Research Article - Biomedical Research (2017) Volume 28, Issue 14
Pitavastatin inhibits proinflammatory cytokines and provides improvement in terms of its motor function in secondary injuries of the spinal cord mouse models
Xianpei Wu* and Jingmin Zhao
The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuangyong Road Nanning, Guangxi, P.R.China
- *Corresponding Author:
- Xianpei Wu
The First Affiliated Hospital of Guangxi Medical University
No. 6 Shuangyong Road Nanning, Guangxi, P.R.China
Accepted date: June 13, 2017
Abstract
Background: Statins are hydroxymethylglutaryl coenzyme A reductase inhibitors that reduce intracellular cholesterol synthesis by competitively inhibiting endogenous cholesterol synthesis rate limiting enzymes. Currently they are most used in the prevention of coronary heart disease. Aside on their cholesterol-lowering effect, statins are also anti-inflammatory in terms of their effects. Recent studies have shown that statins can produce anti-inflammatory effects in acute models of injuries in the spinal cord.
Methods: In order to clarify the function of pitavastatin in secondary spinal cord injuries, we tested the effect of pitavastatin (50 mg/kg) in mouse spinal cord injury. The mice were grouped into control group, sham operation group, injury group, methylprednisolone group and pitavastatin group. We conducted T5-8 laminectomy to mice, then used aneurysms clip at the T6-7 level for epidural compression as our spinal cord injury model. Caspase-3, myeloperoxidase activity, TNF-α, NO level, and BMS score were investigated between groups. The results were analysed statistically.
Results: The activity of caspase-3, myeloperoxidase, TNF-α, and NO had effectively increased after traumatic spinal cord injury, and the above indexes were significantly decreased after the application of pitavastatin. Furthermore, pitavastatin treatment showed improved results in functional tests.
Conclusion: The treatment of pitavastatin in injuries of the acute spinal cord in vivo experiments showed anti-inflammatory effects and ameliorated secondary spinal cord injury.
Keywords
Pitavastatin, Secondary spinal cord injury, Inflammation
Introduction
Spinal Cord Injury (SCI) is recorded as being among the harmful health problems that leads to the failure of the functions of the nervous system. It commonly results from motor, autonomic, and sensory shut down as a result of the inability of the central nervous system (CNS) of an adult to adequately replace lost cells and connections [1,2]. Pathophysiologically, SCI involves both primary and secondary damage. Secondary damage spreads from the site of the primary injury, and includes apoptosis/further cell death, demyelination and axonal degeneration. Oxidative damage is thought to be a key contributor to secondary damage following SCI. Of all the above injuries, inflammation plays the most important role. It is well known that statins have protecting effects on inflammation and some research have shown that simvastatin can be of contribution to the activities that are anti-inflammatory through the PPAR-a pathway [3].
Pitavastatin (Livalo) being a recently developed statin with only several drug-drug interactions, provides a decent feature through the selective uptake of the drug by hepatocytes and the limited metabolism by cytochrome P450 enzymes, which in return reduces the drug potential interaction that were metabolized by these enzymes. Phase III or IV studies in this patient population showed that taking pitavastatin 1-4 mg once daily was generally no less effective than presumed equipotent dosages of atorvastatin and simvastatin. Pitavastatin was generally well tolerated and did not appear to adversely affect glucose metabolism parameters (e.g. fasting blood glucose, fasting plasma glucose, fasting plasma insulin, glycated haemoglobin) in short and longer-term prospective and post-marketing surveillance studies in adults. It is a very promising statin to be widely used in the future. Therefore, we chose pitavastatin as a representative drug of HMG-CoA reductase inhibitor to verify its use in secondary SCI.
In the knowledge of our recent scientific researches, pitavastatin is yet to be expounded in the field of SCI. The main purpose of this study is to examine whether pitavastatin has the ability to make the spinal cord immune from oxidative stress, inflammation and apoptosis in mice once traumatic experiments on SCI have been conducted. A comparison of pitavastatin and Methylprednisolone (MP) has also been conducted and had also been massively studied in traumatic experimental SCI.
Materials and Methods
Experimental groups
Six week old mice, with weights of 18-22 g, were procured from an animal laboratory. The Animal Care Review Board accepted the conduct of the study and the laboratory procedures were in compliance with the Chinese regulations on laboratory animal protection. Random allocation was used to group the mice into:
Group 1: Control (n=8); surgical procedures were not carried out. The control group provides non-traumatized samples of mice spinal cord which are used to determine normal morphological structure and values of biochemical baselines.
Group 2: Sham (n=8); simple laminectomy was performed. Removal of non-traumatized spinal cord was done after 24 h.
Group 3: Trauma (n=8); SCI was induced. Following laminectomy, mice were exposed to injury and traumatized spinal cords were removed after 24 h. Administration of 2 ml distilled water via gavage was done 7 d prior to the induction of SCI and was sustained.
Group 4: MP (n=8); SCI was induced with a one-time intraperitoneal dosage of 30 mg/kg MP (Pfizer Manufacturing Belgium NV) immediately after induction.
Group 5: Pitavastatin (n=8); SCI was induced. Daily administration of 50 mg/kg pitavastatin via gavage was performed 7 d prior to induction and was sustained until sacrifice.
Anaesthesia and SCI procedure
Chloral hydrate, with a dose of 40 mg/kg body weight, was used as anaesthetic. The paravertebral musculature of the mice was exposed via a middle longitudinal incision on its back. The T5-T8 vertebrae were exposed upon dissection of the muscles and laminectomy of the vertebral section was performed in order to expose the spinal cord. The activation of SCI was through the extradural compression of the T6-T7 spinal cord section with the use of an aneurysm clip and a 24 g closure force [4]. A 200 mg/kg dose of pentobarbital was then injected to the mice, 24 h following the operation (Fujinaga Pharm, Japan). Motor function tests were separately conducted on 8 mice from groups 3 and 5 and they were housed for 10 d in a room with a constant temperature of 27°C. Extracted samples of spinal cord samples were immediately placed on ice, transported, and stored in a tissue archiving freezer (-80°C) for preservation until further biochemical analysis.
Biochemical procedures
The homogenization of the tissue samples in physiologically-prepared saline solutions and centrifuged at 4000 rpm for 20 min. The extraction and analysis of the clear supernatants were then conducted.
Tissue caspase-3 analysis
An ELISA kit was used to quantify caspase-3 activity (Uscn Life Science Inc., China). Procedures were done in accordance with the instructions of the manufacturer and obtained results were expressed as ng/mg protein.
Myeloperoxidase activity
The activity of Myeloperoxidase (MPO) is determined through the indication of the accumulation of Polymorphonuclear leukocyte (PMN) and was performed according to the listed procedures [5]. Extracted tissue samples of the spinal cord were weighed after SCI induction. The homogenization of every tissue in a solution of 0.5% (w/v) hexadecyltrimethyl-ammonium bromide which is dissolved in 10 mM potassium phosphate buffer (pH 7) and was centrifuged for 20,000 Xg at 4°C for 30 min. Aliquots of the supernatant were extracted and reacted to a solution of 0.1 mM hydrogen peroxide and tetramethylbenzidine (1.6 mM). A spectrophotometer was used to determine the change rate in the absorbance at a wavelength of 650 nm. The activity of MPO was interpreted as the amount of enzyme that degraded 1 μmol of peroxide per minute at 37°C and the expression was in units/g of wet tissue.
Tumour Necrosis Factor-α concentration measurement
Perilesional zone sections of the extracted cord tissues were collected 24 h after SCI inducted and were homogenized in PBS with 2 mmol/L of PMSF (Sigma Chemical Co.). TNF-α assay was performed in duplicate serial dilutions with the use of a colorimetric commercial kit in accordance with the instructions of the manufacturers.
Tissue nitric oxide analysis
Concentrations of Nitric oxide (NO) in extracted tissue were quantified with the use of a method that was previously discussed by Miranda et al. and obtained results were represented as nmol/mg protein [6].
Neurological evaluation: Motor disturbance grading
Assessment of the functions in motor of trauma-induced mice was performed every day for the next 10 d following injury. Basso Mouse Scale was used in grading motor disturbance recovery (BMS) [7].
Statistical analysis
SPSS version 11.5 was used for the analysis of the data (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was performed to determine normal distribution of continuous variables while the Levene test was carried out for the evaluation of variance homogeneity. All calculated values were reported the standard error of the mean (SEM) of N observations. In the case of vivo study, N represented the number of animal subjects used in the study. One-way ANOVA was performed to determine significant differences between results and a post-hoc Bonferroni test was conducted for the comparisons. A <0.05 pvalue is said to be significant. The analysis of the BMS scale data is through the Mann-Whitney test was performed for the BMS scale analysis and a <0.05 p-value is said to be significant. GraphPad Prism 6.0 was then used to draft the obtained figures (GraphPad Software, Inc., California, USA).
Results
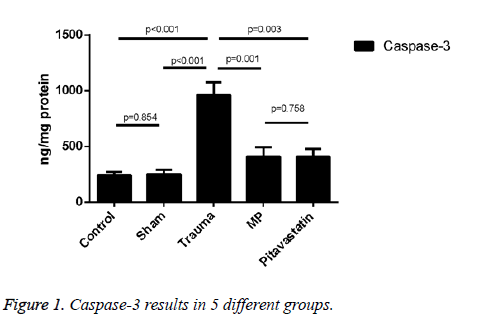
The activity of Tissue caspase-3
An increase in the comparison of the trauma group and both the control group and the sham group were shown as the expression of caspase-3 activity treated on them (both showed p<0.001). Results on pitavastatin treatment showed a massive statistical decrease in the activity of caspase-3 in comparison to the trauma group (showing p=0.003). For the pitavastatin group, activity of caspase-3 in the MP group had also decreased statistically in comparison to the trauma group (showing p=0.001). However, no massive statistical differences were shown between the sham groups and the control groups (showing p=0.854), or between the pitavastatin group and the MP group (showing p=0.758) (Figure 1).
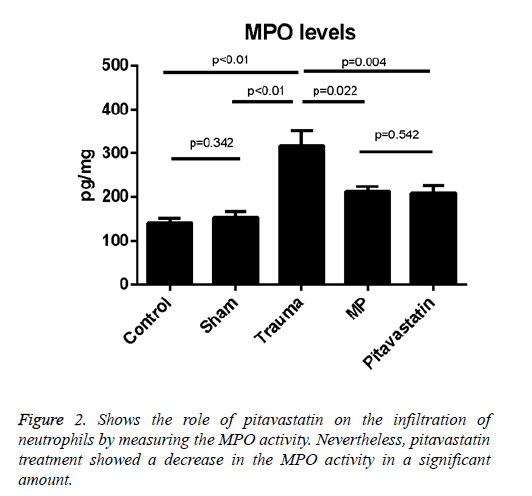
Tissue Myeloperoxidase activity
The comparison of the MPO activities of the sham and control groups with activities of the trauma group showed a difference statistically (p<0.01 for both); after SCI, the data presents that the activity of the tissue MPO has been increased. On the other hand, the treatment with pitavastatin had decreased the activity of the tissue MPO significantly (p=0.004). For the pitavastatin group, the treatment with MP also decreased the activity of MPO in the spinal cord significantly (p=0.022). However, no important differences were observed among the control group and sham groups (p=0.342) or between the MP group and the pitavastatin groups (p=0.542) (Figure 2).
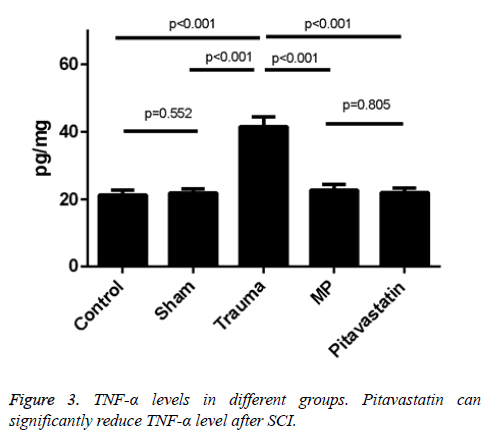
Pitavastatin-induced inhibition of Tnf-α after spinal cord trauma
The levels of TNF-a in the tissues of the trauma groups resulted in a statistical increase in comparison to both the control group and the sham groups (p<0.001 for both). Treatments of both the pitavastatin and MP had massively decreased the levels of TNF-a in the tissues in comparison to the trauma group (p<0.001). Not much differences were shown between the control group and the sham groups (p=0.552), or between the pitavastatin groups and the MP groups (p=0.805). A significant clinical feature of SCI in its secondary level represents these pro-inflammatory cytokines. As a result, huge amounts of TNF-α levels were found in the tissues of the spinal cord of mice 48 h after SCI (Figures 3 and 4).
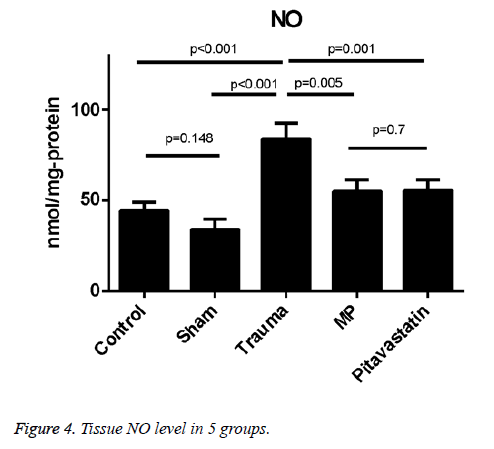
Tissue NO levels
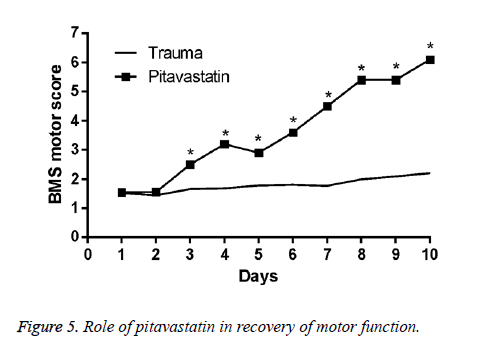
Levels of tissue NO were much higher in the trauma group in comparison to both the control group and the sham group (p<0.001 for both). The treatment of pitavastatin showed a massive reduction in the NO levels of the tissues in comparison to the trauma group (p=0.01). For the pitavastatin group, treatment with MP had massively reduced the NO levels of the tissues in comparison with the trauma group (p=0.005). However, there were not much statistical differences found among the comparison of the control group and the sham group, or among the comparison of MP group and the pitavastatin group in the values of their tissue NO levels (p=0.148 and p=0.7 respectively) (Figure 5).
Effects of pitavastatin treatment on prevention of loss of motor function in SCI mice
For the conclusion of the protection effect of pitavastatin, an application of a modified BMS hind limb locomotors rating scale score was conducted. An assessment of the motor disturbance degree by the BMS criteria was conducted each day until 10 d once SCI was done. The treatment of pitavastatin on the mice had massively decreased the motor disturbance once SCI was done.
Discussion
Initial phase of SCI is established via induction of trauma to the spinal cord that impairs both the bones of the vertebra and associated muscle tissues that lead to the obstruction of axons and extensive neuronal-glial cell degradation causing irreversible damage to the spinal cord [8]. Successive secondary injuries that hinders several pathological processes such as excitotoxicity, inflammation, apoptosis and lipid peroxidation [9-11].
Previous studies have reported that statins lessen the degree of injury on ischemia-reperfusion in multiple organs which includes the spinal cord. The anti-oxidative ability of statin is among its protective mechanisms against neuronal death and cytotoxicity. Hye-Min et al. reported that simvastatin reduced oxidative stress and thus prevented cell death and cytotoxicity in ischemic spinal cord injuries [12]. In another study, statins were shown to decrease injuries in the neurons and deals with experimental neurologic recovery in vitro models of animals in cellular form with cerebral ischemia [13,14]. The significant effects of atorvastatin in the treatment of the pathologies and disabilities on SCI were reported by Pannu et al. [15]. Oral pre-treatment of 10 mg/kg simvastatin for 5 d prior to ischemiare-perfusion injury was reported by Hwang et al. to ameliorate neurologic effects and preserve a greater number of normal motor neurons as compared to control specimens of rats with spinal cord ischemia-reperfusion [16]. The above researches suggest the possible role of pitavastatin in the treatment and prevention of SCI. The reduction of cyclooxygenase-2 mRNA may be another anti-inflammatory mechanism of statins [17]. Disruption of oxidative stress/inflammatory cycles by statins were reported and linked with its ability to impede both lipid peroxidation and inflammatory mediator release [18]. From the evidences above, we can infer that the protective function of pitavastatin was due to its anti-inflammatory effect. To be more specific, pitavastatin significantly reduced interleukin-1β and -6 mRNA protein levels and culture medium expression, which also caused the inhibition of the expression of cyclooxygenase-2 mRNA and their protein levels.
Pitavastatin is a competitive inhibitor of HMG-CoA reductase. Inhibition of this rate-limiting enzyme impedes the cholesterol production in the liver, thereby increasing LDL-C receptor expression and subsequently the uptake of circulating LDL from the blood, which in turn reduces LDL-C (dose dependently) and total cholesterol levels. Pitavastatin was shown to improve postprandial oxidative stress in a study conducted with Japanese men who had abdominal obesity, but did not affect insulin, glucose, or high-sensitivity C-reactive protein levels [19]. The previously stated anti-inflammatory, anti-oxidative, anti-proliferative and immunomodulatory effects of statins, its ability to stabilize plaque, normalize sympathetic outflow and prevent platelet aggregation are all owed to its ability to reduce the amount of circulating isoprenoids which, in turn, inactivates signalling proteins.
MP is a steroid, with anti-oxidative and anti-inflammatory effects, that has been used in treatments for acute SCI [20,21]. MP as a drug treatment is still under debate for the past few years but a few authors have already concluded that MP is not qualified on being a standard treatment for all who suffer from SCI. Present clinical treatments, however, still insist on utilizing high MP doses as a treatment option until further alternative therapies are available. An MP-treated group was used as a comparison to pitavastatin in the present study.
Neuronal death after SCI results from necrosis and apoptosis [22-25]. Apoptosis of spinal cord neurons after induction of trauma results in impaired conduction of impulses through the structural unit of the axon myelin sheath [15]. Caspase-3, an interleukin-converting enzyme, has been reported to be the key mediator of apoptotic death in mammalian cells [15]. Caspase-3 is thus a valuable marker in pointing apoptotic cell activity [26]. In current studies, the activity of caspase-3 had massively increased in the injured spinal cords samples after SCI. A significant down regulation was observed in the caspase-3 activity of the MP group and the pitavastatin group. Results show that inhibition of apoptosis in the segments if the spinal cord that was injured were due to the treatment of both MP and pitavastatin.
The achievement of trans endothelial migration of the leukocytes through the interaction of the CD11b integrin- ICAM-1 which enters the spinal cord have led to promote damage to the cell through the release of proteases, reactive oxygen products, cytokines, and elastase that consists the inflammatory constituents of the injury cascade on its secondary level [27]. The quantity and inflammatory activity of the leukocytes found in the area which is injured is gathered together by the activity of MPO [28]. Moreover, proinflammatory cytokines like TNF-a and MPO, are significant for the quantitative indication of the inflammatory effects of components [29]. In conclusion, the measurement of MPO and proinflammatory cytokine TNF-a levels were conducted for the evaluation and examination of inflammations of the nervous system after SCI. An increase in the mean levels of MPO and TNF-a in the tissues of the trauma group was found in comparison to the control group and the sham group. The significant suppression of the production of MPO and TNF-a was observed during the pitavastatin and MP treatment which likely shows a relation to the anti-inflammatory factor of pitavastatin.
In the present study, we found that pitavastatin can increase BMS scores of mice, decrease the level of MPO, TNF-α and IL-1β in wet tissues after SCI. The results of this study shows that pitavastatin has beneficial effects by stopping the acquisition of apoptosis and increasing inflammation immunity while decreasing oxidative stress. These results showed that pitavastatin had a protective effect on mice after SCI, especially secondary SCI. Our conclusions are consistent with Esposito et al. [3]. They found that the activity of simvastatin is lowered in PPAR-α KO mice, in comparison to the WT controls, it indicates that the contribution of PPAR-α can balance the anti-inflammatory effects of simvastatin in SCI. As our present researches show, this study is the first one to show concern on the pitavastatin’s effect on secondary SCI. We assessed the viable application of pitavastatin in vivo, and opened a new idea of drug treatment of secondary spinal cord injury. But there is limitation in our study; we have not assessed detailed mechanism of drug as well as its safety use in vivo. The number of mice represented in the groups can be increased for the production of a more stable and precise conclusions. Additional investigations can be conducted on the results which are dependent on the precise dosage to perfect. The focus of this study is among the early changes which occurred on the first 24 h after the injury, therefore it does not provide any information that discusses its clinical results, a late assessment of the biochemical and histopathological factors may give a more accurate result of the study. Moreover, the administration of the GLPS was conducted 7 d before the trauma. Hence, the gathered study does not show the proper and realistic process of SCI. In accordance, much more research about GLPS is needed to be gathered for a solid proof of the different effects of the treatment once conducted after SCI.
References
- Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol 2014; 13: 1241-1256.
- Silva NA, Sousa N, Reis RL. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol 2014; 114: 25-57.
- Esposito E, Rinaldi B, Mazzon E. Anti-inflammatory effect of simvastatin in an experimental model of spinal cord trauma: involvement of PPAR-alpha. J Neuroinflammation 2012; 9: 81.
- Genovese T, Esposito E, Mazzon E. Effect of cyclopentanone prostaglandin 15-deoxy-delta12,14PGJ2 on early functional recovery from experimental spinal cord injury. Shock 2008; 30: 142-152.
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 1985; 14: 157-167.
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001; 5: 62-71.
- Basso DM, Fisher LC, Anderson AJ. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23: 635-659.
- Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp 2011; 71: 281-299.
- Fehlings MG, Nguyen DH. Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol 2010; 30: 109-112.
- Sullivan PG, Krishnamurthy S, Patel SP. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma 2007; 24: 991-999.
- Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 2001; 103: 203-218.
- Sohn HM, Hwang JY, Ryu JH. Simvastatin protects ischemic spinal cord injury from cell death and cytotoxicity through decreasing oxidative stress: in vitro primary cultured rat spinal cord model under oxygen and glucose deprivation-reoxygenation conditions. J Orthop Surg Res 2017; 12: 36.
- Sutherland BA, Minnerup J, Balami JS. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke 2012; 7: 407-418.
- Cimino M, Gelosa P, Gianella A. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist 2007; 13: 208-213.
- Pannu R, Barbosa E, Singh AK. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res 2005; 79: 340-350.
- Hwang J, Han JI, Han S. Effect of pre-treatment with simvastatin on spinal cord ischemia-reperfusion injury in rats. J Cardiothorac Vasc Anesth 2013; 27: 79-85.
- Zhou Q, Liao JK. Statins and cardiovascular diseases: from cholesterol lowering to pleiotropy. Curr Pharm Des 2009; 15: 467-478.
- Inoue I, Goto S, Mizotani K. Lipophilic HMG-CoA reductase inhibitor has an anti-inflammatory effect: reduction of MRNA levels for interleukin-1beta, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator-activated receptor alpha (PPARalpha) in primary endothelial cells. Life Sci 2000; 67: 863-876.
- Kakuda H, Kobayashi J, Nakato M. Short-term effect of pitavastatin treatment on glucose and lipid metabolism and oxidative stress in fasting and postprandial state using a test meal in Japanese men. Cholesterol 2013; 2013: 314170.
- Kwon BK, Tetzlaff W, Grauer JN. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004; 4: 451-464.
- Chen HC, Fong TH, Lee AW. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine 2012; 37: 470-475.
- Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest 1978; 39: 236-253.
- Borgens RB, Blight AR, Murphy DJ. Axonal regeneration in spinal cord injury: a perspective and new technique. J Comp Neurol 1986; 250: 157-167.
- Crowe MJ, Bresnahan JC, Shuman SL. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med 1997; 3: 73-76.
- Liu XZ, Xu XM, Hu R. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 1997; 17: 5395-5406.
- Sakurai M, Nagata T, Abe K. Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg 2003; 125: 370-377.
- Isaksson J, Farooque M, Holtz A. Expression of ICAM-1 and CD11b after experimental spinal cord injury in rats. J Neurotrauma 1999; 16: 165-173.
- Gao S, Ding J, Xiao HJ. Anti-inflammatory and anti-apoptotic effect of combined treatment with methylprednisolone and amniotic membrane mesenchymal stem cells after spinal cord injury in rats. Neurochem Res 2014; 39: 1544-1552.
- Harada N, Taoka Y, Okajima K. Role of prostacyclin in the development of compression trauma-induced spinal cord injury in rats. J Neurotrauma 2006; 23: 1739-1749.