Research Article - Biomedical Research (2017) Volume 28, Issue 8
Pharmacokinetics and tissue distribution of microspheres prepared by spray drying technique: targeted drug delivery
Sree Harsha1*, Bandar E. Al-dhubiab1, Anroop B. Nair1, Mahesh Attimarad1, Katharigatta N. Venugopala1,3 and Kedarnath SA21Department of Pharmaceutical Sciences, College of Clinical Pharmacy, King Faisal University, Al-Ahsa-31982, Saudi Arabia
2Nuwill Research and Innovations Pvt Ltd. Janarhana Towers, Bilekahalli, Bangalore, India
3Department of Biotechnology and Food Technology, Durban University of Technology, Durban 4001, South Africa
- *Corresponding Author:
- Sree Harsha
Department of Pharmaceutical Sciences
College of Clinical Pharmacy
King Faisal University, Saudi Arabia
E-mail: sharsha@kfu.edu.sa
Accepted date: October 31, 2016
Abstract
Conventional dosage form after administration, the anti-asthma medication travels freely and distribute equally in highly perfused tissues leading to undesirable side effects. It makes the treatment ineffective and required concentration of drug could not reach the lungs. Microspheres as targeted drug delivery system have emerged as a remedial measure and to achieve higher salbutamol concentration in capillary network of lungs. As an application, salbutamol-loaded albumin microspheres (SAM) were prepared using Buchi B-90 nano spray-drier. Central composite design (CCD) was applied to optimize the spray drying process. The optimized SAM was subjected to surface morphology and found to be shriveled particle. The mean particle size was 8.24 μm, an ideal size to deposit in the capillary bed of the lungs. The maximum drug encapsulation and percentage yield were 72 ± 0.8% and 86 ± 0.4%, respectively. The drug release showed sustained release and was best explained by Korsmeyer peppas equation and showed the highest linearity (R2-0.9915). In vivo results shows sustained release and targeting efficacy of SAM and the concentration of drug in the lung was higher (1311.1 ± 8.12 μg/g, 15 min) in comparison to conventional formulation (90.2 ± 0.76 μg/g, 10 min). The product were stable and no change was observed under (5 ± 2°C) and (25 ± 2°C). Results prove that the microspheres may be an alternative drug delivery to lungs.
Keywords
Targeted drug delivery, Microspheres, Asthma
Introduction
Asthma is considered as most chronic disease of the lungs and serious universal health problem. In asthma the lungs swells and narrow the airways and causes wheezing. It is a long term illness and cannot be cured, but proper management can control the illness and empower individuals to enjoy a quality life. Saudi Initiative for Ashtma (SINA) 2010 reported more than 2 million Saudi population has been affected. Asthma patients are increasing in number day by day in developing countries. Salbutamol is a β2 antagonist and are most prescribed drug for treating bronchial asthma [1]. It helps in widening the air passages of lungs and can make breathing easier. It has relatively shorter duration of action of 4-6 hours after administration. As a result it is administered orally 3-4 times daily at a dose of 2.5-5 mg [2,3]. Even though salbutamols are frequently indicated for the treatment of asthma, their recurrent dosing may decrease compliance. Therefore, formulating and targeting the drug to the lungs are essential. Salbutamol are generally administered in the form of inhaled therapy, however, the use of i.v. route is suggested in patients if the above treatment fails [4]. We have proposed to develop new approach using approved drugs aside preparing them into polymeric drug carriers owing to its biodegradable, nontoxic and biocompatible microspheres by controlling the particle size and targeting lungs passively after intravenous injection. Targeting a formulation to lungs may assist in specific outcomes in patients and can have a benchmark in developing a new drug delivery system. However, it helps in reducing the amount of drug distributed to highly perfused tissues [5-7].
Microspheres offer many significant advantages one of them is site-specific drug delivery. These are small spherical particles vary from micrometer range (1-1,000 microns) in size. Particles between 6 μm and 10 μm are widely distributed throughout the lung tissue after iv bolus injection [8]. Sever methods and techniques have been reported in formulation of microspheres including reduced pressure-solvent evaporation method [9], coprecipitation, coacervation, solvent dispersion, [10,11] solid-in-oil-in-water (S/O/W) emulsion, spray-freezedrying [12], water-in-oil (w/o), [13]. However, the drawback of these methods is generally in use of organic solvents. These organic solvents are highly reactive chemicals forming toxic and explosive [14]. Furthermore, these techniques have poor yield and cannot approach to scale up commercially. Among the several methods of incorporating active ingredients into microspheres, our team works on formulating microspheres by the spray-drying method. Spray-drying is widely used in the pharmaceutical industry for conversion of liquid into dry solid powder. Moreover, the process is single-step, rapid, relatively easy to scale up and may serve a valuable substitute to the multi-step, time-consuming conventional (solvent diffusion, water-in-oil, freeze drying etc.) methods in the area of formulation [15-18]. The pilot study was conducted to supplement to an already existing in one or more prevailing area of site specific drug delivery systems to recommend a complementary alternative method to conventional dosage form, particularly given to asthma patients which is most difficult to cure. The present research work aims at developing and optimizing microspheres of salbutamol using albumin as a polymer (SAM) to overcome its common side effects, including such as upset stomach, runny nose, throat irritation, and shakiness.
Materials and Methods
Salbutamol sulphate, terbutaline sulphate and dialysis tubing cellulose membrane (2,000 molecular weight cut-off) were purchased from Sigma Aldrich Corporation (St Louis, Missouri, United States). Albumin and Glutaraldehyde were purchased from Loba Chemie, Mumbai, India. All other chemicals and solvents used were of analytical grade.
Experimental
Cross-linking albumin with glutaraldehyde
BSA and glutaraldehyde, 0.5% (w/v) BSA in de-ionized (DI) water was cross-linked using glutaraldehyde at 1:15 BSA: glutaraldehyde molar ratios. Cross-linking was carried out in water bath shaker for 24 h at 20°C-25°C in a 100 ml bottle. The mixture was rinsed in DI water for 24 hours to eliminate excess glutaraldehyde [18].
Preparation of spray dried microspheres
A solution of 5% w/v albumin cross-linked with glutaraldehyde and 1% salbutamol added then were prepared in de-ionized (DI) water. The mixture was partially cloudy, so we added 2M HCl drop-wise with stirring to form clear solution. The solution was sprayed using Buchi Nano B-90 spray dryer. Three operating conditions for the experiments were varied so has to achieve desired particle size. The inlet temperature was (80°C-120°C), flow rate was kept between (20-30 mL/h) and the outlet temperature was fixed between (32°C-35°C). The samples were filtered before spraying to avoid blockage of spray nozzle containing 7 μm holes. The dried powder were than collected manually with rubber spatula and stored in amber colored vials at room temperature for further investigation [18-21].
Design of experiments (DoE)
DOE technique was used to provide better and faster means to optimize the spray-drying procedure. It helps in exploring the cause and relationship between the three-process variable (Table 1). The central composite design created constituted 20 of the trials according to the software. The particle size desired for this experiment was (between 5-15 μm). The experiments design technique were carried out using latest statistical software package Stat-Ease software (Design-Expert V.8.0.7.1) [21,22].
| Albumin Conc. % | Inlet temp °C | Feed Flow Ml | Particle size μm |
|---|---|---|---|
| 2 | 120 | 20 | 9.65 |
| 0.7 | 100 | 25 | 1.29 |
| 0.7 | 80 | 20 | 1.32 |
| 1.35 | 100 | 25 | 4.21 |
| 2 | 80 | 30 | 9.97 |
| 1.35 | 100 | 25 | 4.21 |
| 2 | 100 | 25 | 8.7 |
| 0.7 | 120 | 30 | 1.22 |
| 1.35 | 100 | 25 | 6.71 |
| 1.35 | 100 | 25 | 3.98 |
| 1.35 | 100 | 30 | 4.29 |
| 1.35 | 120 | 25 | 6.9 |
| 1.35 | 80 | 25 | 4.9 |
| 1.35 | 100 | 25 | 4.12 |
| 1.35 | 100 | 20 | 7.31 |
Table 1. Design summary for DOE runs of salbutamol microsphere design matrix and performance values.
Particle size analysis
Particle size was determined using Malvern Zetasizer Nano Series Nano-ZS, Malvern Instruments Private Ltd, MA, USA). Two milliliter of suspension containing microspheres in PBS (phosphate-buffed saline) pH 7.4 was transferred in the glass cuvette, and mean size diameter and distribution will be determined [23].
Morphological characteristics
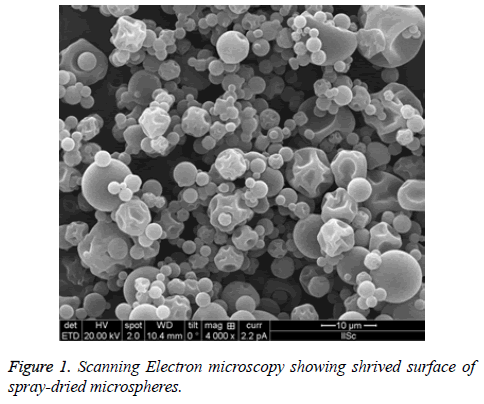
The surface patterns of the spray-dried microspheres were determined after applying ultra-thin coating of electrically conducting metal (platinum) to the microspheres using Quanta 200 scanning electron microscopy system (Philips-FEO, Eindhoven, Netherlands). This helps in determine the external surface of the microspheres for any change in structure, shape, hallow, cracks etc. All the samples will be determined in triplicate [24] (Figure 1).
In vitro Characterization of Spray-Dried Microspheres
Production yield
The spray-dried powder were collected by rubber scapula by scraping them off the collecting vessel and weighed (Figure 2). Production yields of microspheres were determined by calculating the difference between the total amounts of solids sprayed (S1) to the weight of microspheres recovered (S2) [25].
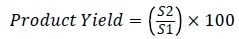
Drug content analysis
A known quantity of 10mg powder were weighted and transferred to mortar and pestle and crushing them to determine the drug content of salbutamol in microspheres. Deionized water was added to the above powder to form suspension. The suspension was transferred to dialysis membrane (molecular weight between 12,000-14,000 kd) and were placed in dissolution basket in phosphate buffered saline (PBS) solution. The basket was allowed to rotate for 2h to release the drug from the microspheres. Drug contents were measured at 276 nm using ultraviolet-visible spectrophotometer 1601, Shimadzu, Tokyo, Japan [26,27].
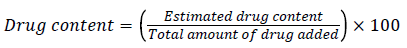
In vitro release mechanism
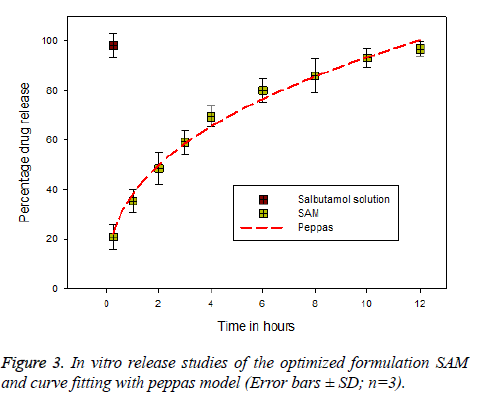
In vitro release profiles were carried out according to our recent published report with slight modification [28]. In vitro release studies were carried out by adding 100 mg of microsphere in 5 ml of PBS (7.4). The suspension was transfer to a 3 × 1 dialysis bag (MWCO-12,000-14,000 Da, Sigma, Germany) and dialyzed against 100 ml of PBS pH 7.4 and stirred at 50 rpm. Sink conditions were maintained during the studies. The temperature of the dissolution medium was maintained at 37 ± 2°C. Aliquots (2 ml) were withdrawn to know the drug content at predetermined time intervals of time. The drug content was determined at 276 nm using ultravioletvisible spectrophotometer 1601, Shimadzu, Tokyo, Japan. Similar procedure was carried out to all the samples in triplicate (mean ± SD, n=3). The data obtained was fitted to different kinetic models to know the release mechanism using Sigmplot Software version 9 (Figure 3).
Pharmacokinetics, tissue distribution and targeting efficiency in vivo
Briefly, healthy New Zealand white rabbits will be used and experiment were carried out according to the published articles [28,29]. In brief, 6 months old healthy male/ female white rabbits weighing between (2.5-3.0 kg) were selected in this experiment. The rabbits were kept under specific environment controlled room temperature (25°C), during this time rabbits had ad libitum access and stand rodent pallets. Animals were divided into groups 1-6 of six animals each to find out the drug release at predetermined time intervals, specifically at 0.5, 1, 3, 6, 10 and 12 hours. Control group-1 received an intravenous dose of 60 mg/kg salbutamol (pure drug), and group 2-6 received the formulation equivalent to 60 mg/ kg of drug [30]. The drug release from different organs (lungs, liver, spleen and blood) were tested after being extracted from the rabbits. The drug content from different organs was estimated by highperformance liquid chromatography [31]. The know quality of salbutamol containing plasma were withdrawn into microtubes and internal standard (terbutaline sulphate) will be added. Then centrifuged and supernatant were separated and injected into C18 coloumn (50 μm, 70 A).
To know the targeting delivery mechanism of clinical application of salbutamol in formulation, in this paper, three targeting parameters (re, te and Te) were used to investigate the influence of salbutamol on the in vivo distribution on rabbits and there pharmacokinetic parameters are calculated according to the following formula (Figure 4) [32,33].
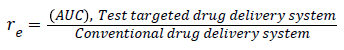
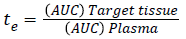
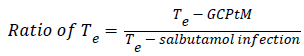
Stability studies
Stability testing was carried out to prove the formulation is stable under different environmental factors such as humidity and temperature. The drug loaded albumin microspheres (SAM) was filled and packed tightly in amber color vials and exposed to stability testing according to International Conference on Harmonization (ICH) guidelines. The packed vials were kept at three different conditions such as (5 ± 2°C), (25 ± 2°C) and (40 ± 2°C/75 ± 5% RH) for one year the formulation were analyzed for physical appearance, drug content and particle size.
Results and Discussion
Formulation of SAM intended for lung targeting
Statistical optimization is an important mathematical tool to solve complex problem and to reduce the time, physical efforts and cost of experiment. Design of experiment (DOE) is one of the mathematical tools used to solve this above problem. Numerical optimization technique was employed to optimize the process parameter such as Inlet temperature, concentration of polymer and feed flow rate. The model containing five center points, four factorial points and six axial points with total 15 experiments. In CCD, the variables was set as (0) intermediate, (+1) highest and (-1) lowest. Moreover, this design offers an improvement that by reducing the error in optimal manner; the responses can be modeled in a quadratic manner and with the selected variable the expected response do not diverge in a linear manner. The experimental designs with responses are shown in Table 2. The particle size from the experimental data point was in the range of 1.22 μm to 9.97 μm. All our experiments were carried out in triplicate (n=3) and in random order. All other process parameters such as spray nozzle (7 μm), gas flow 90-95 L/min, Pressure 68 nbar, Spray 100%, outlet temperature 20°C kept constant throughout the experiment. The polynomial equation obtained to evaluate response variable as in Equation 1.
| Number | Albumin Conc. | Inlet temperature | Feed flow | Particle Size |
|---|---|---|---|---|
| 1 | 1.08 | 116.98 | 21.1 | 7.4 |
| 2 | 1.46 | 114.23 | 24.94 | 6.4 |
| 3 | 1.6 | 80.26 | 28.96 | 6.4 |
| 4 | 2 | 84 | 25 | 8.2 |
| 5 | 1.57 | 82.58 | 20.18 | 7.3 |
| 6 | 1.53 | 94.81 | 21.82 | 6.7 |
| 7 | 1.42 | 91.93 | 20.06 | 6.7 |
| 8 | 1.82 | 97 | 24.13 | 7.6 |
| 9 | 1.64 | 100.25 | 26.93 | 6.2 |
| 10 | 1.25 | 100.69 | 20.98 | 6.1 |
Table 2. List of numerical optimization solution.
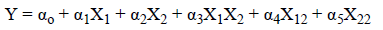
Where, αo-α5 represents effects or regression coefficients, X1- X2 the studied variables and the resultant equation for particle size response is shown in Equation 2.
| Particle Size= | |
|---|---|
| 4.94 | - |
| 3.71 | *A |
| 1 | *B |
| -1.51 | *C |
| -1.57 | *A *B |
| 1.11 | *A *C |
| -0.57 | *B *C |
| -0.31 | *A^2 |
| 0.6 | *B^2 |
| 0.5 | *C^2 |
Equation 2. The resultant equation in terms of coded factors for particle size response.
Effect of albumin concentration on particle size
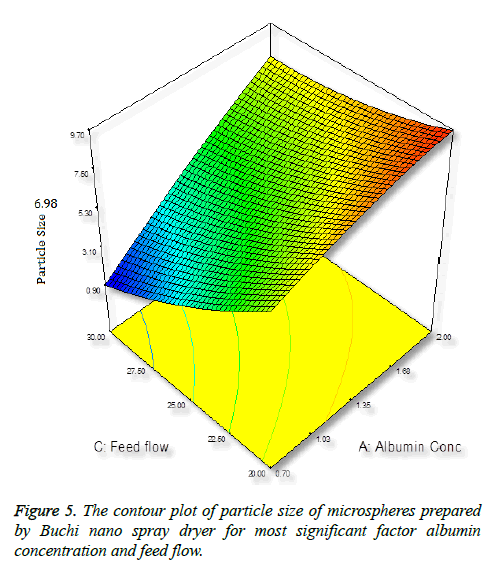
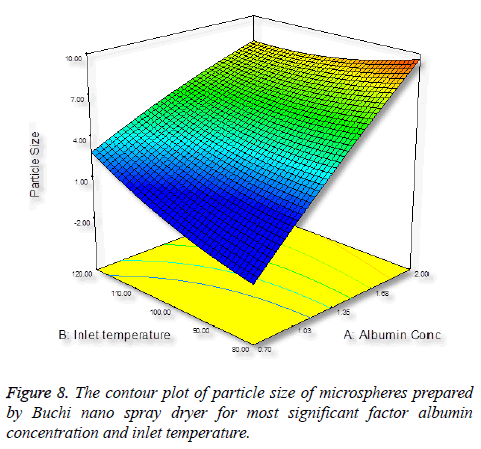
Particle size is important characteristics of targeted drug delivery system. Many studies demonstrated that particles size play important in biodistribution. In this case, we require the size of the particles between 5-15 μm. It was observed that increasing the concentration of albumin results in increasing in size of the particles (Figure 5), this is due to more solid in a droplet may increase the size and also the droplet contains lesser amount of liquid to vaporize [34].
Effect of inlet temperature on particle size
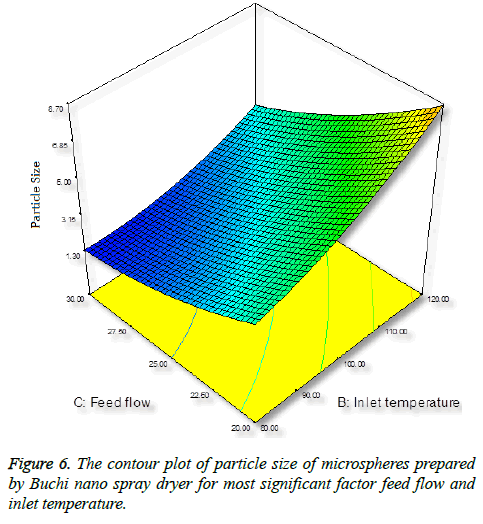
Inlet temperature plays an important role on particle size; the temperature setting in spray-drying preparation should not cause any thermal degradation of the polymeric material. Increase in inlet temperature shows increase in particle size (Figure 6), this is because the particle did not shrink at the early stage during drying, thereby increase in bigger particles [35].
Effect of feed flow on particle size
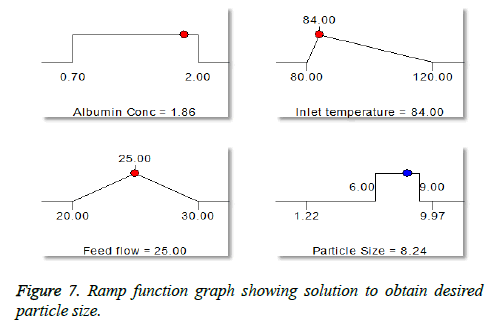
Surprisingly, we found opposite effect with increase in flow rate, decrease in particle size (Figure 7). An increase in feed flow led to a decrease of the transit time of droplet of the particle through the spray-drier and the solvent evaporates instantly and reduce the particle size [36].
Numerical optimization
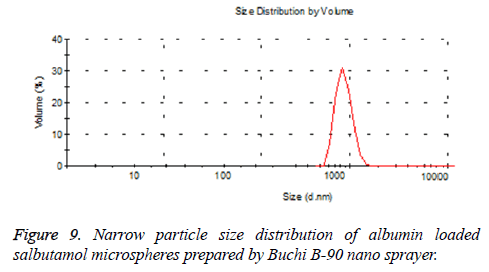
The numerical optimization tool employed to study the low and high level constraints and initial design space of the experimental model. Ten numerical solution obtained by Deign-Expert software in quantitative terms out of this three experiments were carried by optimum conditions. The obtained particle size (8.24 μm) (Figure 5) and predicted size were similar by the equation confirms statistically significant. The analysis of variance (ANOVA) for response surface quadratic model shows the overall model for particle size production is statistically significant for lung targeting. The R2 coefficient of determination is a statistical measure and the obtained value was more than >70%, all the three variables (inlet temp, albumin conc. and flow rate) represents functional relationships. The mean particle size obtained was suitable for lung targeting. Several other studies have published the similar particle size for albumin microspheres. Following intravenous administration, 5-15 μm are entrapped in the capillary network of lungs [37,38]. The obtained formulation further used for characterization.
Determination of encapsulation efficiency and percentage yield
The encapsulation efficiency and percentage yield were found to be 72 ± 0.8% and 86 ± 0.4%, respectively. The low encapsulation and yield was due to millions of uniform droplets, which are formed after atomization comes in contact with hot air and separates the powder from the liquid. These powder stick to the nebulization chamber as shown in the figure. However, the loss can be minimized by increasing the production [39].
Particle size analysis
In our study, particle size is one of the important parameter because targeted drug delivery of microspheres solely depends upon the particle size. Microspheres in size range between 5-15 μm would affect the lung deposition. The particle size obtained after optimization procedure found to be 8.24 μm, which helps to entrap the microspheres after intravenous administration [40,41]. Additionally the obtained particles were narrow in size, this is mainly due to the spray nozzle ejects millions of uniform size droplets and these droplets are dried at constant temperature producing very narrow size particles. So that the drug release from the formulation will not fluctuate largely in vivo, intern maintains the therapeutic level. The spray-drying method found to be superior compared to conventional method of microspheres preparation. The fact is Buchi nano patented spray-drier is young and flourishing drying method works by removal of moisture by applying heat to the spray solution while simultaneously controlling the humidity resulting in free flowing powder. All the process takes place in one single step; there by production cost, reduced [42].
Surface morphology
Morphology is one of the important quality control tests of spray-dried powder, which affects flowability, moisture content, particle size, bulk density. The powder obtained were found to be shriveled particle, which are similar to shrinking core this is due to when the droplet dried at high temperature. However, it can overcome by optimizing the solid feed content. As we could not increase, the concentration of the solid feed which in turn would increase the particle size which was not our objective [43-45].
In vitro and release kinetics
It was found that pure drug (salbutamol) released 95.4% with in first half an hour; however 28.23% of salbutamol from SAM was released in first half hour and remaining drug 99.1% released completely within 12 h. The drug release pattern showed biphasic module, which is important the first phase is burst release or initial release; this is due to the drug loosely embedded in the surface of the particles [46]. However, the initial burst can be decreased by increasing the polymer concentration. In case of acute asthma, it is practiced the loading dose of 4-6 μg kg-1 of salbutamol given intravenously over 10 min period [47]. Clinically initial burst release is important as it acts as a loading dose, which helps in reaching the minimum therapeutic concentration in the blood to show the drug action [48]. The release mechanism was studied by fitting data to various kinetic models to know the release mechanism, (Hixon and Crowell, first-order, Higuchi, Baker and Lonsdale, Korsmeyer’s Peppas). The release mechanism involved all the three process such as swelling, polymer erosion and dissolution and can conclude non-fickian drug release peppas (Figure 8) pattern based on the diffusional exponent n=0.987 [49].
The pharmacokinetic (PK) studies for SAM were analyzed using WinNonline ® 1.5 software (Pharsight Corporation, St Louis, MO, USA). One, two and three PK models for SAM formulation were applied to describe the fate of a drug distributed after 120 μg/kg intravenous administration to the rabbits [50]. Plasma concentration from each rabbit measured at predetermined time intervals and two-compartment model provides the best fit (Figure 9). The in vivo pharmacokinetics parameters illustrated in Table 3. Pharmacokinetics of SAM in comparison with conventional formulation, the SAM formulation altered the circulation pattern of salbutamol in an animal model in vivo. The half-life after iv bolus of SAM (t1/2 (α)=2.12 ± 0.41 h, t1/2 (β)=22.22 ± 3.32) were prominently greater than conventional formulation (t1/2 (α)=0.39 ± 0.19 h, t1/2(β)=9.8 ± 1.11). The data shows sustained release and targeting efficacy of SAM and the concentration of drug in the lung was higher (1311.1 ± 8.12 μg/g, 15 min) in comparison to conventional formulation (90.2 ± 0.76 μg/g, 10 min) (Figure 10). A clinical study shows the initial high salbutamol concentration in the lungs helps to widen the airways and relieves symptoms of asthma [51].
| Parameters | SAM | Salbutamol bolus |
|---|---|---|
| AUC (μg h/mL) | 39.1 ± 2.02 | 132.7 ± 4.21 |
| t1/2 (α) (h) | 2.12 ± 0.41 | 0.39 ± 0.19 |
| t1/2 (β) (h) | 22.22 ± 3.32 | 9.8 ± 1.11 |
| CL (h-1) | 0.18 ± 0.02 | 0.042 ± 0.08 |
| K12 (h-1) | 0.045 ± 0.04 | 0.75 ± 0.14 |
| K10 (h-1) | 1.85 ± 1.05 | 9.01 ± 0.42 |
| K21 (h-1) | 0.40 ± 0.05 | 1.09 ± 0.35 |
Table 3. Pharmacokinetic parameter of SAM and salbutamol injection after intravenous administration.
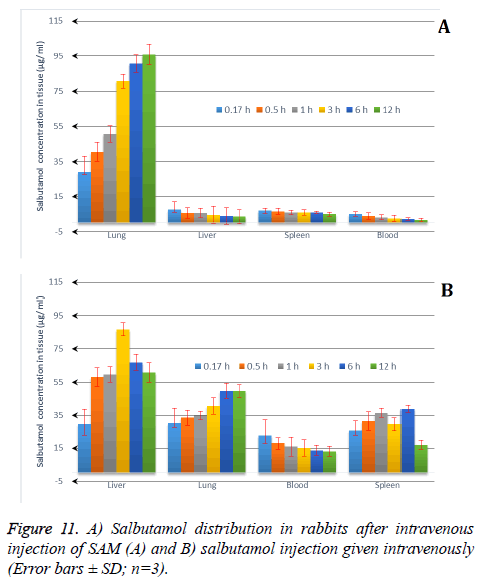
The particle size plays important role in drug distribution. The results proved that the SAM formulation was higher in the lungs in comparison to conventional formulation. This is due to the narrow particle size of microspheres, which was between 5-15 μm obtained from spray drying technique and the study also supported by other researchers [52,53]. The drug distribution from both SAM and conventional formulation, the distribution of salbutamol in blood, spleen, liver and lung the Re (drug exposure) value is >1, indicates that the organ/ tissue was exposed to the drug to a greater extent. The lung-targeting efficacy (Te) of SAM and conventional formulation are shown in Table 4. SAM results in this study showed superior value of Re (10.2) for lungs, 0.43 for liver and 2.0 for spleen, pointing out the SAM formulation exposure to the lung was drastically higher in comparison to liver and spleen. The results showed that the microspheres are more concentrated at the target site, lungs (Figure 11).
| Sample | AUC (µg.min /mL or g) | Te | ||||
|---|---|---|---|---|---|---|
| SAM | Control | Re | SAM | Control | Te-ratio | |
| Plasma | 39.1 ± 2.02 | 132.7 ± 4.21 | 0.14 | 0.03 | 1.75 | 0.021 |
| Spleen | 19.22 ± 3.18 | 8.42 ± 1.1 | 2 | 0.029 | 0.135 | 0.21 |
| Liver | 69.05 ± 3.7 | 152.87 ± 46 | 0.43 | 0.069 | 1.826 | 0.042 |
| Lung | 1311.1 ± 8.12 | 90.2 ± 0.76 | 10.2 | - | - | - |
Table 4. Lung targeting parameters of SAM and salbutamol bolus (control).
Stability testing
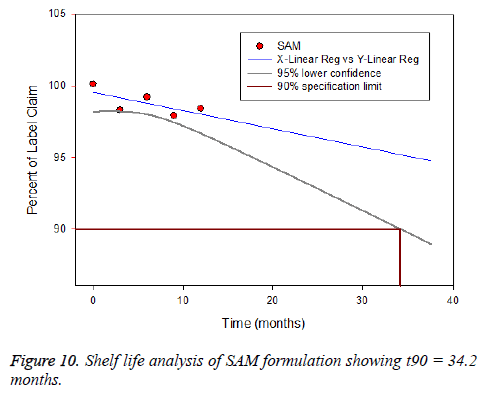
Stability testing was carried out to prove the formulation is stable under different environmental factors such as humidity and temperature. The drug loaded albumin microspheres (SAM) was filled and packed tightly in amber color vials and exposed to stability testing according to International Conference on Harmonization (ICH) guidelines [54]. The packed vials were kept at three different conditions such as (5 ± 2°C), (25 ± 2°C) and (40 ± 2°C/ 75 ± 5% RH) for one year. The formulation was analysed for physical appearance, drug content and particle size. The drug content varied between 3 to 5%, however, it was within a range. The product were stable and no change was observed under (5 ± 2°C) and (25 ± 2°C). However, at (40 ± 2°C/ 75 ± 5% RH) the formulation witnessed liquefaction (Figure 6). There was no variation in the particle size. Based on the report generated from Sigma plot software salbutamol lost its 10% activity and retains the 90% (t90 = 34.2 months).
Conclusion
Salbutamol-loaded albumin optimized microspheres were prepared using patented novel technology Buchi Nano Spray Dryer B-90 that enabled narrow and shriveled particles with small sample volume, higher yield and good drug content. Microspheres found to release drug to a maximum extent in vitro up to 12 hours and were further verified to release the drug in animal model preferable to target lungs than other liver, spleen and blood. Microspheres have a great potential to revolutionize delivery of active compound. This study adds to the previously significant area of site-specific drug delivery systems by slightly modifying the process parameter procedure in preparation of microspheres.
Acknowledgement
This research work was supported by Deanship of Scientific Research (DSR), King Faisal University, Al-Ahsa-31982, Saudi Arabia. Grant number 160005.
References
- Tripathi KD. Essentials of medical pharmacology. Jaypee Bros, India 2006.
- Nair S, Thomas E, Pearson SB, Henry MT. A randomized controlled trial to assess the optimal dose and effect of nebulized albuterol in acute exacerbations of COPD. Chest 2005; 128: 48-54.
- Yazan Y, Demirel M, Güler E. Preparation and in vitro dissolution of salbutamol sulphate microcapsules and tabletted microcapsules. J Microencapsul 1995; 12: 601-607.
- Sellers WF. Inhaled and intravenous treatment in acute severe and life-threatening asthma. Br J Anaesth 2013; 110: 183-190.
- Harsha NS, Rani RH. Drug targeting to lungs by way of microspheres. Arch Pharm Res 2006; 29: 598-604.
- Kumar A, Zhang X, Liang XJ. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol Adv 2013; 31: 593-606.
- Radha GV, Rani TS, Sarvani B. A review on proniosomal drug delivery system for targeted drug action. J Basic Clin Pharm 2013; 4: 42-48.
- Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release 2010; 143: 31-37.
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB. Diabetes in older adults: a consensus report. J Am Geriatr Soc 2012; 60: 2342-2356.
- Asada M, Takahashi H, Okamoto H, Tanino H, Danjo K. Theophylline particle design using chitosan by the spray drying. Int J Pharm 2004; 270: 167-174.
- Su YL, Fu ZY, Zhang JY, Wang WM, Wang H, Wang YC. Microencapsulation of radix salvia miltiorrhiza nanoparticles by spray-drying. Powder Technol 2008; 184: 114-121.
- Morita T, Sakamura Y, Horikiri Y, Suzuki T, Yoshino H. Protein encapsulation into biodegradable microspheres by a novel s/o/w emulsion method using poly(ethylene glycol) as a protein micronization adjuvant. J Control Release 2000; 69: 435-444.
- Hong X, Wei L, Ma L, Chen Y, Liu Z. Novel preparation method for sustained-release PLGA microspheres using water-in-oil-in-hydrophilic-oil-in-water emulsion. Int J Nanomedicine 2013; 8: 2433-2441.
- National Research Council Committee on Prudent Practices for Handling SDCL. Prudent practices in the laboratory: Handling and disposal of chemicals. National Academy Press, 1995.
- Arpagaus C. A novel laboratory-scale spray dryer to produce nanoparticles. Drying Technol 2012; 30: 1113-1121.
- Branchu S, Forbes RT, York P, Petrén S, Nyqvist H. Hydroxypropyl-beta-cyclodextrin inhibits spray-drying-induced inactivation of beta-galactosidase. J Pharm Sci 1999; 88: 905-911.
- Freitas S, Merkle HP, Gander B. Ultrasonic atomisation into reduced pressure atmosphere-envisaging aseptic spray-drying for microencapsulation. J Control Release 2004; 95: 185-195.
- Littringer EM, Zellnitz S, Hammernik K, Adamer V, Friedl H, Urbanetz NA. Spray drying of aqueous salbutamol sulfate solutions using the nano spray dryer b-90-the impact of process parameters on particle size. Drying Technol 2013; 31: 1346-1353.
- Jain I. Crosslinking albumin for drug release from spray dried particles. University of Louisville, 2014.
- Harsha S. Dual drug delivery system for targeting H. pylori in the stomach: preparation and in vitro characterization of amoxicillin-loaded Carbopol® nanospheres. Int J Nanomedicine 2012; 7: 4787-4796.
- Harsha S, Al-Khars M, Al-Hassan M, Kumar NP, Nair A, Attimarad M. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: Lung targeting via intravenous route. Arch Pharm Res 2013.
- Hooda A, Nanda A, Jain M, Kumar V, Rathee P. Optimization and evaluation of gastroretentive ranitidine HCl microspheres by using design expert software. Int J Biol Macromol 2012; 51: 691-700.
- Khare P, Jain S. Influence of rheology of dispersion media in the preparation of polymeric microspheres through emulsification method. AAPS PharmSciTech 2009; 10: 1295-1300.
- Wu G, Chen L, Li H, Deng CL, Chen XF. Comparing microspheres with different internal phase of polyelectrolyte as local drug delivery system for bone tuberculosis therapy. Biomed Res Int 2014; 2014: 297-808.
- Jones AK, Bejugam NK, Nettey H, Addo R, D'Souza MJ. Spray-dried doxorubicin-albumin microparticulate systems for treatment of multidrug resistant melanomas. J Drug Target 2011; 19: 427-433.
- Venkatesan P, Manavalan R, Valliappan K. Preparation and evaluation of sustained release loxoprofen loaded microspheres. J Basic Clin Pharm 2011; 2: 159-162.
- Zhang X, Chen F, Ni J. A novel method to prepare magnetite chitosan microspheres conjugated with methotrexate (MTX) for the controlled release of MTX as a magnetic targeting drug delivery system. Drug Deliv 2009; 16: 280-288.
- Harsha S, Al-Khars M, Al-Hassan M, Kumar NP, Nair AB. Pharmacokinetics and tissue distribution of spray-dried carboplatin microspheres: lung targeting via intravenous route. Arch Pharm Res 2014; 37: 352-360.
- Fan Y, Shan-Guang W, Yu-Fang P, Feng-Lan S, Tao L. Preparation and characteristics of erythromycin microspheres for lung targeting. Drug Dev Ind Pharm 2009; 35: 639-645.
- Perreault S, Ong H, du Souich P. Salbutamol disposition and dynamics in conscious rabbits: Influence of the route of administration and of the dose. J Pharmacokinet Biopharm 1992; 20: 461-476.
- Soriano-Ursúa MA, Correa-Basurto J, Romero-Huerta J, Elizalde-Solis O, Galicia-Luna LA, Trujillo-Ferrara JG. Pharmacokinetic parameters and a theoretical study about metabolism of br-aea (a salbutamol derivative) in rabbit. J Enzyme Inhibit Med Chem 2010; 25: 340-346.
- Gupta PK, Hung CT. Quantitative evaluation of targeted drug delivery systems. Int J Pharm 1989; 56: 217-226.
- Zhao RZ, Yuan D, Liu SJ, Chen YJ, Liu LJ. Liver targeting effect of vinegar-baked Radix Bupleuri on rhein in rats. J Ethnopharmacol 2010; 132: 421-428.
- Patel BB, Patel JK, Chakraborty S, Shukla D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharmaceut J 2015; 23: 352-365.
- Nijdam JJ, Langrish TAG. The effect of surface composition on the functional properties of milk powders. J Food Eng 2006; 77: 919-925.
- Bilancetti L, Poncelet D, Loisel C, Mazzitelli S, Nastruzzi C. A statistical approach to optimize the spray drying of starch particles: application to dry powder coating. AAPS PharmSciTech 2010; 11: 1257-1267.
- BozdaÄŸ S, Calis S, Kas HS, Ercan MT, Peksoy I. In vitro evaluation and intra-articular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul 2001; 18: 443-456.
- Ramaiah B, Nagaraja SH, Kapanigowda UG, Boggarapu PR, Subramanian R. High azithromycin concentration in lungs by way of bovine serum albumin microspheres as targeted drug delivery: lung targeting efficiency in albino mice. Daru 2016; 24: 14.
- Li X, Anton N, Arpagaus C, Belleteix F, Vandamme TF. Nanoparticles by spray drying using innovative new technology: the Büchi nano spray dryer B-90. J Control Release 2010; 147: 304-310.
- Harsha S. Dual drug delivery system for targeting h. Pylori in the stomach: Preparation and in vitro characterization of amoxicillin-loaded carbopol® nanospheres. Int J Nanomedicine 2012; 7 :4787-4796.
- Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci 2011; 73: 355-366.
- Arpagaus C. Nano spray dryer b-90: Literature review and applications. Büchi Inf Bull 2011; 63: 8.
- Anandharamakrishnan C, Ishwarya PS. Spray drying techniques for food ingredient encapsulation. John Wiley & Sons, New Jersy, USA, 2015.
- De Jaeghere F, Allémann E, Doelker E, Gurny R, Cerny R, Galli B. Ph-dependent dissolving nano- and microparticles for improved peroral delivery of a highly lipophilic compound in dogs. AAPS J 2001; 3: 92-99.
- Miller DA, Gil M, Williams Iii ROO, Watts ABB, Miller DAA. Spray-drying technology formulating poorly water soluble drugs. Springer, New York; 2012.
- Yeo Y, Park K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res 2004; 27: 1-12.
- Skinner DV. Cambridge textbook of accident and emergency medicine. Cambridge University Press, 1997.
- Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release 2001; 73: 121-136.
- Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opinion Drug Deliv 2010; 7: 429-444.
- Perreault S, Ong H, du Souich P. Salbutamol disposition and dynamics in conscious rabbits: influence of the route of administration and of the dose. J Pharmacokinet Biopharm 1992; 20: 461-476.
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part ii: The role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 2003; 56: 600-612.
- Wang H, Xu Y, Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: In vitro and in vivo evaluation. Int J Mol Sci 2014; 15: 3519-3532.
- Yang F, Kang J, Yang F, Zhao Z, Kong T. Preparation and evaluation of enrofloxacin microspheres and tissue distribution in rats. J Vet Sci 2015; 16: 157-164.
- Tyagi LK, Kori ML. Stability study and in-vivo evaluation of lornoxicam loaded ethyl cellulose microspheres. Int J Pharmaceut Sci Drug Res 2014; 6: 26-30.