Case Report - Archives of General Internal Medicine (2023) Volume 7, Issue 4
Posterior Nutcracker Syndrome in a 16-year-old male with PRES
Smaran Kasireddy*, Boney J. Lapsiwala, Ketna R. Chudasama, Bhaswanth Bollu, Ayushi Mishal, Ankitha Vanaparti, Simran NimalDepartment of Internal Medicine, University Hospital Leicester, Leicester
- *Corresponding Author:
- Smaran Kasireddy
Department of Internal Medicine
University Hospital Leicester, Leicester
E-mail: smaran.psbb@gmail.com
Received: 29-Jul-2023, Manuscript No. AAAGIM-23- 108866; Editor assigned: 01-Aug-2023, PreQC No. AAAGIM-23-108866(PQ); Reviewed: 16-Aug-2023, QC No. AAAGIM-23-108866; Revised: 21-Aug-2023, Manuscript No. AAAGIM-23-108866(R); Published: 28-Aug-2023, DOI:10.35841/aaagim-7.4.183
Citation: Kasireddy S. Posterior Nutcracker Syndrome in a 16-year-old male with PRES. Arch Gen Intern Med. 2023;7(4):183
Keywords
Nutcracker syndrome, Hematuria, Renovascular hypertension.
Introduction
Nutcracker Syndrome (NCS) is a clinical condition that is infrequently observed. It is caused by the compression of the Left Renal Vein (LRV) by the Superior Mesenteric Artery (SMA) as it passes between the SMA and the Abdominal Aorta (AA) (anterior NCS). A distinct form of this anatomical structure, referred to as posterior NCS, arises in cases where the LRV is situated retroaortic and experiences compression due to the proximity of the spine and the AA [1]. Renal venous hypertension is a consequence of LRV compression in both scenarios. Prolonged exposure to elevated pressures may result in renal vasculature impairment, ultimately leading to the manifestation of hematuria [2]. Left-sided varicocele can occur in males when the left testicular vein empties into the LRV. Pelvic Congestion Syndrome (PCS) is a condition that can manifest as dysmenorrhea, dyspareunia, lower abdominal pain, and pelvic varices in females. This condition can be detected through Nerve Conduction Studies (NCS).
The precise prevalence of NCS remains uncertain due to the absence of uniform diagnostic criteria and the heterogeneity of symptoms among individuals with the condition. The condition typically exhibits a higher incidence rate among the female population, with its prevalence reaching its peak during the young and middle-aged adult stages [3,4]. The utilization of Doppler ultrasound is commonly recommended as the primary imaging modality for identifying left renal vein stenosis, potential renal failure, and varicocele resulting from venous congestion in the LRV. However, further investigation with contrast-enhanced computed tomography (CE-CT) is necessary for more precise evaluation of these conditions [5-7]. According to previous research, CE-CT scans have been found to be a reliable method for identifying vascular structures and their proximity to neighboring organs, as well as ruling out alternative sources of compression [6].
The management of NCS may vary between conservative and invasive approaches, contingent upon the severity of hematuria and pain, as documented in sources [8,9]. Conservative treatment of PNCS may be considered in younger patients exhibiting mild hematuria, owing to the high rate of spontaneous remission. In cases of recurrent gross hematuria, severe flank pain, or ineffective conservative treatment, invasive treatments such as open surgical and endovascular interventions have been suggested [9].
Case presentation
A 16-year-old male patient presented to the emergency department with complaints of convulsions, headache, and blurred vision, with a BP of 220/100 mmHg on admission.
Physical examination was significant for bilateral extensor plantar responses. The patient appeared confused and conscious, and the cardiopulmonary examination yielded normal results. The abdomen was soft and non-tender. Initial blood tests, including a complete blood count and metabolic panel, showed normal values, with a creatinine level of 0.97 mg/dl (0.9-1.3 mg/dl). A complete urine analysis indicated the presence of blood (60-80 rbc/hpf) and +1 proteinuria. Additional examination was conducted to assess serum renin and aldosterone levels in order to rule out pheochromocytoma, as shown in Tables 1-4 provided. One month prior to being hospitalised, the patient experienced a single episode of generalised tonic-clonic seizures with normal vital signs and physical examination findings. As the patient had no other complaints at that time, he was discharged without medication and advised for further follow-up.
| Blood Count And Indices | Result | Reference Range |
|---|---|---|
| Haemoglobin | 13 | 13.0-18.0 gm% |
| RBC | 5.02 | 4.6-6.2 mill/c.mm |
| PCV | 38.6 | 40.0-54.0 % |
| MCV | 77 | 80.0-96.0 Fl |
| MCH | 25.8 | 27.0-31.0 Pg |
| MCHC | 33.6 | 32-36 % |
| RDW | 15 | 10-15% |
| Platelet | 21600 | 1.5-4.0 lacs/c.mm |
| WBC | 9100 | 4000-10000 /c.mm |
| Neutrophils | 68% | 55-70% |
| Lymphocytes | 30% | 20-40% |
| Eosinophils | 1% | 01-06% |
| Monocytes | 1% | 02-08% |
| Platelet in Smear | Adequate |
Table 1. Results of complete blood count
| LFT | ||
| ALT | 16 | <45 U/ml |
| Total Bilirubuin | 0.2 | <1.3 mg/dl |
| Direct Bilirubin | 0.1 | <0.4 mg/dl |
| Indirect bilirubin | 0.1 | <1.3 mg/dl |
| ELECTROLYTES | ||
| Sodium | 139.09 | 136-145 mmol/L |
| Potassium | 3.56 | 3.5-5.1 mmol/L |
| RFT | ||
| Creatinine | 0.97 | 0.9 -1.3 |
Table 2. Results of complete metabolic panel
| Physical Characteristics | Lab Values | Reference ranges |
|---|---|---|
| Colour | Pale yellow | |
| Specific Gravity | 1.015 | 1.001-1.080 |
| pH | 6 | 5.0-8.0 |
| Chemical Characteristics | ||
| Proteins | Present 1+ | Absent |
| Glucose | Absent | Absent |
| Blood | Present +++ | Absent |
| Ketones | Absent | Absent |
| Bile Salts | Absent | Absent |
| Bile Pigment | Absent | Absent |
| Urobilinogen | Absent | Absent |
| Nitrate | negative | Negative |
| RBC | 60-80 | 0-3 |
| RBC morphology | Normal | |
| WBC cells | Occasional | 0-5 |
| Epithelial cell | 0-2 | 0-5 |
| Cast | Absent | Absent |
| Crystal | Absent | Absent |
Table 3. Results of Complete Urine Analysis
| Upright | Supine | ||
|---|---|---|---|
| Aldosterone, Serum | 2.57 | 2.52 -39.2 ng/dl | 1.76 - 23.2 ng/dl |
| Renin | 4.65 | 4.4-46.1 microIU/ml | 2.8-39.9 microIU/ml |
Table 4. Results of Complete Serum Renin and Aldosterone levels.
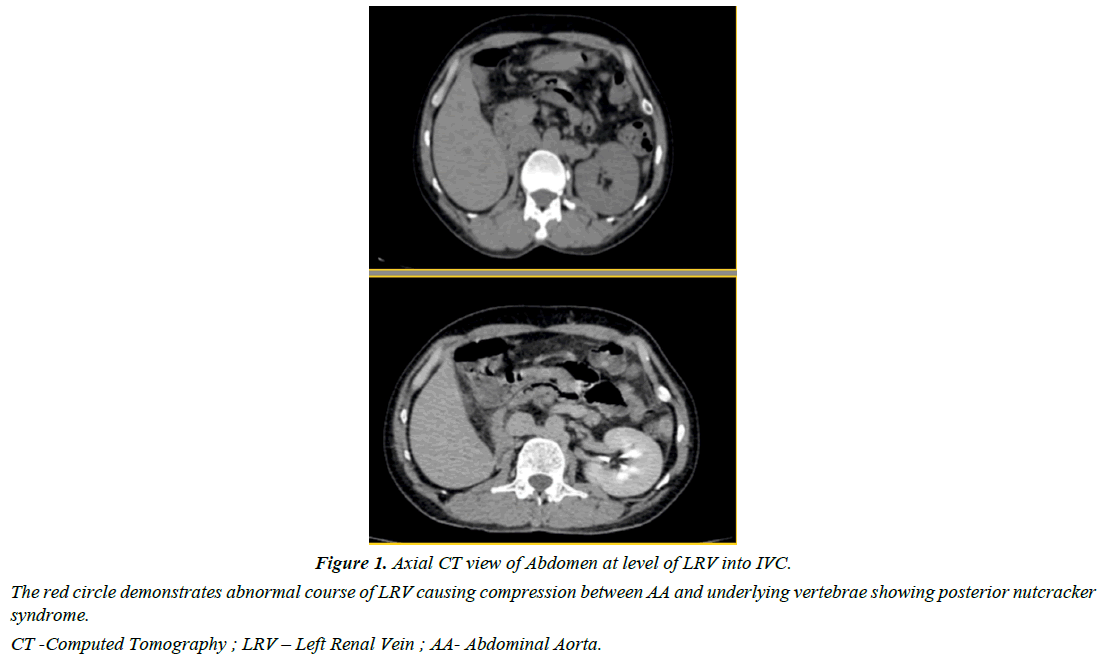
On further radiological examination, USG showed normal sized kidneys with preserved CMD. Renal Artery doppler was performed to rule out renal artery stenosis which revealed elevated acceleration time of bilateral renal arteries at ostium hilum as indirect marker of renovascular hypertension. Hence, an abdominal CT Renal arteriography was done to look for renovascular hypertension which showed retro-aortic renal vein compression between aorta and vertebrae suggestive of Posterior nutcracker syndrome Table 1.
MRI Brain was done for abnormal CNS examination which was suggestive of multifocal abnormal signal intensities in bilateral parieto-occipito-temporal lobes without post contrast enhancement suggestive of posterior reversible encephalopathy syndrome Figure 1.
Based On these examinations, a decision was made to treat the patient symptomatically using anticonvulsant and antihypertensive medications. Late BP control was achieved by alpha blockers and ACE inhibitors. The patient is being closely monitored and has not experienced any additional seizures. As a result, the use of anti-seizure medications was eventually discontinued. The patient was discharged on an ACE inhibitor (Enalapril 5mg) and Alpha-2 Agonist (Clonidine 100mcg) and advised for further follow up.
Discussion
The extrinsic constriction of the LRV between the abdominal aorta and proximal Superior Mesenteric Artery (SMA) is called the "nutcracker phenomenon or renal vein entrapment syndrome. LRV compression is known as anterior nutcracker syndrome. Another variation, posterior nutcracker syndrome, is the retroaortic location of the LRV with compression between the aorta and the vertebral column”. Symptomatic patients have "Nutcracker Syndrome" (NCS), while non-symptomatic patients have "Nutcracker Phenomenon" (NCP) [10,11].
Venous compression induces outflow blockage and LRV hypertension, causing collateralization and varies. Venous collateralization mostly includes the left gonadal vein and connecting lumbar vein. Hematuria happens when high blood pressure in the collaterals and venous sinuses near the renal calyces breaks the thin-walled septum between the small veins and the renal collecting system [12]. A welldeveloped collateral circulation that lowers LRV hypertension may prevent renal symptoms. Left flank pain, proteinuria, varicoceles in males, pelvic venous congestion in females, and anemia are other common symptoms [13].
Doppler ultrasonography of the renal veins is the initial investigative examination. Venography with renal vein pressure gradient monitoring is the gold standard, but its invasiveness makes it a last resort that is sometimes unnecessary for diagnosis. Nutcracker syndrome diagnostic criteria include LRV-IVC venous pressure gradient <3 mmHg; LRV maximal flow velocity five times higher than the renal hilum when it passes the SMA; Aortic and SMA angle less than 45º in CT or MR angiography.
In our case report, blood (60–80 RBC/hpf) and proteinuria were found in the urine analysis of the patient. In order to rule out pheochromocytoma, serum renin, and aldosterone were measured. The patient had a single episode of generalized tonic-clonic seizures with normal vital signs and physical examination findings one month before being hospitalized. The USG indicated kidneys with preserved CMD. To rule out renal artery stenosis, renal artery Doppler was done, which showed that both renal arteries at the ostium hilum had a faster acceleration time. This is an indirect sign of renovascular hypertension. For renovascular hypertension, an abdominal CT Renal arteriography demonstrated retro-aortic renal vein compression between the aorta and vertebrae, suggesting posterior nutcracker syndrome. Also, an MRI of the brain was done for abnormal CNS examination, which showed that both parietal-occipital-temporal lobes had multiple spots with abnormally high signal intensities but no post contrast enhancement. This suggests that the person has posterior reversible encephalopathy syndrome.
Symptom intensity determines NCS management. Conservative treatment frequently resolves symptoms. Patients less than 18 years old are conservatively managed for 24 months. Mild hematuria or spontaneous resolution can be managed conservatively. In young patients, eventual physical development, growth of connective and adipose tissue near the SMA origin and between it and the LRV, and generation of collateral veins may relieve compression and venous hypertension, resulting in spontaneous symptom remission. In our patient, symptomatic anticonvulsant and anti-hypertensive treatment was chosen. Alpha and ACE inhibitors controlled hypertension, and the patient's convulsions stopped. Thus, anti-seizure drugs were discontinued. The patient was released with Enalapril 5mg and Clonidine 100 mcg and told to follow up.
Conclusion
The morbidity associated with NCS is substantial, but the condition is uncommon and often misdiagnosed. Patients often have to go through a number of investigations and procedures before an accurate diagnosis can be made because there aren't any broadly recognized clinical diagnostic criteria. The financial burden on the patient and their family is greatly increased by unnecessary investigations and consultations. It adds to the small number of case reports already described in the literature by presenting a clinical variant of an intriguing phenomenon. Due to the prevalence of features such as flank pain and hematuria in pediatrics and adolescence, PNCS is often missed by uninformed physicians and, as we saw in our case study, can have devastating outcomes if left untreated. In addition to summarizing diagnostic and therapeutic options for patients presenting with microscopic hemorrhage and hypertension, this case serves as a reminder for healthcare professionals to keep a thorough differential diagnosis list for these cases.
References
- Macedo GL, Santos MA, Sarris AB, et al. Diagnóstico e tratamento da síndrome de quebra-nozes (nutcracker): revisão dos últimos 10 anos. J Vasc Bras. 2018;17:220-8.
- Cuéllar i Calàbria H, Quiroga Gómez S, Sebastià Cerqueda C, et al. Nutcracker or left renal vein compression phenomenon: Multidetector computed tomography findings and clinical significance. Eur Radiol. 2005;15:1745-51.
- Ananthan K, Onida S, Davies AH. Nutcracker syndrome: An update on current diagnostic criteria and management guidelines. Eur J Vasc Endovasc Surg. 2017;53(6):886-94.
- Kurklinsky AK, Rooke TW. Nutcracker phenomenon and nutcracker syndrome. Mayo Clin Proc. 2010;85(6):552-559.
- Lamba R, Tanner DT, Sekhon S, et al. Multidetector CT of vascular compression syndromes in the abdomen and pelvis. Radiographics. 2014;34(1):93-115.
- Gozzo C, Giambelluca D, Cannella R, et al. CT imaging findings of abdominopelvic vascular compression syndromes: what the radiologist needs to know. Insights into imaging. 2020;11:1-3.
- Farina R, Iannace FA, Foti PV, et al. A case of nutcracker syndrome combined with wilkie syndrome with unusual clinical presentation. Am J Case Rep. 2020;21:e922715-1.
- Marone EM, Psacharopulo D, Kahlberg A, et al. Surgical treatment of posterior nutcracker syndrome. J Vasc Surg. 2011;54(3):844-7.
- Erben Y, Gloviczki P, Kalra M, et al. Treatment of nutcracker syndrome with open and endovascular interventions. J Vasc Surg Venous Lymphat Disord. 2015;3(4):389-96.
- Gulleroglu K, Gulleroglu B, Baskin E. Nutcracker syndrome. World J Nephrol. 2014;3(4):277.
- Dunphy L, Penna M, Tam E, et al. Left renal vein entrapment syndrome: nutcracker syndrome!. BMJ Case Rep. 2019;12(9):e230877.
- Orczyk K, Wysiadecki G, Majos A, et al. What each clinical anatomist has to know about left renal vein entrapment syndrome (nutcracker syndrome): a review of the most important findings. Biomed Res Int. 2017;2017.
- Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvic venous disorders. J Vasc Sur. 2001;34(5):812-9.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref