Research Article - Microbiology: Current Research (2020) Volume 4, Issue 1
Linking the metabolic pathways of Escherichia coli with virulence by altering glucose availability, inhibiting the acetyl-CoA carboxylase gene accA with asRNA, and through the quantification of luxS
Tatiana Hillman*Biological Sciences, The LAB, Inc., Los Angeles, California, United States
- *Corresponding Author:
- Tatiana Hillman
Biological Sciences, The LAB Inc Los Angeles, California, United States
E-mail: thillman@usc.edu
Tel: +7472734349
Accepted Date: December 10, 2019
Citation: Tatiana Hillman. Linking the metabolic pathways of Escherichia coli with virulence by altering glucose availability, inhibiting the acetyl-CoA carboxylase gene accA with asRNA, and through the quantification of luxs. Microbiol Curr Res. 2020;4(1):1-8.
Abstract
The present study determines to confirm a link between the metabolic pathways of bacteria and virulence. Commensal bacteria convert ingested D-glucose into short-chain fatty acids and long- chain fatty acids. The long-chain fatty acids are produced for plasma membrane and biofilm construction. The genetic activity of accA produces the acetyl-CoA caroboxylase enzyme needed for long-chain fatty acid elongation. In this study, Luria broth liquid cultures of Escherichia coli were enhanced with Dglucose. The 15 mM glucose sample yielded 4,210 ng/µL of accA as compared to 196 ng/µL for the control. The gene accA was inhibited with antisense RNA with a qPCR gene copy number of 63. The inhibition of accA suppressed the expression of the luxS gene. The luxS gene is vital for transferring intercellular quorum-sensing signals through its increasing the synthesis of autoinducer-2. Bacterial cells that expressed antisense inhibition of accA were more susceptible to antibiotics. The purpose of the study is to advocate for an antibiotic design that targets bacterial metabolic genes and enzymes. An antibiotic design that inhibits bacterial metabolism may help to overcome the issue of bacterial antibiotic resistance.
Keywords
Antibiotic resistance, Antibiotics, Biofilm, Carboxylase enzymes, Autoinducers, Quorum sensing, Antisense RNA, Bacterial metabolism.
Introduction
Penicillin was invented by Alexander Fleming in 1928. His discovery coincided with the use of antibiotics which saved many lives; however, the issue of antibiotic resistance has emerged in tandem with the overuse of antibiotics. The synthesis of many novel and a typical antibiotics increased between the 60s and the 80s of the 20th century [1]. Fleming's discovery came after witnessing a mold growth that reduced the staphylococci population cultivated in a petri-dish [2]. In 1940, scientists published the methods and protocols required for extracting and formulating penicillin [3].
After the first use of penicillin in 1945, the resistance to antibiotics has increased [4]. Bacteria adapt to many diverse habitats and environments. Adding antibiotics to a bacterium's atmosphere creates pressure that induces gene mutations called antibiotic-resistant genes. These antibiotic-resistant genes are also shared by the bacterium with other bacteria. The natural selection process is extremely effective and essential to antibiotic resistance development.
Persistent cells, for example, are the bacterial cells that survived their antibiotic contact. The persistent cells live after being exposed to antibiotics to cause and propagate antibiotic resistance [5]. A. baumanii, a bacterium, inhabit hospitals, are highly contagious, and develop antibiotic resistance through horizontal gene transfer. The A. baumanii bacterium cause 64% of urinary tract infections during the use of catheters [6]. Microbial biofilm communities are mainly antibiotic resistant. Biofilms are a community of bacterial colonies attached to a biotic or an abiotic extracellular matrix. Because the extracellular biofilm matrix halts the penetration of antibiotics, and is mostly anaerobic, bacteria within the biofilm are largely resistant to antibiotics [7].
Bacterial motility and biofilm formation are a product of SRibosylhomocysteinase or the enzyme termed Lux-S. Lux-S produces autoinducer-2. The AI-2 is released to transport quorum sensing signals between bacterial cells. Though quorum sensing bacterial cells have an intercellular interaction. When the genetic expression of luxS is increased, the level of AI-2 production is amplified, causes more quorum sensing, and forms more biofilm to spread infection. Gram-positive bacteria produce antoinducers called oligopeptides and gram-negative bacteria release homoserine lactones as autoinducers [8]. After treating mutant cultures with the antibiotic of kanamycin, researchers concluded that the biofilm had less forms of antibiotic resistance. Adversely, the resistant and mutant bacteria repopulated at the base to the surface of the biofilm after 250 generations of reproduction [9]. The metabolic pathways of bacteria affect the production of AI-2 and biofilm. A study by Peng et al. showed that bacterial cultures without glucose or lacking alanine were less sensitive to kanamycin [10].
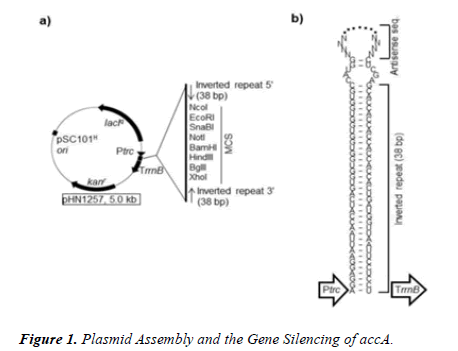
As a result, we examined the effect of glucose availability upon the internal gene expression of E. coli by altering the osmolarity of glucose. We extracted RNA after exposures to glucose and gene inhibition. We inhibited the gene accA that is translated into the acetyl-CoA carboxylase transferase enzyme called the ACCA transferase subunit. The ACCase enzyme catalyzes the first step of the synthesis of bacterial fatty acids, converting an acetyl-CoA into a malonyl-CoA. The accA gene was suppressed with antisense RNA or asRNA, shown in Figure 1. The asRNA codons attach to the sequences that enclose the ribosome-binding site and the promoter site of the DNA target [11]. The detection of the mRNA target is blocked by the asRNAs applied [11]. Next, the intercellular communication was quantified by the qPCR analysis of luxs.
The glucose concentration was altered and the accA gene was inhibited in order to confirm the link between the extracellular (glucose), intracellular (accA), and the intercellular (luxs) metabolic pathways of E. coli with virulence. Antibiotic resistance was tested to demonstrate the antibiotic susceptibility of E. coli cells expressing the asRNA of accA. The author hypothesizes that the inhibition of accA and decreasing the availability of glucose will significantly reduce the production of luxs. It is further hypothesized that inhibiting accA can augment antibiotic sensitivity. Here, the purpose is to describe and demonstrate the profound potential for inhibition metabolic genes and enzymes for possibly overcoming antibiotic resistance.
The PCR products of accA were ligated into the PTasRNA expression vector of the digested plasmid pHN1257 at the multiple cloning site.
Materials and Methods
Cell culture of bacteria and RNA extraction
The bacterial strain TOP10 for Escherichia coli was plated onto agar plates. Three to five colonies were selected and cultured in Luria-Bertani broth. The liquid culture was then enhanced with 200 μM, 50 μM, and 0 μM of D-glucose. The RNA OD260 readings were recorded and replicated (n=7). Additional sets of liquid cultures were treated with 15 mM, 7.5 mM, 5 mM, and a control of glucose. The plasmid pHN1257 for the expression of antisense RNA to block accA, was used to transform competent bacterial cells and were plated onto agar enhanced with the kanamycin antibiotic. The cells expressing asRNA were inoculated into 4 mL of LB broth, and then RNA was extracted with the Omega E.Z.N.A extraction kit.
cDNA preparation
The cDNA was synthesized with a kit by the Applied Biological Materials Inc. or ABM. One microgram of each RNA extraction was added to the reaction mixture and to oligonucleotides(dT) (10 μM) combined with the primers for luxs and accA. The cDNA was prepared for PCR and for qPCR analysis of luxs and accA. The primer/RNA mix included 1 μg of RNA, 1 μL of dNTP (10 mM) mix, 1 μL of oligo(dt), and nuclease-free water added upto 20 μL.
PCR
The Promega PCR MasterMix kit was used and added to the upstream and downstream primers of the antisense cDNA of accA. The PCR mastermix of 25 μL was added to the primers, the cDNA of 190 ng, and with nuclease-free water (n=3). The upstream primer, downstream primer, a DNA template of 1–5 μl, and nuclease-free water were added to the PCR reaction mixture up-to 50 μl.
Agarose gel electrophoresis and assembly of plasmid
An agarose electrophoresis gel was used to confirm the PCR products of asRNA-accA were without primer dimers or contamination. The plasmid pHN1257 was digested with XhoI (upstream) and NcoI (downstream) with the New England Biolabs XhoI-catalog R0146S and NcoI-catalog R0193S as shown in Figure 1. The PCR amplification of the antisense codons for accA were cut with XhoI and NcoI. The PCR products were ligated into the pHN1257 multiple clonal site. The DNA insert was mixed with 2 μL of the plasmid with 5 μL of the ligation mix, and then placed on a heat block for 15 minutes at 90°C.
Real-Time PCR
The cDNA from bacterial samples enhanced with glucose at 15 mM, 7.5 mM, 5 mM, and a control (n=3) was diluted to a an 8- fold serial dilution. The standards for each sample were prepared into 10-fold dilutions. An absolute quantification method was applied for measuring the number of gene copies of luxs and accA. The 8-fold dilutions of cDNA were pipetted into clear 96-well plates. The upstream and downstream primers of 0.4 μl for luxs and accA were pipetted with 10 μL of SensiMix SYBR MasterMix, and with 1.2 μL of nucleasefree water. The SYBR mixture was then micropipetted into the wells with the cDNA. The qPCR protocol for the Roche Lightcycler 480 machine was applied.
Absolute quantification of LUX-S by qPCR
The concentration of the luxs gene was measured through qPCR analysis. RNA was extracted from liquid cultures of asRNA-accA expressing bacterial cells enhanced with 25 μM and 5 μM concentrations of glucose. RNA was also extracted from bacterial cells cultured without glucose and treated with asRNA-accA. A control without glucose (-) and without (-) asRNA was compared to each sample. An absolute quantification of luxs was analyzed through the qPCR amplification protocol (Roche Lightcycler 480 machine).
Antibiotic resistance test
The asRNA-accA expression vector of pHN1257 transformed competent bacterial cells. The transformed cells were plated unto agar with the kanamycin antibiotic. Three to five transformant bacterial colonies were chosen and placed in a liquid culture with kanamycin (50 μg/mL). One hundred microliters of the bacterial liquid cultures, expressing the pHN1257- asRNA-accA vectors, were plated onto nutrient agar plates with kanamycin. The bacterial liquid cultures were plated with a swab to form a lawn of bacterial growth. Disks containing tetracycline (10 μg/mL), carbenicillin (100 μg/mL), and chloramphenicol (25 μg/mL) were placed unto the plates with the bacterial cell pHN1257-asRNA-accA expression vectors and onto the control groups. The plates were cultured for 18-24 hours at 37°C. The zone of inhibition for each disk was measured (mm) and analyzed based on criteria specific for the determination of antibiotic resistance and susceptibility.
Statistical analysis
Microsoft excel and Graph Pad Prism were used to calculate the mean and standard deviations. Graph Pad Prism produced the p-values and the ANOVA results. Microsoft excel was used to apply linear regression coefficients to the standard curves for the qPCR results of accA and luxs. The OriginPro 2019B software gave the descriptive statistics and generated paired-ttests results for the antibiotic resistance tests. A density graph was created through OriginPro 2019B for the antibiotic susceptibility of pHN1257-(+)asRNA for accA.
Results and Discussion
Glucose, RNA concentration and accA
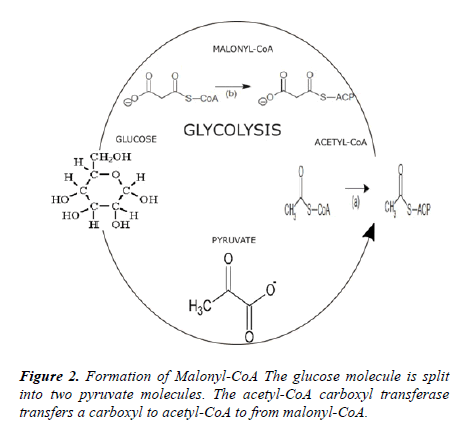
into pyruvate molecules. The ACCA subunit repositions a carboxyl from the pyruvate to the acetyl-CoA to generate malonyl-CoA as shown in Figure 2. The gene accA translates into the ACCA subunit of the ACCase enzyme. By increasing the concentration of glucose, the genetic activity of accA was augmented. For the H-Glucose, M-Glucose, the L-Glucose, and the control samples, the RNA concentrations ranged in a decreasing range from 1392, 797, 608, and to 199 ng/μL, respectively. Because the genes accA and accD are independent of the accBC operon, each gene can sustain overexpression without suppression [12]. Conversely, if the accB is overexpressed the activity at the accBC operon is terminated. Although increased glucose osmolarity inhibits accBC expression, the total yield of accA was not discontinued.
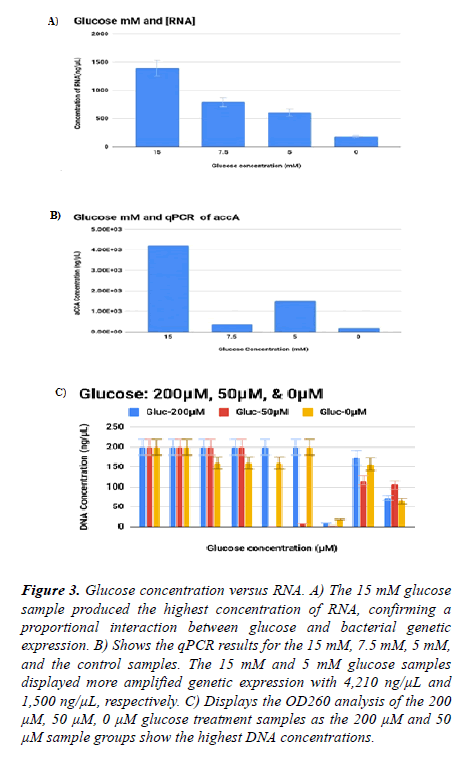
RNA was extracted from bacterial sample cultures enhanced with glucose (N=20, 335.4 ± 329.2) shown in Figures 3 and 4. The Real-Time PCR protocol was administered to measure the intensity of accA expression. The qPCR results for H-glucose, M-glucose, L-glucose, and for the control included: 4210, 375, 1500, and 196, respectively. A t-test of 200 μM contrasted to its control group showed a statistical significance with a pvalue of 0.038. The 200 μM and 50 μM sample produced more double-stranded DNA, or dsDNA, compared to the control. Standard curves were graphed in a logarithmic mode to provide the qPCR analysis for the absolute quantification of each treatment group. Ferrais et al. (1990) confirmed that concentrations of glucose in an animal’s diet, averaging 0.4-24 mM, delivered the maximum level of glucose uptake. However, there was a substantial reduction of glucose uptake at an osmolarity of 30 mM of glucose [13].
Figure 3. Glucose concentration versus RNA. A) The 15 mM glucose sample produced the highest concentration of RNA, confirming a proportional interaction between glucose and bacterial genetic expression. B) Shows the qPCR results for the 15 mM, 7.5 mM, 5 mM, and the control samples. The 15 mM and 5 mM glucose samples displayed more amplified genetic expression with 4,210 ng/µL and 1,500 ng/µL, respectively. C) Displays the OD260 analysis of the 200 µM, 50 µM, 0 µM glucose treatment samples as the 200 µM and 50 µM sample groups show the highest DNA concentrations.
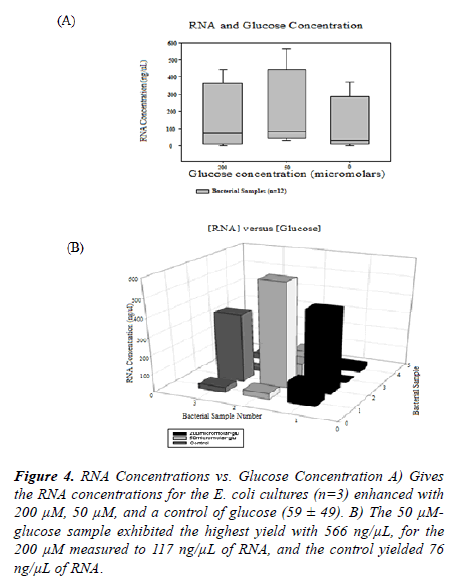
Figure 4. RNA Concentrations vs. Glucose Concentration A) Gives the RNA concentrations for the E. coli cultures (n=3) enhanced with 200 µM, 50 µM, and a control of glucose (59 ± 49). B) The 50 µMglucose sample exhibited the highest yield with 566 ng/µL, for the 200 µM measured to 117 ng/µL of RNA, and the control yielded 76 ng/µL of RNA.
Ferrais et al. used the Michaelis-Menten kinetic enzyme equation and found that high levels of hypertonic and hypotonic glucose lowered uptake glucose by a lowered Vmax value at a constant Km [13].
The conclusion for the antisense RNA inhibition of the target gene ACCA by qPCR
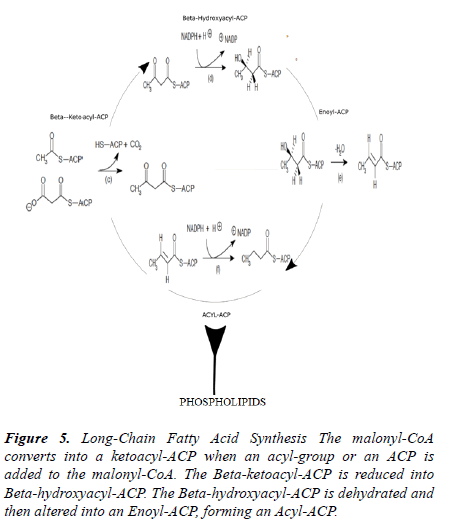
The ACCase complex and the ACCA transferase subunit conveys a carboxyl group to acetyl-CoA that forms malonyl- CoA shown in Figure 5. The PCR product of the antisense for accA was inset into the pHN1257 plasmid during ligation shown in Figure 1. The control ((-) asRNA) yielded 739.44 ng/μL compared to 279.28 ng/μL of the (+) asRNA expressing E. coli cells. The antisense inhibition of accA (n=3) limited its activity with only 63 ng/μL of qPCR gene copies (37.8 ± 35.6). The control gave an acquisition of 421.69 ng/μL for accA. A 147% difference between the control and the antisense treatment group was obtained (p=0.027). As a result, it seems inhibiting ACCase enzymatic activity can reduce lipid synthesis and elongation. The asRNA of accA can adjust the generation of fatty acids by its suppression of the ACCase enzyme.
Figure 5. Long-Chain Fatty Acid Synthesis The malonyl-CoA converts into a ketoacyl-ACP when an acyl-group or an ACP is added to the malonyl-CoA. The Beta-ketoacyl-ACP is reduced into Beta-hydroxyacyl-ACP. The Beta-hydroxyacyl-ACP is dehydrated and then altered into an Enoyl-ACP, forming an Acyl-ACP.
Quantification of LUX-S gene expression
The fatty acids produced create a more fluid membrane and the fluid membrane causes the biofilm to expand. As the biofilm expands the number of microcolonies of bacteria settle and grow proportionally to the size of the matrix. The microcolonies proliferate and induce a substantial release of autoinducers, displaying much bioluminescence. The bioluminescence is an indication of quorum sensing activity. The gene luxs assist with the production of AI-2. The quality of biofilm, flagellum mobility, and the release of virulent factors are all regulated by luxs. The ACCA subunit assist with the elongation of fatty acids needed for initiating the release of AI-2. The AI-2 molecules are released during quorum sensing.
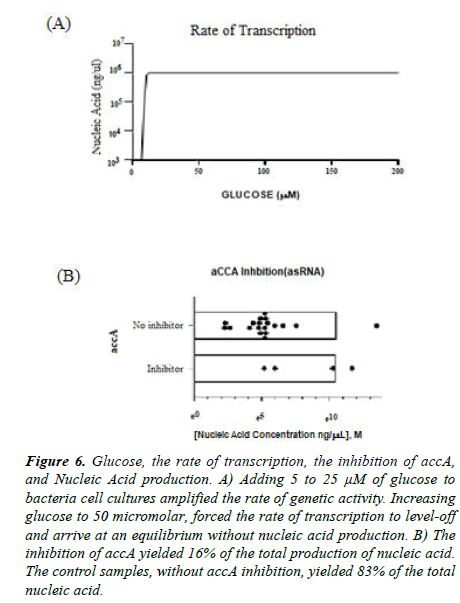
There is an important link between bacterial cell metabolism and pathogenesis. After inhibiting accA, the gene copies for luxs were measured, confirming a connection of the metabolism of microbials with virulence. Adding glucose amplified the rate of transcription shown in Figure 6. Liquid cultures of asRNA-accA pHN1257 vector expressing E. coli were enhanced with 25 μM and 5 μM of glucose. Samples with 5 μM glucose-(+)asRNA yielded a high qPCR product of luxs. Gene copies of 199 and 2511 for luxs were produced from (-) glucose-(+) asRNA and (-) glucose- (-)asRNA, respectively (n=3, 277 ± 37). The gene copies of luxs calculated to 39,810 and 316,228 gene copies for 25 μM glucose-(+)asRNA (n=3, 658,114 ± 483,499) and 5 μM glucose-(+)asRNA (n=3, 171571 ± 204574), respectively. The largest number of luxs gene copies produced included the control samples (n=3, 658,114 ± 483,499) and the 5 μM glucose- (+)asRNA (n=3, 171571 ± 204574) (p=0.025). Wang et al. added 0.8% of glucose to bacterial cultures and this triggered increased activity of luxs at its promoter site [14]. Jesudhansan et al. concluded that adding more glucose increased levels of AI-2 but cannot produce more gene expression of the luxs for the Salmonella typhimurium [15].
Figure 6. Glucose, the rate of transcription, the inhibition of accA, and Nucleic Acid production. A) Adding 5 to 25 µM of glucose to bacteria cell cultures amplified the rate of genetic activity. Increasing glucose to 50 micromolar, forced the rate of transcription to level-off and arrive at an equilibrium without nucleic acid production. B) The inhibition of accA yielded 16% of the total production of nucleic acid. The control samples, without accA inhibition, yielded 83% of the total nucleic acid.
The increased level of AI-2 released initiates all the bacterial cells to share quorum signals mimicking a positive feedback loop [16]. As the bacterial population increases, the AI-2 molecules accumulate in the external of the microbial environment [17]. The bacteria sense the level of population growth, count the capacity of the cells, and then transport signals for controlling genetic activity. The level of genetic expression is regulated through the process of quorum sensing. Quorum sensing involves the production of bioluminescence, antibiotic secretion, biofilm formation, spore development, and the release of virulent factors [17]. The biofilm allows for a more rapid mobility of pathogenic bacteria that spreads into a systemic infection of the blood circulation [18].
DNA concentrations, present in biofilms at large volumes, sustain a high quality of the extracellular matrix and the biofilm [19]. Pathogenic bacteria rely on biofilm production to attach to epithelial cells that assist with spreading the infection. As the AI-2 accumulates more bacteria can infect and produce more biofilm [20]. The increased genetic activity of luxs rapidly expresses the protein formation of the bacterial flagella that resides in the lungs and bloodstream during an infection. A mutation of luxs causes bacteria to quickly infect the lungs and the blood versus its wild type [21]. This increased activity of luxs allows for the transport and spread of pathogenic bacteria by accelerating its motility.
Increased amounts of biofilm are created as mutations of luxs lead to more luxs expression. The biofilm helps virulent bacteria to cross the mucosal layers into host epithelial cells, causing a development of a systemic infection. As the Lux-S/ AI-2 system is less regulated, pathogenic bacteria can continue to grow in environments with high concentrations of acids and salts [22]. Kendall et al. showed that S. pyogenes can survive in high acidic conditions because of a downregulated Lux-S/AI-2 system [23]. For example, Haemophilus influenza expresses a mutation of luxs that spreads the infection that causes middle ear infections [24]. Therefore, the data and results demonstrate that the production of luxs is essential for the development of biofilm that is a significant factor for virulence. Decreasing the glucose availability while inhibiting accA, reduced the genetic activity of luxs [25].
Antibiotic resistance test
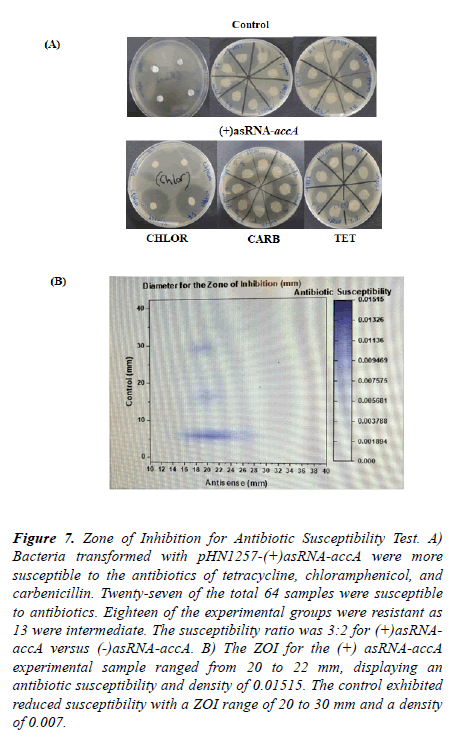
The antibiotic susceptibility of asRNA-accA expressing bacterial cells was tested. The experimental samples with asRNA-accA expression pHN1257 vectors displayed high levels of antibiotic susceptibility to tetracycline, chloramphenicol, and to carbenicillin Figure 7. The antibiotic disks (N=64) were placed onto bacterial lawns of agar plates. The median was 20 mm for the experimental groups (with asRNA-accA). The control groups showed more resistance to the antibiotics measuring an average Zone of Inhibition at 6 mm.
Figure 7. Zone of Inhibition for Antibiotic Susceptibility Test. A) Bacteria transformed with pHN1257-(+)asRNA-accA were more susceptible to the antibiotics of tetracycline, chloramphenicol, and carbenicillin. Twenty-seven of the total 64 samples were susceptible to antibiotics. Eighteen of the experimental groups were resistant as 13 were intermediate. The susceptibility ratio was 3:2 for (+)asRNAaccA versus (-)asRNA-accA. B) The ZOI for the (+) asRNA-accA experimental sample ranged from 20 to 22 mm, displaying an antibiotic susceptibility and density of 0.01515. The control exhibited reduced susceptibility with a ZOI range of 20 to 30 mm and a density of 0.007.
The pHN1257-(+)asRNA-accA transformants displayed more susceptibility to the tetracycline, carbenicillin, and to the chloramphenicol. The 59% of the (+)asRNA-accA samples were sensitive to the antibiotics (21 ± 4, SEM=0.7). The experimental groups exhibited an intermediate sensitivity at 28% and only 3% were antibiotic resistant (13.75 ± 10, SEM=1.8) [26,27].
Twenty-five percent of the control groups were antibiotic susceptible compared to 12.5% with intermediate susceptibility and to 53% for resistant. When comparing the experimental groups to the control groups, the experimental showed more antibiotic sensitivity with an 82% percent difference and change. The control yielded at 77% percent change of resistance compared to the treatment groups (p=0.000554). The paired t-test gave a t-score of 3 with 31 degrees of freedom. The DF and the t-score were combined to calculate the p-value [28,29].
S. Aureus expresses a lysine at the 164th loci, forming a resistance to the thailandamide antibiotic. This change in expression denatures the acetyl-CoA carboxylase enzyme that deactivates the ACCA subunit. Salmonellae also expresses this similar mutation of the accA gene [30]. Antibiotics called capistruin and malleilactone are newer, atypical, and synthetic antibiotics. These atypical antibiotics can inhibit the transformation of microbials. By blocking the bacterial transformation, additional antibiotics are released [31,32].
Conclusion
The role of accA was examined and a connection between bacterial cell metabolism and virulence was demonstrated. The decrease of RNA concentration and cell growth after inhibiting accA with asRNA can model a bacterial metabolic process that leads to less antibiotic resistance. After treating bacterial cells with asRNA for accA the qPCR results yielded 63 gene copies. A strong metabolic state of a population of bacteria can reduce antibiotic susceptibility. The hypothesis was confirmed by the data results. When decreasing the glucose and inhibiting accA, the expression of luxs decreased. The luxs is important for transporting quorum signals through the release of autoinducer-2. The amplified production of AI-2 transports quorum signals that then increases biofilm formation. The biofilm positions pathogenic bacteria to attach, grow in population, detach, and create a systemic infection. The bacteria that expressed asRNA of accA were more sensitive to tetracycline, carbenicillin, and to chloramphenicol. Therefore, targeting accA may serve to make bacteria more sensitive to antibiotics. In addition, commensal bacteria can interact with host cells of the GI tract by releasing a form of chemical communication. Chemical signals used for communication of bacteria with host cells includes the homoserine lactones. During an infection, the homoserine lactones released by bacteria interrelate the immune cells of the host.
When the accA is translated into the ACCA carboxyl transferase subunit, it allows for the transfer of a carboxyl from a pyruvate to the acetyl-CoA, forming malonyl-CoA. The increase in glucose amplifies the synthesis of ACCA and produces more AI-2 for quorum signaling. The elongation of long-chain fatty acids that is catalyzed by the malonyl-CoA enzyme form biofilm. By limiting the expression of ACCA, biofilm creation can be reduced. Zuberi et al. inhibited the luxs gene in E. coli cells, and they determined that biofilm formation heavily depended upon luxs expression.They used a crystal violet test to demonstrate the production of biofilm after inhibiting luxs. They found that biofilm creation was significantly reduced when inhibiting luxs. Researchers discovered a genetic source for the antibiotic resistance and sensitivity initiated by biofilms. The discovery and synthesis of antibiotics has drastically decreased over the past 30 years. Future research can be implemented to research the metabolic genes and enzymes that contribute to the production of the bacterial ACCase complex.
Acknowledgements
Special thanks are given to the staff from The LAB in Los Angeles, CA. Thank you to their staff. They were always available for answering questions and providing much guidance. Thank You!
Funding
The author received no specific funding for this work.
Conflicts of interest
The author declares no conflict of interest.
References
- Calero-Caceres W, Ye M, Balcazar JL. Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol. 2019.
- Lobanovska M, Pilla G. Focus: Drug Development: Penicillin’s Discovery and Antibiotic Resistance: Lessons for the Future? Yale J Biol Med. 2017; 90:135.
- Aminov R. History of antimicrobial drug discovery: Major classes and health impact. Biochemical pharmacology. 2017;133:4-19.
- Landecker H. Antibiotic resistance and the biology of history. Body & Society. 2016;22:19-52.
- Verstraeten N, Knapen W, Fauvart M, et al. A historical perspective on bacterial persistence. In Bacterial Persistence. Humana Press, New York, NY. 2016:3-13.
- Eichenberger EM, Thaden JT. Epidemiology and Mechanisms of Resistance of Extensively Drug Resistant Gram-Negative Bacteria. Antibiotics. 2019;8:37.
- Rojo-Molinero E, Macia MD, Oliver A. Social behavior of antibiotic-resistant mutants within Pseudomonas aeruginosa biofilm communities. Front Microbiol. 2019;10:570.
- Xu L, Li H, Vuong C, et al. Roleof the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. 14PeerJ reviewing PDF | (2018:12:33750: 1:2: NEW 8 Mar 2019) Manuscript to be reviewed Infect Immun. 2006;74:488-496.
- France MT, Cornea A, Kehlet-Delgado H, et al. Spatial structure facilitates the accumulation and persistence of antibiotic-resistant mutants in biofilms. Evol Appl. 2019;12:498-507.
- Peng B, Su YB, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21:249-262.
- Nakashima N, Tamura T. Conditional gene silencing of multiple genes with anti- senseRNAs and generation of a mutator strain of Escherichia coli. Nucleic Acids Res. 2009;37:103.
- James ES, Cronan JE. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J Biol Chem. 2004;279:2520-2527.
- Ferraris RP, Yasharpour S, Lloid KCK, et al. Luminal glucose concentration in the gut under normal conditions. Am J Physiol. 1990;259:820–837.
- Wang L, Hashimoto Y, Tsao CY, et al. Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli. J Bact. 2005;187:2066-2076.
- Jesudhasan PR, Cepeda ML, Widmer K, et al. Transcriptome analysis of genes controlled by luxS/autoinducer-2 in Salmonellaenterica serovar Typhimurium. Foodborne pathogens and disease. 2010;7:399-410.
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319-346.
- Rutherford ST, Bassler BL. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb Perspect Med. 2012;2: a012427.
- Soares A, Caron F, Etienne M. Commentary: tolerance and resistance of pseudomonas aeruginosa biofilms to antimicrobial agents–how p. Aeruginosa can escape antibiotics. Front Microbiol. 2019;10:2164.
- Trappetti C, Potter AJ, Paton AW, et al. LuxS mediatesiron- dependent biofilm formation, competence, and fratricide in Streptococcus pneumoniae. Infect Immun. 2011;79:4550-4558.
- Vidal JE, Howery KE, Ludewick HP, et al. Quorum- sensing systems LuxS/autoinducer 2 and Com regulate Streptococcus pneumoniae biofilms in a bioreactor with living cultures of human respiratory cells. Infect Immun. 2013;81:1341-1353.
- Vendeville A, Winzer K, Heurlier K, et al. Making' sense of metabolism: autoinducer-2, LuxS, and pathogenic bacteria. Nat Rev Microbiol. 2005;3:383.
- Siller M, Janapatla RP, Pirzada ZA, et al. Functional analysis of the group A streptococcal luxS/AI-2 system in metabolism, adaptation to stress, and interaction with host cells. BMC Microbiol. 2008;8:188.
- Kendall MM, Rasko DA, Sperandio V. Global effects of the cell-to-cell signaling molecule autoinducer-2, autoinducer-3, and epinephrine in a luxS mutant of enterohemorrhagic Escherichia coli. Infect Immun. 2007;75:4875-4884.
- Vidal JE, Ludewick HP, Kunkel RM, et al. The LuxS dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strainD39. Infect Immun. 2011;79:4050-4060.
- Wozniak CE, Lin Z, Schmidt EW, et al. Thailandamide, a fatty acid synthesis antibiotic that is coexpressed with a resistant target gene. Antimicrob Agents Chemother. 2018;62:e00463-18.
- Baquero F, Martínez JL. Interventions on metabolism: making antibiotic-susceptible bacteria. mBio 2017;8:e01950-17.
- Jayaraman A, Thomas KW. Bacterial quorum sensing: signals, circuits, and implications for biofilms and disease. Annu Rev Biomed Eng. 2008;10:145-167.
- Telford G, Wheeler D, Williams P, et al. The Pseudomonas aeruginosaquorum-sensing signal moleculen(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66:36-42.
- Wilson JW, Schurr MJ, LeBlanc CL, et al. Mechanisms of bacterial pathogenicity. Postgrad Med J. 2002;78:216-224.
- Zuberi A, Misba L, Khan AU. CRISPR interference (CRISPRi) inhibition of luxS gene expression in E. coli: an approach to inhibit biofilm. Front Cell Infect Microbiol. 2017;7:214.
- Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41:276-301.
- Polyak SW, Abell AD, Wilce MC, et al. Structure, function, and selective inhibition of bacterial acetyl-CoA carboxylase. Appl Microbiol Biotech. 2012;93:983-992.