reactions of manganese oxides in different types and the standard free energies of formation of these chemical reactions in different temperature ranges.
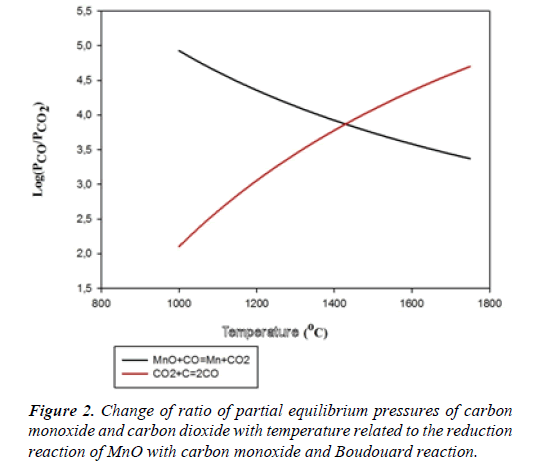
A very high carbon monoxide pressure is required for the reduction of MnO with carbon monoxide. Change of ratio of partial equilibrium pressures of carbon monoxide and carbon dioxide with temperature is presented in a diagram given in Figure 2 related to the reduction reaction of MnO with carbon monoxide and Boudouard reaction [3].
As understood from the diagram, the reduction of MnO with carbon monoxide can only be achieved at temperatures over 1430°C at which the ratio P_CO/P_([CO]_2 ) is 7400. Since the reduction, if done with carbon monoxide, can only be achieved in the abundance of carbon, at temperatures over 1430°C and at an extremely high carbon monoxide pressure, the reduction of MnO with carbon monoxide can not be accomplished in many industrial applications. For this reason, reduction of MnO with solid carbon or iron carbide, as given in Table 1, comes forward [3-9]. Furthermore, manganese carbides are also formed during the carbothermic reduction of manganese oxides. The temperature required for manganese carbide formation (1280°C) is lower than that required for metallic manganese formation (1430°C). Therefore, formation of metallic manganese is inevitable.
References
- Ari ME. Determination of ferromanganese/ferrosilicon-manganese production parameters from manganese ore of Tavas-Turkey in laboratory-type arc furnace. Master?s Thesis, Institute of Science and Technology, Istanbul Technical University, Istanbul. 1996.
- Goel R. Thermodynamic considerations in the production of bulk ferroalloys (Fe-Mn, Fe-Cr, Fe-Si). In: 4th Refreshor Course on Ferro Alloys, Jamshedpur, India. 1994.
- Olsen SE, Olsen S, Tangstad M, et al. Production of manganese ferroalloys. Tapir Academic Press, Trondheim, Norway. 2007.
- Gaskell DR. Introduction to metallurgical thermodynamics. Scripya Publishing Company, Washington D.C., USA. 1940.
- Karapetyants MK. Examples and problems in chemical thermodynamics, MIR Publishers, Moscow. 1974.
- Samsonov GV. The oxide handbook. (Turton, C. N., Turton, T. I., Translated), IFI/PLENUM, USA. 1973.
- Aytekin V. Metallurgy thermodynamics, ITU Metallurgy Faculty Offset Printing Studio, Istanbul, 1989.
- Dikeç F, Aydin S. Problems of solution metallurgy thermodynamics. Offset Atelier of ITU Chemistry and Metallurgy Faculty, Istanbul. 1991.
- Grimsley WD, See JB, King PP. The mechanism and rate of reduction of Mamatwan manganese ore fines by carbon. J South African Inst of Min and Metallurgy. 1977; p. 51-62.