Research Article - Biomedical Research (2018) Volume 29, Issue 6
Pain scales enhance diagnostic accuracy of coronary artery disease-an observational study
Kai-Chun Cheng1,2,3#, Kai-Yuan Cheng4#, Mei-Chu Lai5, Tsung-Hsien Lin6,7, Ho-Ming Su5,6,7, Wen-Ter Lai8, Kai-Hung Cheng6,7,9*1Department of Ophthalmology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
2Department of Ophthalmology, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung, Taiwan
3Department of Optometry, Shu-Zen Junior College of Medicine and Management, Kaohsiung, Taiwan
4Department of Otolaryngology-Head and Neck Surgery, Ministry of Health and Welfare Pingtung Hospital, Pingtung, Taiwan
5Department of Cardiology, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung Medical University, Taiwan
6Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
7Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
8Department of Internal Medicine, Kaohsiung Municipal United Hospital, Kaohsiung, Taiwan
9Cardiology Division, Massachusetts General Hospital, Harvard Medical School, Boston, MA
#These authors contributed equally to this work
- *Corresponding Author:
- Kai-Hung Cheng
Division of Cardiology
Department of Internal Medicine
Kaohsiung Medical University Hospital, Taiwan
Accepted date: December 19, 2017
DOI: 10.4066/biomedicalresearch.29-17-750
Visit for more related articles at Biomedical ResearchAbstract
Background: Hypoalgesia has been identified in patients with DM and hypertension. In addition, endogenous opioid system has been proven to be activated during myoischemia. The aim of the study was to test if pain scales could enhance the diagnostic accuracy of CAD.
Methods: Patients (n=249) with symptomatic chest pain with Myocardial Infarction (MI) suspicion, or with positive stress test were prospectively enrolled for diagnostic coronary angiography by the left trans-radial approach. The pain elicited by arterial puncture was assessed using 3 different pain-scale questionnaires, namely the Numerical Rating Scale (NRS), Verbal Rating Scale (VRS), and Visual Analogue Scale (VAS), immediately after the procedure. The pain scales were compared between patients with CAD and non-CAD to find the associations.
Results: All the values of pain scales, including NRS, VRS, and VAS were significantly lower in patients with CAD (n=138) compared to those without CAD (n=111). The optimal cut-off points (sensitivity/ specificity) of pain scales were 3.25 (0.74/0.75) in NSR, 1.5 (0.69/0.79) in VRS, and 4.25 (0.68/0.78) in VAS. In addition, these three pain scales improved c-statistics for CAD prediction from 0.50 to 0.73~0.75. Patients with low pain scales of NSR 3.25 (or 3) had >8-fold higher risk of CAD than those with NSR>3.25 (or 3).
Conclusions: These findings suggest that low pain scales can enhance diagnostic accuracy in patients with symptomatic chest pain suspicious of MI or with positive stress tests. NSR was the best pain scale among these three for enhancing diagnosis of CAD.
Keywords
Hypoalgesia, Coronary artery disease, Transradial access, Angina pectoris, Pain scales.
Introduction
Hypoalgesia, a decreased sensitivity to painful stimuli, occurs when nociceptive (painful) stimuli are interrupted or decreased. It is noted in patients with DM (as a Coronary Artery Disease (CAD) equivalent) [1-4] and hypertension (one of major risk factor for CAD) [5], and it can be mediated by some chemicals such as opioids. The endogenous opioid system consists of 3 three opioids, beta-endorphin, enkephalins, and dynorphins, and 3 families of receptors, μ (MOR), δ (λ, DOR), and κ (KOR). The endogenous opioid system including beta-endorphin and opioid receptors is being activated and increased during myoischemia [6-11]. The CAD is reported to be the most common type of heart disease, which is the leading cause of death for both men and women. (CDC, NCHS. Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015.). Chest pain is a common complaint in patients, with one of the relevant causes being coronary artery disease. While many studies were focused on how to differentiate what patterns of chest pain responsible for CAD relevant chest pain in the past [12-14], it is interesting and is a reverse thinking to find out if the sensitivity to painful stimuli is helpful to improve the diagnostic accuracy of CAD. Since little is known about the influence of pain assessment for CAD prediction, the aim of the study is to test the pain scales on the diagnostic accuracy of CAD in patients with symptomatic chest pain.
Methods
Study populations and the protocol
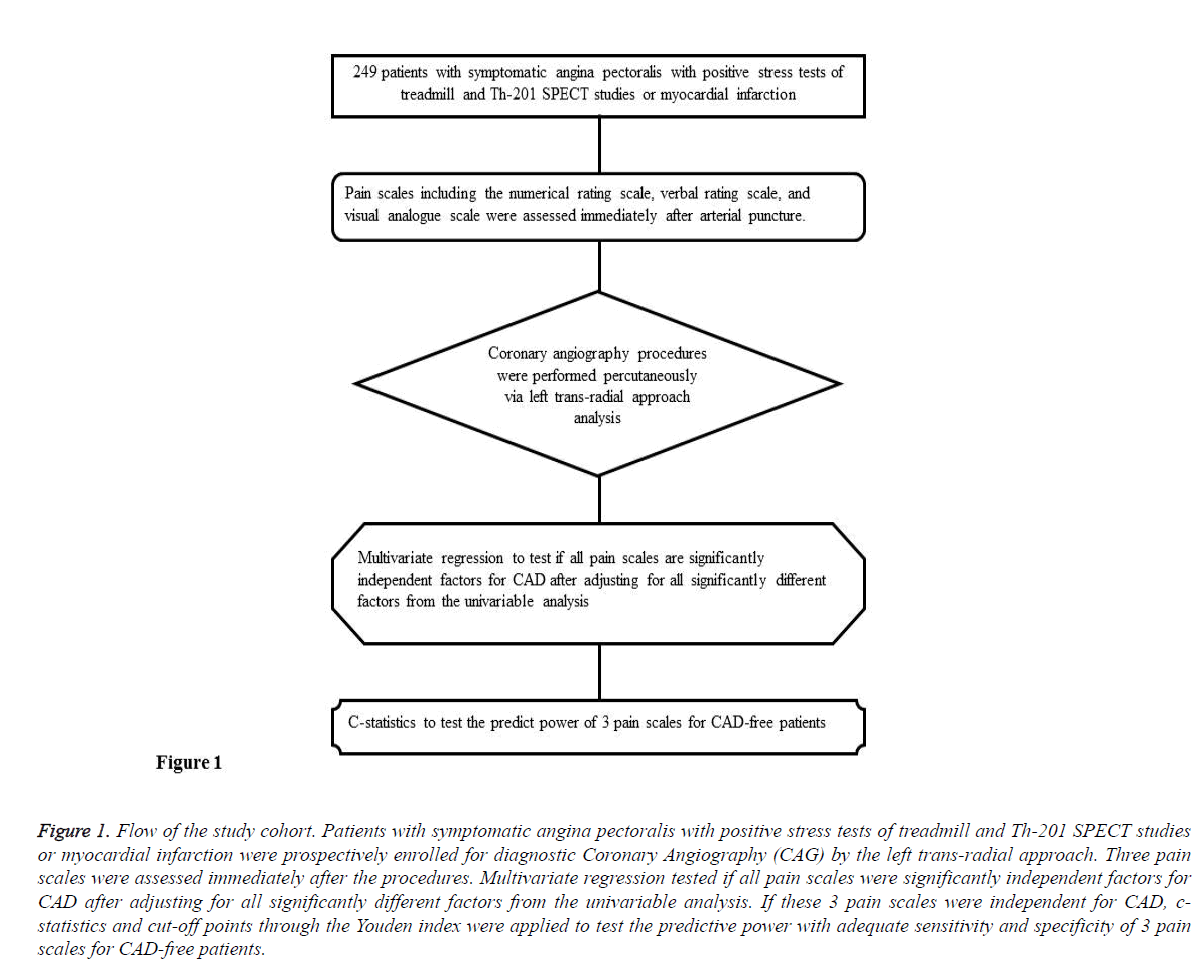
In total, 249 patients with symptomatic angina pectoralis with positive findings in stress tests, treadmill and Th-201 SPECT studies, or with suspicion of myocardial infarction, were prospectively enrolled for coronary angiography. All diagnostic coronary angiography procedures were performed percutaneously via the left trans-radial approach, and pain scales including the numerical rating, verbal rating, and visual analogue scales were assessed immediately following the procedures. After being verified by angiography, patients were allocated into CAD and Non-CAD groups respectively. This study was approved by the Institutional Review Board at Kaohsiung Medical University Hospital (Approval No. KMUHIRB-E(I)-20150178). The flow of the cohort is outlined in Figure 1.
Figure 1: Flow of the study cohort. Patients with symptomatic angina pectoralis with positive stress tests of treadmill and Th-201 SPECT studies or myocardial infarction were prospectively enrolled for diagnostic Coronary Angiography (CAG) by the left trans-radial approach. Three pain scales were assessed immediately after the procedures. Multivariate regression tested if all pain scales were significantly independent factors for CAD after adjusting for all significantly different factors from the univariable analysis. If these 3 pain scales were independent for CAD, cstatistics and cut-off points through the Youden index were applied to test the predictive power with adequate sensitivity and specificity of 3 pain scales for CAD-free patients.
Pain scales
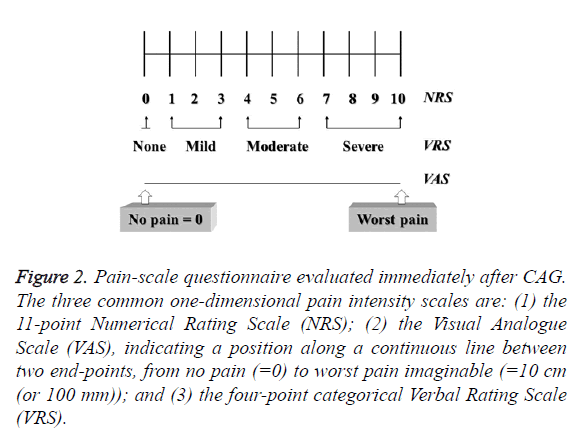
The Numerical Rating Scale (NRS) used a scale of 1-10 to assess the degree of pain; the Verbal Rating Scale (VRS) of 0-3 was determined by patients’ subjective responses in the words “no”, “mild”, “moderate”, and “severe” in referring to pain intensity; and the Visual Analogue Scale (VAS) was based on patients’ marks on a horizontal 10 cm line indicating their pain intensity, in which 0 indicated “no pain” and 10 indicated “worst pain” [15] (Figure 2).
Figure 2: Pain-scale questionnaire evaluated immediately after CAG. The three common one-dimensional pain intensity scales are: (1) the 11-point Numerical Rating Scale (NRS); (2) the Visual Analogue Scale (VAS), indicating a position along a continuous line between two end-points, from no pain (=0) to worst pain imaginable (=10 cm (or 100 mm)); and (3) the four-point categorical Verbal Rating Scale (VRS).
Statistical analysis
Demographic and clinical data were presented as mean ± SD. Categorical variables were compared using contingency tables (Pearson chi-square test) and continuous variables using student t-test. Spearman’s correlation coefficients were calculated. Multiple logistic regression analysis with Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were calculated to evaluate the independence property of the association between CAD and pain scales. The discriminatory capacity of the risk scores were assessed by using AUC (cstatistic) as an index of model performance [16] and optimal cut-off points by maximal values of Youden index (Youden index=sensitivity+specificity-1) between pain scales and CAD. The c-statistic reflected the concordance of predictions with actual outcomes in rank order, with a c-statistic of 1.0 indicating perfect discrimination. The P value<0.05 was considered statistically significant. Statistical analysis was performed by SPSS 12.0 software (Chicago, IL, USA).
Results
Patient characteristics
The demographic and clinical characteristics at baseline of the study patients are summarized in Table 1. The traditional atherosclerotic risks including male gender, concomitant diabetes, End-Stage Renal Disease (ESRD), dyslipidemia, and habit of cigarette smoking were significantly higher in patients with CAD.
| CAD (n= 138) | Non-CAD (n=111 ) | P value | |
|---|---|---|---|
| Male gender, n (%) | 106 (76.8) | 55 (49.5) | <0.001# |
| Age (y) | 61.0 ± 11.1 | 58.8 ± 11.9 | 0.14 |
| DM, n (%) | 63 (45.7) | 22 (19.8) | <0.001# |
| Hypertension, n (%) | 97 (70.3) | 70 (63.1) | 0.28 |
| Current smoking, n (%) | 86 (62.3) | 44 (39.6) | <0.01* |
| Dyslipidemia, n (%) | 73 (52.9) | 42 (37.8) | 0.02* |
| ESRD history, n (%) | 11 (8) | 2 (2) | 0.04* |
Table 1: The baseline characteristics of patients with CAD and Non-CAD.
Correlation analysis between pain scales and number of stenotic coronary arteries
All study parameters of the pain scales, including numerical rating, verbal rating and visual analogue scales were significantly lower in patients with CAD (Table 2). In addition, significantly negative correlations among numerical rating, verbal rating, and visual analogue scales with number of affected coronary vessels in CAD group were found (r=-0.308, -0.338 and -0.342 respectively, all p<0.001).
| Independent Student t-test | |||
|---|---|---|---|
| Variables | CAD (n=138) | Non-CAD (n=111) | P value |
| Numerical rating scale | 3.4 ± 2.0 | 4.8 ± 1.8 | <0.001# |
| Verbal rating scale | 1.2 ± 0.6 | 1.8 ± 0.6 | <0.001# |
| Visual analogue scale | 3.7 ± 2.0 | 5.4 ± 2.0 | <0.001# |
| Data are displayed as mean ± SD or n (%). *p<0.05, #p<0.001 | |||
| Correlation analysis between pain scales and involved CAD number | |||
| Variables | correlations coefficient | P value | |
| Numerical rating scale | -0.308 | <0.001# | |
| Verbal rating scale | -0.338 | <0.001# | |
| Visual analogue scale | -0.342 | <0.001# | |
| *p<0.05, #p<0.001 | |||
Table 2: The association of pain scales with Coronary Artery Disease (CAD).
Performance of pain-scale models prediction in CADfree population using c-statistics and cut-off points through Youden index
C-statistics of pain-scales for predicting CAD-free group and optimal cut-off points of pain-scales are presented in Table 3. The c-statistics (ROC index) (and its 95% Confidence Interval (CI)) were 0.5 (0.43-0.57) for stress test alone (data not shown), 0.73 (0.67-0.80) when incorporating NRS, 0.73 (0.66-0.79) when incorporating VRS, and 0.75 (0.69-0.82) when incorporating VAS respectively. The optimal cut-off point (sensitivity/specificity) of pain scales were 3.25 (0.74/0.75) in NSR, 1.5 (0.69/0.79) in VRS and 4.25 (0.68/0.78) in VAS. These pain scale results appeared able to predict CAD-free status with good sensitivity and specificity. Taking gender into consideration, the sensitivity and the specificity were relatively lower in women’s treadmill testing [17], and our results compensated for this discrepancy with better sensitivity and specificity in the women’s group. While the optimal cut-off points (sensitivity/specificity) of pain scales were 3.5 (0.62/0.76) in NSR, 1.5 (0.56/0.79) in VRS, and 4.15 (0.60/0.75) in VAS for the men’s group, the cut-off points (sensitivity/specificity) of pain scales were 3.25 (0.74/0.73) in NSR, 1.5 (0.68/0.77) in VRS, and 4.15 (0.68/0.76) in VAS for the women’s group (Table 3).
| ROC curves of 3 pain-scale questionnaires for diagnosing free of CAD in whole population | |||||
|---|---|---|---|---|---|
| Pain-scale questionnaire | c-statistics (ROC index) | 95% CI | Optimal cut-off point | Sensitivity | Specificity |
| Numerical rating scale | 0.73 | 0.67-0.80 | 3.25 | 0.74 | 0.75 |
| Verbal rating scale | 0.73 | 0.66-0.79 | 1.5 | 0.69 | 0.79 |
| Visual analogue scale | 0.75 | 0.69-0.82 | 4.25 | 0.68 | 0.78 |
| ROC curves of 3 pain-scales for diagnosing free of CAD in male population | |||||
| Pain-scale questionnaire | c-statistics (ROC index) | 95% CI | Optimal cut-off point | Sensitivity | Specificity |
| Numerical rating scale | 0.67 | 0.59-0.76 | 3.5 | 0.62 | 0.76 |
| Verbal rating scale | 0.67 | 0.58-0.76 | 1.5 | 0.56 | 0.79 |
| Visual analogue scale | 0.89 | 0.60-0.78 | 4.15 | 0.6 | 0.75 |
| ROC curves of 3 pain-scales for diagnosing free of CAD in female population | |||||
| Pain-scale questionnaire | c-statistics (ROC index) | 95% CI | Optimal cut-off point | Sensitivity | Specificity |
| Numerical rating scale | 0.72 | 0.65-0.79 | 3.25 | 0.74 | 0.73 |
| Verbal rating scale | 0.72 | 0.65-0.79 | 1.5 | 0.69 | 0.77 |
| Visual analogue scale | 0.75 | 0.68-0.82 | 4.25 | 0.69 | 0.76 |
Table 3: Performance of free of CAD prediction pain-scale models using c-statistics and cut-off points through Youden index.
Multivariate regression analysis for clarifying the independent association among pain scales for the CAD-free population
Independence of association among all pain scales in the CAD population were found to be significant after being adjusted for risk factors including age, gender, DM, hypertension, smoking, dyslipidemia, and ESRD. Compared to NSR>3.25, NSR ≤ 3.25 was the strongest predictor among the pain scales for CAD (OR, 8.291; 95% CI, 4.227~15.981, p<0.001). Since NSR was not a continuous scale, NSR value of 3.0 was further analysed, and the same result was found with NSR ≤ 3.25. The ORs (95% CI) of VRS ≤ 1.5 and VAS ≤ 4.25 (compared with VRS>1.5 and VAS>4.25) were 7.943 (4.120~15.315) and 6.781 (3.572~12.873) respectively (both p<0.001). The ORs of these 3 scales were almost double or even more than doubled compared to other risk factors (Table 4). In addition, 3-vessel disease (3VD) was differentiated from single-vessel disease (1VD), NSR ≤ 3.25 (3.0), VRS ≤ 1.5, and VAS ≤ 4.25 had significantly and independently higher risks of 3-vessel disease than did NSR>3.25 (3.0), VRS>1.5 and VAS>4.25 with ORs (95% CI) of 4.922 (2.123~11.409), 5.403 (2.190~13.330) and 5.627 (2.283~13.868) respectively (all p<0.001) (Table 5).
| Variables | OR | 95% Confidence Interval (CI) | P-value |
|---|---|---|---|
| NSR 3.25 (or 3) | 8.219 | (4.227~15.981) | <0.001# |
| DM | 3.587 | (1.747~7.364) | <0.001# |
| Current smoking | 2.776 | (1.313~5.868) | 0.008* |
| Gender | 1.69 | (0.782~3.652) | 0.182 |
| Dyslipidemia | 2.028 | (1.057~3.888) | 0.033* |
| ESRD | 4.445 | (0.737~26.811) | 0.073 |
| Age | 1.018 | (0.989~1.048) | 0.235 |
| Hypertension | 1.248 | (0.608~2.562) | 0.547 |
| VRS 1.5 | 7.943 | (4.120~15.315) | <0.001# |
| DM | 3.783 | (1.834~7.801) | <0.001# |
| Current smoking | 2.521 | (1.199~5.298) | 0.015* |
| Gender | 1.926 | (0.896~4.139) | 0.093 |
| Dyslipidemia | 1.886 | (0.989~3.598) | 0.054 |
| ESRD | 4.882 | (0.821~29.017) | 0.081 |
| Age | 1.018 | (0.989~1.048) | 0.214 |
| Hypertension | 1.182 | (0.578~2.418) | 0.646 |
| VAS 4.25 | 6.781 | (3.572~12.873) | <0.001# |
| DM | 3.705 | (1.817~7.555) | <0.001# |
| Current smoking | 2.494 | (1.195~5.206) | 0.015* |
| Gender | 1.985 | (0.930~4.239) | 0.076 |
| Dyslipidemia | 1.752 | (0.928~3.305) | 0.084 |
| ESRD | 3.588 | (0.627~20.515) | 0.151 |
| Age | 1.108 | (0.989~1.047) | 0.23 |
| Hypertension | 1.176 | (0.582~2.375) | 0.651 |
Table 4: Multiple logistic regression analysis for prediction of CAD by three pain scale cut-off points.
| Variables | OR | 95% Confidence Interval (CI) | P-value |
|---|---|---|---|
| NSR 3.25 (or 3) | 4.922 | (2.123~11.409) | <0.001# |
| DM | 2.943 | (1.425~6.079) | 0.004* |
| Current smoking | 1.088 | (0.471~2.510) | 0.844 |
| Gender | 1.722 | (0.658~4.509) | 0.268 |
| Dyslipidemia | 1.738 | (0.841~3.591) | 0.136 |
| ESRD | 0 | - | 0.998 |
| Age | 1.014 | (0.983~1.045) | 0.393 |
| Hypertension | 1.886 | (0.818~4.353) | 0.137 |
| VRS 1.5 | 5.403 | (2.190~13.330) | <0.001# |
| DM | 3.069 | (1.490~6.320) | 0.002* |
| Current smoking | 1.02 | (0.439~2.366) | 0.964 |
| Gender | 1.88 | (0.719~4.914) | 0.198 |
| Dyslipidemia | 1.693 | (0.820~3.496) | 0.154 |
| ESRD | 0 | - | 0.998 |
| Age | 1.015 | (0.984~1.046) | 0.342 |
| Hypertension | 1.831 | (0.797~4.211) | 0.154 |
| VAS 4.25 | 5.627 | (2.283~13.868) | <0.001# |
| DM | 3.051 | (1.475~6.315) | 0.003* |
| Current smoking | 1.072 | (0.465~2.467) | 0.871 |
| Gender | 1.743 | (0.675~4.505) | 0.251 |
| Dyslipidemia | 1.616 | (0.784~3.333) | 0.194 |
| ESRD | 0 | - | 0.998 |
| Age | 1.102 | (0.982~1.044) | 0.436 |
| Hypertension | 1.815 | (0.788~4.178) | 0.161 |
Table 5: Multiple logistic regression analysis for prediction of single or 3-vessel CAD by three pain scale cut-off points.
Discussion
All pain scales, including NSR, VRS and VAS were significantly lower in patients with CAD, and all showed significant negative correlations with CAD. To the best of our knowledge, this is the first study to investigate the pain scales on improvement of diagnostic accuracy of CAD. It validated that low pain scales could further enhance CAD diagnostic accuracy in patients with symptomatic chest pain of suspicion of MI or with positive stress tests. The optimal cut-off points (sensitivity/specificity) of pain scales were 3.25 (0.74/0.75) in NSR, 1.5 (0.69/0.79) in VRS, and 4.25 (0.68/0.78) in VAS. In addition, these three pain scales improved c-statistics for CAD prediction from 0.50 to 0.73~0.75. All pain scales were significantly associated with CAD after adjusting for risk factors including age, gender, DM, hypertension, smoking, dyslipidemia, and ESRD. The ORs of these 3 scales were almost double or even more than doubled compared to other risk factors. Compared to NSR>3.25, NSR ≤ 3.25 was the strongest predictor among these pain scales for CAD (OR, 8.291; 95% CI, 4.227~15.981, p<0.001). In addition, these pain scales might be independently helpful in differentiating multivessel disease (MVD) from single-vessel disease (1VD).
Wide variations in the sensitivity and specificity of treadmill testing have been reported in the related literature. In a largescale analysis, the reported sensitivities ranged from 23-100% (mean, 68%), and the specificities ranged from 17-100% (mean, 77%) [17]. Dipyridamole-thallium testing has a reported sensitivity of 67-95% (mean, 86%) and a specificity ranging from 41-100% (mean, 71%) [17,18]. Our study showed a gain of 68%-74% in sensitivity and 75%-78% in specificity on top of these stress tests including treadmill and dipyridamole-thallium tests (Table 3). In addition to the estimation of CAD risks (Table 4), physicians can also easily estimate the risk of MVD through the pain scale assessments (Table 5). The sensitivity was relatively lower in treadmill testing in the women’s group [17], and our study results provide a solution for this discrepancy (Table 3).
The possible mediator for low pain scales in CAD is the activated endogenous opioid system through increased betaendorphin and opioid receptor activations in the situation of myoischemia [6-11]. In addition to generalized hypoalgesia to visceral and somatic stimulations mechanism [19], competitive inhibition from visceral nociception transmitted through sympathetic afferent fibers entering the dorsal root ganglions might compete with and reduce peripheral painful stimuli intensity [20-23]. This competitive inhibition has been verified and used in a reverse way for many clinical neuromodulation therapies, such as acupuncture, spinal cord stimulation, and transcutaneous electrical nerve stimulation, and all of which have been proven to reduce symptoms of visceral pain induced by angina pectoris [20,23-25] by competing against the visceral nociception inputs.
Factors such as concomitant DM, smoking habit, and male gender were significantly higher in patients with CAD in our study, which are also major CAD risk factors documented in previous studies [26-28]. From the viewpoint of these risk factors, hypoalgesia has been documented in patients with DM (CAD equivalent) [1-3]. In addition, smoking is an important source of acrylamide exposure, which is a possible cause of hypoalgesia by interrupting inputs from demyelinating and axonal changes in peripheral nerves [29]. Current human findings regarding gender differences in experimental pain indicate greater pain sensitivity among females as compared with males for most pain modalities and hypoalgesia [30-34]. This advantage of higher pain sensitivity in female population led to a greater pain test sensitivity in our study that compensated for the relatively low sensitivity in treadmill testing.
Study Limitations
This is the first study to unveil the lower pain in patients with myoischemia through assessment of the pain during arterial puncture procedure. The low-pain scale improved the diagnostic accuracy of CAD in patients with symptomatic chest pain suspicious of MI or with positive stress tests; consequently, it would also assist physicians in being aware of the possibility of MVD. NSR value of 3.25 presents as the best cut-off point for predicting the risk of CAD.
Acknowledgments
The authors thank the volunteers and staff who participated in this study.
Conflict of Interests
No potential conflicts of interest relevant to this article were reported.
Funding/Supports
None
Author Contributions
The authors, Dr. Kai-Chun Cheng and Dr. Kai-Yuan Cheng, participated in generating original ideas, in study design and analysis of data, in drafting of the manuscript, in revising it critically for important intellectual content and in final approval of the manuscript submitted. All the authors were involved in 1) making substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; and 3) reviewing and providing final approval of the version to be published. Kai-Chun Cheng and Kai-Hung Cheng wrote the manuscript, researched data and held the meetings for discussion. Kai-Chun Cheng and Kai-Hung Cheng (guarantors) take responsibility for the content of this article.
References
- Ig O. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics 2009; 6: 638-647.
- Heller GV. Evaluation of the patient with diabetes mellitus and suspected coronary artery disease. Am J Med 2005; 118: 9-14.
- Haffner S. Rationale for new American Diabetes Association Guidelines: are national cholesterol education program goals adequate for the patient with diabetes mellitus? Am J Cardiol 2005; 96: 33-36.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229-234.
- Bruehl SBJ, Chung OY, Magid E, Chont M, Gilliam W, Matsuura J, Somar K, Goodlad JK, Stone K, Cairl H. Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement. J Behav Med 2010 33: 168-176.
- Oldroyd KGHK, Gray CE, Beastall GH, Cobbe SM. Beta endorphin release in patients after spontaneous and provoked acute myocardial ischaemia. Br Heart J 1992; 67: 230-235.
- McCormack KJCC. Opioid receptors and myocardial protection: do opioid agonists possess cardioprotective effects? Clin Drug Investig 1998; 15: 445-454.
- Hikita HEH, Takase B, Satomura K, Kurita A, Nakamura H. Extent of ischemic stimulus and plasma beta-endorphin levels in silent myocardial ischemia. Am Heart J 1998; 135: 813-818.
- Falcone CGL, Ochan M, Codega S, Tortorici M, Angoli L, Bergamaschi R, Montemartini C. Beta-endorphins during coronary angioplasty in patients with silent or symptomatic myocardial ischemia. J Am Coll Cardiol 1993; 22: 1614-1620.
- Chen YTLC, Lee AY. Plasma levels of endogenous opioid peptides in patients with acute myocardial infarction. Jpn Heart J 1995 36: 421-427.
- Chang MCLA, Lin WY, Chen TJ, Shyu MY, Chang WF. Myocardial and peripheral concentrations of beta-endorphin before and following myocardial ischemia and reperfusion during coronary angioplasty. Jpn Heart J 2004 45: 365-371.
- Bösner S, Becker A, Haasenritter J, Abu Hani M, Keller H, Sonnichsen AC, Karatolios K, Schaefer JR, Seitz G, Baum E, Donner-Banzhoff N. Chest pain in primary care: epidemiology and pre-work-up probabilities. Eur J Gen Pract 2009; 15: 141-146.
- Bosner S, Becker A, Abu Hani M, Keller H, Sonnichsen AC, Haasenritter J, Karatolios K, Schaefer JR, Baum E, Donner-Banzhoff N. Accuracy of symptoms and signs for coronary heart disease assessed in primary care. Br J Gen Pract 2010; 60: 246-257.
- Verdon F, Herzig L, Burnand B, Bischoff T, Pecoud A, Junod M, Muhlemann N, Favrat B, GMIRG. Chest pain in daily practice: occurrence, causes and management. Swiss Med Wkly 2008; 138: 340-347.
- Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth 2008; 101: 17-24.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29-36.
- Chou TMAT. Evaluating coronary artery disease noninvasively-which test for whom? West J Med 1994; 161: 173-180.
- Jadvar H, Strauss HW, Segall GM. SPECT and PET in the evaluation of coronary artery disease. Radiographics 1999; 19: 915-926.
- Dimcevski GSK, Tage-Jensen U, Funch-Jensen P, Krarup AL, Toft E, Thorsgaard N, Arendt-Nielsen L, Drewes AM. Hypoalgesia to experimental visceral and somatic stimulation in painful chronic pancreatitis. Eur J Gastroenterol Hepatol 2006; 18: 755-764.
- Qin CFJ, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal dorsal column and somatic afferents in rats. J Pain 2008; 9: 71-78.
- de Vries JDM, Tio RA. An open label, single-centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial. Eur Heart J 2006; 27: 1631-1632.
- McNab DKS, Sharples LD, Ryan JY, Freeman C, Caine N, Tait S, Hardy I, Schofield PM. An open label, single-centre, randomized trial of spinal cord stimulation vs. percutaneous myocardial laser revascularization in patients with refractory angina pectoris: the SPiRiT trial. Eur Heart J 2006; 27: 1048-1053.
- Bueno EAMR, Frishman WH. Alternative approaches to the medical management of angina pectoris: acupuncture, electrical nerve stimulation, and spinal cord stimulation. Heart Dis 2001; 3: 236-241.
- Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg 2004; 100: 254-267.
- Meng J. The effects of acupuncture in treatment of coronary heart diseases. J Tradit Chin Med 2004; 24: 16-19.
- Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens 1994; 7: 7-12.
- Jenner JLOJ, Lamon-Fava S, Schaefer MM, Wilson PW, Castelli WP, Schaefer EJ. Effects of age, sex, and menopausal status on plasma lipoprotein(a) levels. The Framingham Offspring Study. Circulation 1993; 87: 1135-1141.
- Wilson PW, Castelli WP, Kannel WB. Coronary risk prediction in adults (the Framingham Heart Study). Am J Cardiol 1987; 59: 91-94.
- Kjuus HGL, Heier MS, Sjoholm H, Ovrebo S, Skaug V, Paulsson B, Tornqvist M, Brudal S. Effects on the peripheral nervous system of tunnel workers exposed to acrylamide and N-methylolacrylamide. Scand J Work Environ Health 2004; 30: 21-29.
- Fillingim RBKC, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009; 10: 447-485.
- Heitkemper MM, Jarrett M. Gender differences and hormonal modulation in visceral pain. Curr Pain Headache Rep 2001; 5: 35-43.
- Silverstein B. Gender differences in the prevalence of somatic versus pure depression: a replication. Am J Psychiatry 2002; 159: 1051-1052.
- Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med 2001; 16: 266-275.
- Ge HYMP, Cairns BE, Arendt-Nielsen L. Hypoalgesia in the referred pain areas after bilateral injections of hypertonic saline into the trapezius muscles of men and women: a potential experimental model of gender-specific differences. Clin J Pain 2006; 22: 37-44.