- Journal of RNA and Genomics (2006) New Methods and Technologies
Long-term transgene expression and inhibition of HIV-1 replication by a Cre/loxP-EBNA-1/oriP HIV-1-dependent ribozyme vector: Applications for HIV-1 gene therapy
| Takashi Nagawa1,§, Yuichiro Habu2,3,§, Norihiko Matsumoto1, Naoko Miyano-Kurosaki1,2, and Hiroshi Takaku1,2,* 1Department of Life and Environmental Sciences and 2High Technology Research Center, Chiba Institute of Technology, 2-17-1 Tsudanuma, Narashino, Chiba 275-0016, Japan, and 3Japanese Foundation for AIDS Prevention, Toranomon, Minato-ku, Tokyo, 105-0001, Japan |
| *Correspondence to: Hiroshi Takaku, Email: hiroshi.takaku@it-chiba.ac.jp, Tel: +81 47 478 0407, Fax: +81 47 471 8764 |
| §Joint first authors |
| Journal of RNAi and Gene Silencing (2006), 2(1), 146-153 |
| © Copyright Takashi Nagawa et al |
| (Received 01 November 2005; Revised 10 December 2005; Accepted 15 December 2005; Available online 13 January 2006; Published 28 February 2006) |
Abstract
The cleavage of target mRNA by ribozymes is being exploited as a means of gene silencing in nucleic-acidbased therapies. We previously established an HIV-1-dependent ribozyme-expression vector system, based on Cre-loxP technology with an LTR-gag-p17 promoter as a molecular switch for use in acute HIV-1 infection. The simultaneous expression of the Cre protein and loxP homologous recombination induced a high level of HIV-1-replication inhibition, but ribozyme expression was transient. In the current study, we overcame this limitation by inserting EBNA-1 and oriP genes from the Epstein-Barr virus (EBV) into the vector. When this plasmid was introduced into HeLa CD4+ cells, we observed long-term expression of both the EGFP reporter gene and the ribozyme. Moreover, HIV-1 replication was inhibited in the long-term in transfected cells. These data suggest that the HIV-1-dependent ribozyme-expression vector containing EBNA-1/oriP sequences would be a useful tool in HIV-1 gene therapy applications.
Keywords |
| Cre/loxP recombination, EBNA-1/oriP, gene therapy, HIV-1, ribozyme |
Introduction |
| In the application of therapeutics to HIV-1 infection, prevention is of greater importance than treatment. With this aim, many antiviral RNA-expression vector systems have been developed for use in anti-HIV-1 gene therapy (Banerjea et al, 2003; Barnor et al, 2004; Boden et al, 2004; Cordelier P et al, 2004; Habu et al, 2005; Mautino and Morgan, 2002; Takaku, 2004). Recently, small RNA molecules such as, siRNAs and snRNAs, have also been shown to affect gene silencing by RNA interference (Boden et al, 2003; Chang et al, 2002; Li et al, 2003). |
| Increasing the efficiency of transgene expression is of prime importance for human gene therapy (Davis and McNeilly, 2001). The Epstein-Barr Virus (EBV) is an autonomously replicating episomal vector that has been utilized to overcome the problem of rapid elimination of intracellularly delivered plasmid DNA in nonviral gene transfer. EBV is a gammaherpesvirus that is maintained as an episome of approximately 172 kb in size in a small number of resting B cells and epithelial cells in most of the human population. It latently infects human B cells with a high efficiency, after which its linear doublestranded genome circularizes and is sustained as a stable episome (Hirai and Shirakata, 2001). The EBV replication system is present at a frequency of about 1- 100 copies per cell, and maintains a non-covalent attachment to the host chromosome. |
| The latent origin of replication oriP and the viral transactivator protein EBNA-1 are essential components for EBV latent replication and maintenance of the viral genome (Daikoku et al, 2004; Lee et al, 1999). Both elements have been employed for long-term transgene expression in gene-therapy studies (Otomo et al, 2001; Tsujie et al, 2001). |
| Previously, we described an HIV-1-dependent ribozymeexpression vector capable of achieving site-specific excision of loxP sequences by using the HIV-1 minimal LTR-Cre-loxP system as a molecular switch in an acute HIV-1 infection (Habu et al, in press). However, we were unable to detect long-term expression of the anti-HIV-1 ribozyme. We hypothesized that the length of HIV-1- dependent transgene expression could be significantly increased in mammalian cells by introducing EBNA- 1/oriP sequences to the vector. |
| In this study, we constructed an HIV-1-dependent longterm transgene (RNA ribozyme) expression vector (LTRgag- p17/Cre-loxP-Rz-U5-EBNA-1-oriP-EGFP (EOG)) using the EBV replicon system, which was propagated in Escherichia coli and transfected into mammalian cells. We measured transgene-expression levels, including EBNA-1 and oriP, in the presence and absence of HIV-1 infectious molecular clone (pNL4-3, Adachi et al, 1986). The potential anti-HIV-1 activity of the expression vector was evaluated with a view to establishing a highly effective therapeutic agent that could be further developed for HIV gene-therapy applications. |
Materials and Methods |
| Construction of plasmids |
| The retroviral vector pLEGFP-C1 (Clontech, Mountain View, CA) was digested with Nhe I and Xho I to release the DNA fragment encoding enhanced green fluorescent protein (EGFP). This was inserted into the Nhe I/Xho I sites of pCEP4 (Invitrogen, Carlsbad, CA), which contains EBNA-1 and oriP, to create pCEP4-EGFP. An Ssp I fragment containing EGFP, EBNA-1, and oriP was cloned into the Stu I sites of pLTR-gag-p17-Cre/loxPRz- U5 (Habu et al, in press) and ploxP-Rz-U5, which been previously described with a high cleavage affinity (Habu et al, 2002) to yield pLTR-gag-p17-Cre/loxP-Rz- U5-EOG (Figure 1A) and ploxP-Rz-U5-EOG (Figure 1C), respectively. The control plasmid vector ploxP-Rz- U5-EOG lacks the LTR-gag-p17-Cre gene and so does not trigger expression of the ribozyme. A Pvu II fragment containing the EGFP-expression unit was excised from pCMV-EGFP previously constructed (unpublished data) and cloned into the Stu I sites of ploxP-Rz-U5 (Habu et al, 2002) or pLTR-gag-p17- Cre/loxP-Rz-U5 to generate ploxP-Rz-U5-G (Figure 1D) and pLTR-gag-p17-Cre/loxP-Rz-U5-G (Figure 1B), which are the EBNA-1 and oriP negative-control plasmids, respectively. |
| Cell culture and transfections |
| HeLa CD4+ cells were grown in RPMI 1640 medium (Sigma, Saint Louis, MO) supplemented with 10% (v/v) heat-inactivated FBS, 100 U/ml penicillin, and 100 Jg/ml streptomycin. HEK 293T cells were grown in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 Jg/ml streptomycin. All cells were maintained at 37 °C in a 5% CO2 atmosphere. HeLa CD4+ and 293T cell transfections were carried out using FuGENE™6 (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s protocol. |
| Luciferase assay |
| Luciferase activity was measured with the PicaGene kit (Toyo-inki, Tokyo, Japan) according to the manufacturer’s protocol. The target gene-expressing plasmid pNL4-3-luc (Akkina et al, 1996), lacking an env gene and with a firefly luciferase gene replacing the nef gene, was co-transfected into HeLa CD4+ cells with the pLTR-gag-p17-Cre/loxPRz- U5-EOG plasmid, which expresses the ribozyme following Cre/loxP homologous recombination. Transfected HeLa CD4+ cells were lysed in 200 Jl PicaGene cell lysis buffer for 15 min and then harvested. Cell debris was removed by centrifugation. Centrifuged lysate (10 Jl) was added to 100 Jl luminous substrate, and the luminescent signal was immediately quantitated with a luminometer (Lumat LB 9507; Berthold, Bad Wildbad, Germany). |
| Flow cytometry |
| Transfected HeLa CD4+ cells were trypsinized, washed twice in PBS, and fixed in PBS containing 1% formaldehyde. Direct fluorescence of EGFP was analyzed by FACS Calibur (Clontech). Data acquisition and analysis were performed with CellQuest software (Clontech). Gates for detection of EGFP were established using mock-transfected cells as background. |
| Fluorescent microscopy |
| To evaluate the self-replicating function of the loxP ribozyme as an index for stable transgene expression in cells, transfected HeLa CD4+ cells were trypsinized and seeded at a low cell density. Direct fluorescence microscopy of EGFP was carried out at the mitotic stage of cell division, after each passage on days 1, 3, 7, 13, 19 and 25 and the data were acquired with a DP12 digital microscope camera (Olympus Company, Tokyo, Japan). |
| RNA isolation and RT-PCR |
| Total cellular RNA was isolated from transfected HeLa CD4+ cells with the GenElute Mammalian Total RNA kit (Sigma) using the manufacturer’s protocol. RNA samples were treated with DNase I (Promega, Madison, WI) according to the manufacturer’s instructions. RT-PCR assays were carried out using previously described primers (Habu et al, 2002) and the RT-PCR high-Plus-kit (Toyobo, Osaka, Japan) according to the manufacturer’s protocol. |
| Assay of HIV-1 replication |
| HIV-1 production was monitored by determining the HIV- 1 p24 antigen concentration. The culture medium from HeLa CD4+ cells co-transfected with pNL4-3 and pLTRgag- p17-Cre/loxP-Rz-U5-EOG was harvested on days 1, 3, 5, 7, 9, 11, 13 and 15 post-transfection. p24 Gag protein production was detected by the HIV-1 p24 CLEIA assay (Lumipulse, Fujirebio Inc., Tokyo, Japan), according to the manufacturer’s protocol (Sakai et al, 1999). |
Results And Discussion |
| Design and construction of an HIV-1-dependent Creexpression vector |
| Tissue-specific gene transfer and expression are crucial for the development of safe and effective gene-therapy protocols. To this end, the HIV-1 LTR can serve as an efficient and inducible promoter dependent on the HIV-1 trans-activation factor, Tat. In a previous study, we constructed the HIV-1-dependent RNA ribozyme expression Cre/loxP vector, pLTR-gag-p17-Cre/loxP-Rz- U5-G, which targets mRNAs encoded by the U5 region (548–578) of the LTR (Figure 1E). This vector showed HIV-1-dependent ribozyme expression in HeLa CD4+ cells (Habu et al, 2002; Habu et al, in press), but expression was not long term. Hence, in the current study, we constructed an HIV-1-dependent expression vector containing Cre/loxP and EBNA-1/oriP sequences, with the aim of increasing the duration of transgene expression. Moreover, we inserted the reporter gene EGFP to enable visualization of transgene expression (Figure 1A). The plasmid vectors pLTR-gag-p17- Cre/loxP-Rz-U5-G (Figure 1B), ploxP-Rz-U5-EOG (Figure 1C), and ploxP-Rz-U5-G (Figure 1D) served as controls. The advantage of this vector system over previously reported ribozyme vector systems (Chang et al, 2002; Li et al, 2003) is that it is not constitutively expressed to trigger off non-specific inhibition, but specifically expressed only in the event of HIV infection. |
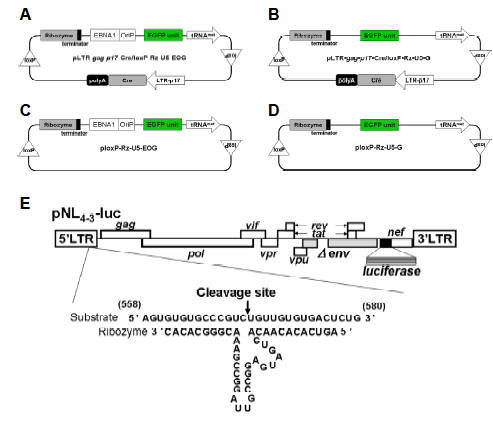 |
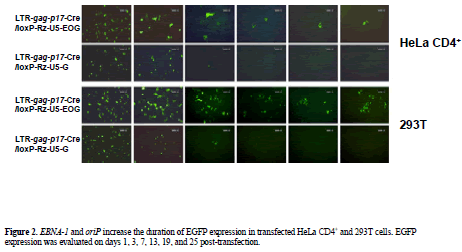
| Figure 1. Schematic representation of HIV-1-dependent ribozyme-expression vectors. (A) The ribozyme expression (off switch) vector pLTR-gag-p17-Cre/loxP-Rz-U5-EOG, containing EBNA-1 and oriP genes for long-term expression. (B) Control ribozyme-expression vector pLTR-gag-p17-Cre/loxP-Rz-U5-G, which lacks EBNA-1 and oriP genes. (C) Control vector ploxPRz- U5-EOG, which lacks the LTR-gag-p17-Cre gene. (D) Control vector ploxP-Rz-U5-G, which lacks EBNA-1/oriP and LTRgag- p17-Cre genes. (E) HIV-1 NL4-3 molecular clone pNL4-3-luc containing the luciferase reporter gene, showing the target site and structure of the constructed ribozyme. |
| Long-term transgene expression of pLTR-gag-p17- Cre/loxP-Rz-U5-EOG in 293T and HeLa CD4+ cells |
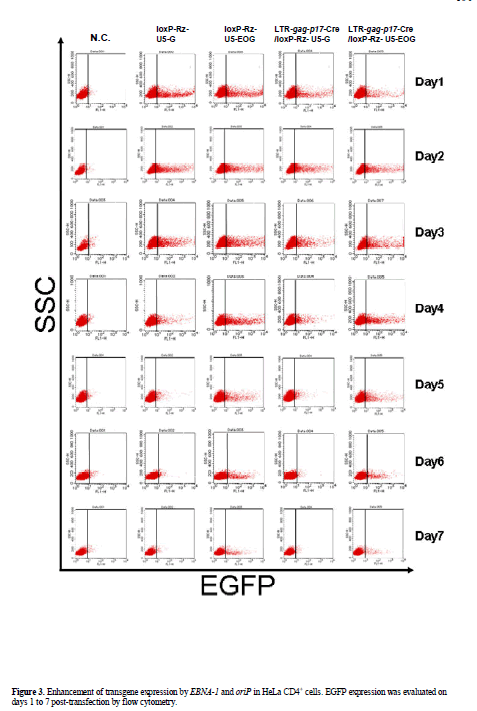
| To characterize the ability of the EBV replication system to effect long-term transgene expression we compared EGFP expression of control vector (pLTR-gag-p17- Cre/loxP-Rz-U5-G) with that of the EBNA-1/oriP plasmid pLTR-gag-p17-Cre/loxP-Rz-U5-EOG. EGFP expression in pLTR-gag-p17-Cre/loxP-Rz-U5-G-transfected 293T cells was observed for a maximum of 3 days, while EGFP expression persisted for more than 25 days in pLTR-gagp17- Cre/loxP-Rz-U5-EOG-transfected 293T cells (Figure 2). As it has been reported that the EBNA-1 protein potentiates gene transcription (Mackey and Sugden, 1999), we measured the enhancement of gene expression by determining EGFP fluorescence intensity. FACS analysis showed that HeLa CD4+ cells transfected with the EBNA- 1-containing plasmids demonstrated longer-term EGFP expression (until day 7; Figure 3) than HeLa CD4+ cells transfected with plasmids lacking EBNA-1. This is of therapeutic importance, because EBNA retains the therapeutic molecule to sensor for infective HIV-1 to release the catalytic ribozyme for cleavage. |
| Measurement of the mean fluorescence intensity (MFI) revealed a twofold increase in the EGFP expression level of HeLa CD4+ cells transfected with pLTR-gag-p17- Cre/loxP-Rz-U5-EOG compared with pLTR-gag-p17- Cre/loxP-Rz-U5-G-transfected cells (data not shown). These results indicate that EBNA-1/oriP sequences mediate efficient and stable replication of transgene expression by enhancing nuclear localization of EBNA-1 (Mackey and Sugden, 1999; Marechal et al, 1999). The nuclear localization of this vector system is of cardinal importance since its function is induced by HIV-1 tat which is nuclear-based. |
| Dose-dependent inhibition of HIV-1 replication by pLTR-gag-p17-Cre/loxP-Rz-U5-EOG |
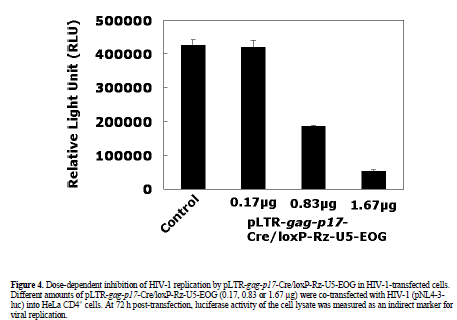
| The effect of pLTR-gag-p17-Cre/loxP-Rz-U5-EOG on HIV-1 (pNL4-3-luc) replication was measured in a transient assay following its co-transfection with pNL4-3- luc into HeLa CD4+ cells. At 72 h post-transfection, the luciferase activity of cell lysate was measured as an indirect marker of viral replication. The plasmid vector ploxP-Rz-U5-EOG (Figure 1C), which does not trigger ribozyme expression as it lacks the LTR-gag-p17-Cre gene, was used as a control. Our analysis showed a dosedependent inhibition of HIV-1 replication by pLTR-gagp17- Cre/loxP-Rz-U5-EOG with a maximum inhibitory efficacy of >90% at a vector DNA concentration of 1.67 Jg (Figure 4). Control vector alone had no inhibitory effect. This result suggests that the Rz-U5 ribozyme was expressed using Cre/loxP recombination and EBNA/oriP systems in HIV-1 infected cells, and successfully cleaved its target HIV-1 mRNA (Figure 1E). |
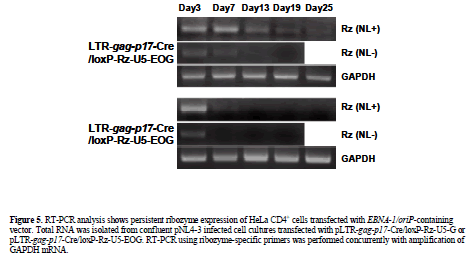
| EBNA-1 mediates long-term ribozyme expression in HIV-1-dependent vector-transfected HeLa CD4+ cells |
| As long-term ribozyme-U5 (Rz-U5) expression is an important determinant of its efficiency, we compared the level of expression in HeLa CD4+ cells transfected with pLTR-gag-p17-Cre/loxP-Rz-U5-EOG or pLTR-gag-p17- Cre/loxP-Rz-U5-G in the presence or absence of pNL4-3 (Figure 5). RT-PCR analysis showed that Rz-U5 expression persisted for more than 19 days following pLTR-gag-p17-Cre/loxP-Rz-U5-EOG transfection in the absence of pNL4-3 (Figure 5). By contrast, pLTR-gagp17- Cre/loxP-Rz-U5-G-transfected cells demonstrated Rz- U5 expression for only 3 days. Ribozyme expression was not observed following transfection of either plasmid DNA in the absence of pNL4-3. These results confirm that the Cre/loxP vector containing EBNA-1 has the potential to confer long-term transgene expression in HIV-1 infected cells. This property therefore puts our vector system in the class of prophylactics, since the vector will function to prevent infection of the target cells. |
 |
 |
 |
 |
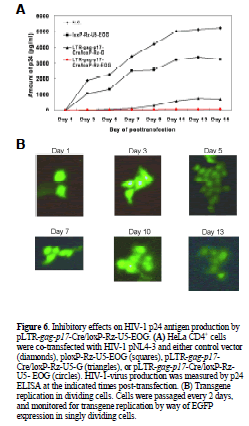
| Long-term inhibition of HIV-1 replication by pLTR-gagp17- Cre/loxP-Rz-U5- EOG in pNL4-3 infected HeLa CD4+ cells |
| To evaluate further the persistency of the EBNA-1/oriPdependent ribozyme effect on HIV-1 replication we cotransfected 0.3 Jg pNL4-3 with 5.0 Jg pLTR-gag-p17- Cre/loxP-Rz-U5-EOG, pLTR- gag-p17-Cre/loxP-Rz-U5-G or ploxP-Rz-U5-EOG into HeLa CD4+ cells. As shown in Figure 6A, pLTR-gag-p17-Cre/loxP-Rz-U5-EOG inhibited HIV-1 replication for 15 days. By contrast, ploxP-Rz-U5- EOG (lacking ribozyme expression) showed little inhibitory activity towards HIV-1 replication compared with the negative control (pNL4-3 only). |
| EGFP reporter gene expression was monitored as an indicator of transgene expression (Figure 6B). Its persistence throughout the experiment (up to 13 days) confirms our earlier findings, and suggests that the HIV-1-dependent ribozyme-expression vector containing EBNA-1/oriP sequences mediates long-term ribozyme expression, inhibits HIV-1 replication and would be a useful tool for HIV-1 gene-therapy applications. |
 |
Conclusions |
| • Ribozyme expression is HIV-1 Tat dependent, as we were only able to detect it in the presence of pNL4-3. |
| • Transgene expression is long-term and is extended by the presence of oriP and EBNA-1. Hence, inhibition is also shown to be long-term. |
| • The Cre/loxP system induces ribozyme-mediated inhibition of HIV-1 replication in a dose-dependent manner. |
| • These results demonstrate the anti-HIV-1 effect of the expressed ribozyme from the Cre/loxP system, clearly illustrating the potential of the Cre/loxP-oriP/EBNA-1 system as a gene-therapy tool for controlling HIV-1 infection. |
Acknowledgements |
| This work was supported by a Grant-in-Aid for High Technology Research (HTR) from the Ministry of Education, Science, Sports, and Culture, Japan, by research grants from the Human Science Foundation (HIV-K-14719), by a Grant-in-Aid for AIDS research from the Ministry of Health, Labor, and Welfare, Japan (H17-AIDS-002), and by the Sasakawa Scientific Research Grant from The Japan Science Society. Y. H. was a Research Fellow of the HTR until June 2005 and has been a Research Fellow of the Japanese Foundation for AIDS Prevention since July 2005. |
Statement of Compteting Interests |
| The authors declared no competing interests. |
List of Abbreviations |
| EBV; Epstein-Barr virus |
| EBNA-1; EBV nuclear antigen 1 |
| EOG; EBNA-1/OriP/EGFP |
| MFI; Mean fluorescence intensity |
| OriP; EBV latent origin of replication |
References
|