Research Article - Biomedical Research (2017) Volume 28, Issue 10
Kinetic characterization of malate dehydrogenase in normal and malignant human breast tissues
Najme Sadat Abtahi1, Ali Shahriari1*, Abdolhassan Talaiezadeh2 and Payam Fathizadeh2
1Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran
2Cancer, Petroleum and Environmental Pollutants Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- *Corresponding Author:
- Ali Shahriari
Department of Biochemistry and Molecular Biology
Faculty of Veterinary Medicine
Shahid Chamran University of Ahvaz
Iran
Accepted date: February 10, 2017
Abstract
Malate Dehydrogenase (MDH), as a critical enzyme in cancer metabolism, can be the main foundation for NAD, along with the well-known LDH, which allows the prolonged process of glycolysis, even in the absence of oxygen. The kinetic characteristics of the enzyme are related to the surrounding environment of the enzyme. In this regard, the tumor microenvironment has noticeable properties, in connection with the neighboring normal tissues. The goal of this research is to elaborate the kinetic parameters of MDH in human breast cancer by taking into account two major perspectives as follows: 1. the possible role of MDH in supporting the NAD pool; 2. the impact of different tumor microenvironments on the kinetic characteristics of the enzyme. For this purpose, the MDH activity was measured from the normal and malignant crude human breast tissues, which were directly collected from the operating room. The Michaelis-Menten constant (Km) and the maximum velocity (Vmax) were determined in the crude extracts. It was realized that the affinity of cancerous MDH in the forward reaction was identical to the normal MDH, while the Vmax of cancerous MDH was higher than the normal MDH. In the reverse reaction, the affinity of cancerous MDH for malate and NAD+ was less than the normal MDH. The increasing affinity of MDH for malate and the decreasing MDH activity and expression in the forward reaction may be an acceptable molecular target for eliminating its possible impact on tumorigenesis. Therefore, the kinetic characteristics of MDH can be demonstrated as a novel diagnostic parameter for human breast cancers. However, further research seems necessary to validate this proposition. In addition, various kinetic parameters of different cancer cell lines should be investigated in the culture conditions, where both oxygen and nutrients are limited, similar to the tumor microenvironment.
Keywords
MDH, Tumor, Metabolism, NAD, Km
Introduction
High aerobic glycolysis is one of the most significant characteristics of cancer cells, which is known as the Warburg effect [1,2]. This bioenergetic and metabolic characteristic, while allowing the cancer cells to continue to exist in unfavorable tumorous conditions (like hypoxia), assists them to grow and invade, and can lead to subsequent distant metastasis [3]. The control point of glycolysis is glyceraldehyde-P-dehydrogenase, which demands NAD in the glycolytic direction. Cancer cells supply the required NAD via Lactate Dehydrogenase (LDH) that converts pyruvate to lactate, along with the concomitant regeneration of NAD, which is a noticeable reaction in cancer cells. In addition, the gene expression and the activity of LDH are higher in different types of tumors [4-6]. On the other side, there is another approach that might provide the needed NAD for glycolysis; Malate Dehydrogenase (MDH). MDH as a part of the malate-aspartate shuttle catalyses the reverse reaction of Oxaloacetate (OAA) to malate in the presence of NADH. The enzyme has two distinctive isoforms: 1. Mitochondrial; 2. Cytosolic. The later isoform is engaged in the oxidation of NADH to NAD, which can be expressed as another pool of NAD for the continuous glycolysis progression [7].
The environmental parameters (such as pH, oxygen, and nutrient availability) can impact on the enzyme kinetics through different procedures [8]. The tumor microenvironment has a highly heterogeneous oxygen pressure and pH distribution, and other metabolites are also available [9]. However, the nature and substantial influence of the tumor environment on the enzyme kinetics have not been amply emphasized in enzyme studies. It might be because of the cell culture conditions, in which the pH is located in the normal range without any variation and also, there is always an excessive level of oxygen and nutrients. Moreover, the alterations of the enzyme kinetics in the tumor microenvironment, which can transform the intrinsic characteristics of the enzyme, have been inadequately investigated. Therefore, the objective of this research is to compare the kinetic parameters of MDH in breast cancer and normal mammary tissues with respect to two focal points as follows: 1. The possible role of MDH in providing NAD; 2. The challenging tumor microenvironment that engages the enzyme, since the enzyme function and kinetics can be extensively affected by the composition of the intracellular milieu, where the enzyme operates.
Material and Methods
Clinical sample collection
The human breast tumor samples were acquired from Apadana Hospital in the city of Ahvaz, Iran, during a mastectomy. The normal tissues, away from the tumor, were used as controls. Two independent expert pathologists from the pathological laboratory of Apadana hospital performed the pathological examination of the tumor tissue. The samples were immediately placed in liquid nitrogen and then, transported to the laboratory and stored at-80 °C. The process of the study was completely approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences. The investigations were carried out according to the Guide for Human Study by the National Academy of Sciences (National Institutes of Health), and informed consent was obtained from all patients involved in the survey.
Sample preparation
The frozen normal and malignant tissues were homogenized (1:5 w:v) in ice-cold homogenization buffer (20 mM Tris-HCl, pH 8.0, 10 mM 2-mercaptoethanol, 10% v:v glycerol, 2 mM EDTA, 2 mM EGTA, and 20 mM β-glycerophosphate) and a few crystals of Phenylmethylsulfonyl Fluoride (PMSF) were added during the homogenization. The samples were homogenized using a Miccra homogenizer (Miccra, Germany), centrifuged for 30 min at 15,000 g at 4°C, and the supernatant was transferred and kept on ice until use. The low-molecular-weight metabolites and ions were separated from the supernatant by the Sephadex G-25 columns (1 × 5 cm) (Sigma, Germany), equilibrated in the homogenizing buffer. Afterward, the samples were pooled and maintained at 4°C until use for the subsequent kinetic characterization of MDH.
Enzyme assay and kinetic parameters
The MDH activity was measured in the presence of oxaloacetate with NADH (as the substrates for the forward reaction), and malate with NAD+ (as the substrates for the reverse reaction). The lowest concentration of each substrate, which indicated the maximum velocity, the constant rate of product formation, and the linear regressions of activities for serial dilutions of the enzyme, was determined as the optimal substrate concentration.
The reactions were started by adding 10 μl of the purified enzyme to a 200 μl total reaction volume and by using 20 mM Tris-HCl buffer (pH 8) in a microplate well. The process was watched at 340 nm to examine the conversion of NADH to NAD+ (or vice versa) with the aid of a Biotek PowerWave XS microplate reader (Biotek, USA) and Gen5 software (version 2.0, USA) (kinetic mode, reading interval=39 s). Next, the enzyme activity was revealed as n moles of oxaloacetate or malate formed/min for the reverse and forward reactions, respectively.
The data were analysed by means of Microplate Analysis (MPA) and Kinetics computer programs 3.51 [10,11]. For this purpose, Kinetics computer program adjusted the data through a nonlinear least-squares regression for the determination of Km (substrate concentration providing the half-maximal activity; Michaelis-Menten constant) and Vmax (maximum velocity).
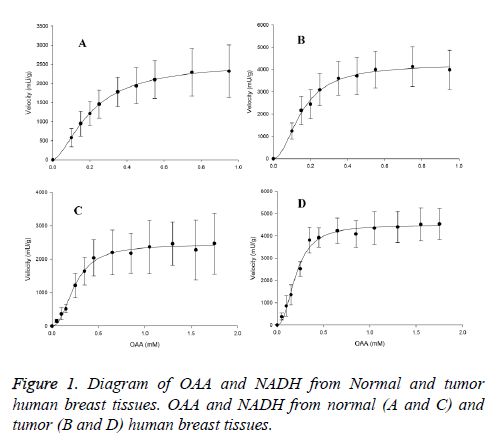
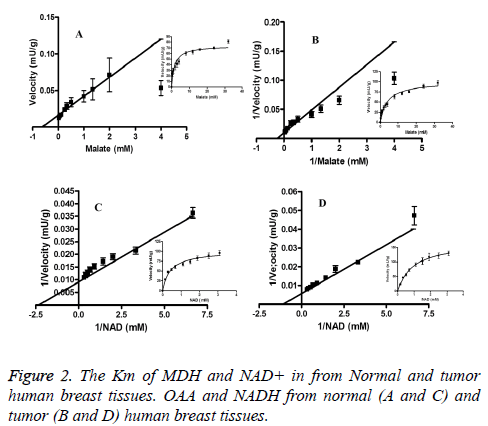
The Km of OAA was obtained at 0.5 mM NADH and OAA concentrations, which varied from 0.05 to 1.75 mM. The Km of NADH was achieved at 1.5 mM OAA and NADH concentrations, ranging from 0.1 to 0.95 mM in both tissues. The Km of malate was gained at 2.5 mM NAD+ and malate concentrations, varying from 0.25-32 mM, the Km of NAD+ was obtained at 24 mM malate and NAD+ concentrations, which ranged from 0.1 to 3.1 mM in both tissues.
The Km and Vmax were calculated from the average of the three distinct series of determinations. The total protein content was measured using the Bradford method and bovine serum albumin as standard.
With respect to the likely existence of the endogenous interconversion of NADH to NAD+ (i.e., NADH oxidation by complex І activity) in partially-purified samples, the interconversion of NADH to NAD+ in each sample was studied in order to eliminate the possible existence of its influence. This interconversion was realized by adding NADH (0.5-1 mM) or NAD+ (3-5 mM) in the Blue-Sepharose-purified samples and observing the absorbance alterations at 340 nm.
Statistical analysis
The data were expressed as mean ± SEM from the independent determinations on the separate preparations of the enzyme. The data were analysed with the aid of the Student’s t-test and the significance level of all tests was adjusted at p<0.05.
Results
Optimization of the experimental conditions
The optimal assay conditions for MDH in the forward reaction were 1.5 mM OAA and 0.5 mM NADH in both tumor and normal tissues. In the reverse reaction, the optimal conditions were 24 mM malate and 2.5 mM NAD+. In addition, it should be noticed that no NADH-to-NAD+ interconversion activity (or vice versa) in the crude extract samples was observed.
Kinetic properties of MDH in forward reaction
The maximal activity of Cancerous-MDH (C-MDH) for malate formation (4509.8 ± 88 mU/g for OAA and 4233.3 ± 111 mU/g for NADH) was about two fold higher than the values in normal tissues (N-MDH) (2456 ± 46 mU/g for OAA and 2549 ± 37 mU/g for NADH) (Table 1). The enzyme in forward reaction in both tissues displayed sigmoidal kinetics with respect to OAA and NADH (Figure 1).
| Kinetic parameters | Groups | |
|---|---|---|
| Tumor | Normal | |
| S 0.5 OAA (mM) | 0.2 ± 0.009 | 0.23 ± 0.007 |
| S 0.5 NADH (mM) | 0.16 ± 0.007 | 0.2 ± 0.005 |
| Vmax OAA (mU/g) | 4509.8 ± 88* | 2456 ± 46 |
| Vmax NADH (mU/g) | 4233.3 ± 111* | 2549 ± 37 |
| Assays were conducted at 25°C and data are presented as means ± SEM, n=3 independent determinations on each of tumor and normal samples.*Significant difference in each row at p<0.05. | ||
Table 1: Kinetic parameters of MDH in forward reaction from breast tumors) and normal tissues.
The Hill coefficients for OAA and NADH in tumor samples were 2.3 ± 0.22 and 2 ± 0.19, respectively. Furthermore, in normal samples, they were 2.22 ± 0.17 and 1.7 ± 0.06, respectively. However, the S0.5 of OAA (0.2 ± 0.009 mM for tumor and 0.23 ± 0.007 mM for normal samples) and NADH (0.16 ± 0.007 mM for tumor and 0.2 ± 0.0051 mM for normal samples) were significantly indifferent among the normal and cancerous tissues (Table 1).
Kinetic properties of MDH in reverse reaction
The highest activity of MDH for malate (78 ± 2.13 mU/g in tumor and 75 ± 2.7 U/g in normal tissues) and NAD (110 ± 3.32 mU/g and 102 ± 4.4 mU/g) were not meaningfully different between normal and cancerous tissues (Table 2). The Km for malate of N-MDH (3.13 ± 0.4 mM) was significantly greater than of C-MDH (1.6 ± 0.2 mM) (p<0.05) (Table 2). The Km of NAD+ in normal tissues (0.43 ± 0.06 mM) was considerably less than in tumor tissues (0.93 ± 0.17 mM) (p<0.05) (Figure 2).
| Kinetic parameters | Groups | |
|---|---|---|
| Tumor | Normal | |
| Km malate (mM) | 3.13 ± 0.4* | 1.6 ± 0.2 |
| Km NAD+ (mM) | 0.63 ± 0.05* | 0.43 ± 0.06 |
| Vmax malate (mU/g) | 78 ± 2.13 | 75 ± 2.7 |
| Vmax NAD+ (mU/g) | 110 ± 3.32 | 102 ± 4.4 |
| Assays were conducted at 25°C and data are presented as means ± SEM, n=3 independent determinations on each of tumor and normal samples.*Significant difference in each row at p<0.05. | ||
Table 2: Kinetic parameters of MDH in reverse reaction from breast tumors and normal tissues.
Discussion
The enzyme rate is generally controlled and adjusted via two distinct processes: 1. Changing the total amount of the enzymes present (i.e., changing the Vmax); 2. Changing one or more kinetic constants (Km or Vmax). These processes and the enzyme stability can be significantly affected by the composition of the intracellular milieu, where the enzyme operates [8]. Considering the dissimilar microenvironmental conditions of the normal and malignant tissues, the enzyme function might be different between these tissues. The obtained data confirmed this theory and demonstrated that the kinetics of MDH, as one of the important enzymes in the metabolism, has special features in tumors.
According to some arguments, it can be presumed that the maximum activity recorded by us was mainly because of the cytosolic fraction. In fact, the highest concentration of OAA, applied in the assay method (i.e., 1.5 mM), was well above 0.04 mM, used to inhibit the activity of the mitochondrial fraction [12]. Furthermore, in order to manifest that our samples were free of the mitochondrial enzymes, the supernatant created from specimens was studied for NADH oxidase activity as the mitochondrial marker and the disruption of dehydrogenase. The findings disclosed that there was not any NADH oxidase activity and consequently, the supernatant can be displayed free of the mitochondrial particles. Finally, the centrifugation speed was applied based on the methods, performed in other similar studies, and the mitochondrial particles in the pellet (not in the supernatant) were divided [13-15].
The achieved data signified that in the forward reaction, the highest activity of MDH was higher in tumors compared to normal tissues. This finding is well in compliance with the proposed hypothesis on the role of MDH in supporting the NAD pool. Based on the obtained results, the needed NAD for the constant flow of glycolysis can be supplied by the MDH activity along with the high LDH activity. One of the major and unavoidable necessities for the development of the Warburg effect is the NAD replacement. In this regard, the high LDH expression and activity can supply the essential NAD. However, the results exhibited that there is another potential approach, which is MDH.
Recently, several studies have been carried out to eliminate the LDH activity and expression, and to remove its influence on supporting the glycolysis pathway [16,17], This has been done by considering the possible role of MDH, associated with glycolysis, and removing the MDH activity and expression, along with LDH, to halt the glycolysis pathway. However, these achievements are contrary to the Balinisky et al.’s findings, where the cytosolic MDH presented similar activity in tumor and normal breast tissues and there was no significant difference between tissues [11]. This dissimilarity between these two surveys might refer to the diverse methods employed.
Moreover, the gained data on the Km of the forward reaction were in contradiction with the results of Grisham et al. which is one of the main studies on the kinetics of MDH in tumors. The obtained results demonstrated that the forward reaction obeys the Hill equation and both tissues have approximately the same S 0.5. However, Grisham et al. stated that the Km of malate is higher in tumors compared to normal tissues. Thus, the difference between two studies can be concluded by considering three viewpoints as follows;
First, Grisham et al. [15] used 0.2 mM and 2 mM OAA in normal and tumor tissues (respectively) as the highest concentration of OAA, whereas in the current study, the highest OAA concentration in both tissues was 1.5 mM. The obtained findings revealed that the concentrations above 1.5 mM have an inhibitory effect on the MDH activity and 1.5 mM was the optimal concentration for both tissues. As a result, it was presumed that the reaction reached the apparent Vmax. It seems that Grisham and his colleagues have not optimized the reaction, because the highest OAA concentration was applied at a very low level in normal species (0.2 mM), which can affect the real kinetic results. The other reason can be associated with the NADH concentration, used in these two studies. In the present study, the NADH concentration was 0.5 mM, while in the Grisham et al.’s work, it was 0.14 mM. A higher concentration of NADH can influence the kinetics of MDH by changing the reaction curve shape from hyperbolic (Michaelis-menten) to sigmoidal (Hill equation) (the data not presented). The last reason can be connected to the type of the curve, applied in the Grisham et al.’s study. They employed a Lineweaver-Burk plot, but plot drawing was associated with an essential problem. The line drawn in the plot was not straight and did not include all points, while a correct curve must have a straight line and consists almost all of the points in the plot.
In the reverse reaction, the Km of malate in tumors was higher compared to normal tissues; i.e., the affinity of MDH for malate was lower in tumors. The role of malate in cell metabolism can be regarded for the lower affinity of malate in tumors. The malate generated from OAA in cytosol may have three possible fates as follows: 1. Conversion to OAA in the reverse reaction by the cytosolic MDH; 2. Decarboxylation to pyruvate by NADP-ME in the cytosol, crossing the mitochondria, and then, conversion to OAA by the mitochondrial MDH; 3. Oxidization to pyruvate and CO2 via an intra-mitochondrial NAD(P)-ME. Which of the three possible ways can correctly interpret the lower affinity of malate in tumors?
The first approach could not properly explain the higher Km of malate, since our data showed that the affinity of the reverse reaction in tumors was lower compared to normal tissues. However, the second pathway could appropriately explain the obtained results. One of the well-known characteristics of metabolism in cancer cells is related to the Warburg effect. For the first time, Warburg demonstrated that cancer cells possess a high rate of glycolysis, even in the presence of sufficient oxygen. Since then, numerous other investigations have confirmed the Warburg effect in various types of cancer cells. It has been also suggested that the Warburg effect can induce many other benefits for cancer cells [18]. The control point of glycolysis is glyceraldehyde-P-dehydrogenase that needs NAD in the glycolytic condition. In cancer cells, the NAD essentially comes via lactate dehydrogenase that converts pyruvate to lactate, which is a prominent reaction in cancer cells.
According to the obtained results, another approach for providing NAD for a high rate of glycolysis can be malate dehydrogenase activity in the reverse reaction. The high Km of malate in the reverse reaction implied that cancer cells do not intend to change malate to OAA and consume NAD; instead, they aim to keep NAD for providing the needed NAD for glycolysis. The low affinity of MDH for malate may also increase the pool of malate in the cytosol. This malate can be converted to pyruvate by Malic Enzyme (ME) and then, to lactate by LDH. Consequently, it can be a source for lactate production in cancer cells for providing NAD and lactate, which are both highly effective in tumorigenesis and metastasis. This understanding on the fate of malate in cancer cells is extremely likely, since the ME and LDH activity and expression are high in tumors [14] Thus, MDH in the reverse reaction, by maintaining malate, can play an indirect role in providing abundant NAD for supporting the glycolysis rate. Mitochondrial MDH and ME, whose expression and activity are higher in tumors compared to normal tissues [5,14], can also apply malate, which is preserved by MDH. This would be the third fate of malate as mentioned previously. Therefore, MDH by keeping malate reveals a dual role in supporting the glycolysis pathway and the mitochondrial function.
Logic behind the low tendency for malate in tumors can be uncovered by considering malate as an effective metabolite that impact on gene expression. Recently, a number of studies have concentrated on the relationship between metabolites and gene expression [19]. One of these surveys claimed that pyruvate, as a critical metabolite, can induce apoptosis by inhibiting histone deacetylase activity [20], Such a role might be also found in other metabolites like malate, since based on the Darwinian process, any feature in the population can provide an advantage for them. Hence, in order to maintain malate and avoid converting to OAA, some benefits should be granted for cancer cells, one of which may be related to providing NAD for a high rate of glycolysis, as elucidated above. Another gain may be accompanied with the role in gene expression. The noteworthy point about the later hypothesis is related to the role of malate dehydrogenase in the regulation of p53 transcriptional activity in response to metabolic stress [21].
Furthermore, the higher Km of malate in cancerous MDH displayed that cancerous MDH is less inhibited by the high concentration of malate and could consequently tolerate higher malate levels. This feature of the cancerous MDH may probably reflect the high concentration of malate in the tumor microenvironment.
The kinetic variations expressed above may suggest that MDH from both normal and tumor tissues can be seen in distinct structural conditions and may be associated with different conditions of the tumor microenvironment. As stated earlier in this paper, the conditions of the milieu where the enzyme operates can influence on the enzyme kinetics. Therefore, different conditions of the tumor microenvironment can play remarkable roles for the distinct kinetic properties of the cancerous MDH, which was observed in this study.
Conclusion
Overall, it is clear that the hypothesis elaborated in this paper about the possible role of malate dehydrogenase in providing NAD and the effect of malate on gene expression must remain unanswered, until the enzyme is well purified and fully characterized and the effect of malate is completely checked on gene regulators (such as HDAC, p53, and CDKs). In this regard, various kinetic properties of MDH in tumors can be the beneficial diagnostic means, which need to be examined among more patients with/without metastasis to illuminate the possible correlation with the metastasis incidence.
Acknowledgements
This research was financially supported by Shahid Chamran university research council.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646-674.
- Warburg O. On the origin of cancer cells. Science 1956; 123: 309-314.
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4: 891-899.
- Koukourakis MI, Kontomanolis E, Giatromanolaki A, Sivridis E, Liberis V. Serum and tissue LDH levels in patients with breast/gynaecological cancer and benign diseases. Gynecol Obstetr Investig 2008; 67: 162-168.
- Balinsky D, Platz CE, Lewis JW. Isozyme patterns of normal, benign, and malignant human breast tissues. Cancer Res 1983; 43: 5895-5901.
- Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumor Biology 2013; 34: 1523-1530.
- Murray RK, Granner DK, Mayes PA, Rodwell VW. Harpers illustrated biochemistry. McGraw-Hill 2014.
- Storey KB BP. Principles of medical biology. Cell Chem Physiol 1995; 147-169.
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 2007; 101: 937-949.
- Brooks S. A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 1992; 13: 906-911.
- Brooks SP. A program for analysing enzyme rate data obtained from a microplate reader. Biotechniques 1994; 17: 1154-1161.
- Belfiore F, Borzi V, Vecchio LL, Napoli E, Rabuazzo AM. Enzyme activities of NADPH-forming metabolic pathways in normal and leukemic leukocytes. Clinical Chemistry 1975; 21: 880-883.
- Zelewski M, Swierczynski J. Malic enzyme in human liver. Intracellular distribution, purification and properties of cytosolic isozyme. Eur J Biochem 1991; 201: 339-345.
- Jiang P, Du W, Mancuso A, Wellen KE, Yang X. Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature 2013; 493: 689-693.
- Grisham M, Bernstein L, Everse J. The cytoplasmic malate dehydrogenase in neoplastic tissues; presence of a novel isoenzyme. Br J Cancer 1983; 47: 727.
- Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Molecular Cancer 2010; 9: 1.
- Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol 2011; 2: 49.
- DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med 2008; 10: 767-777.
- Zelezniak A, Sheridan S, Patil KR. Contribution of network connectivity in determining the relationship between gene expression and metabolite concentration changes. PLoS Comput Biol 2014; 10: 1003572.
- Palmnas MS, Vogel HJ. The future of NMR metabolomics in cancer therapy: towards personalizing treatment and developing targeted drugs. Metabolites 2013; 3: 373-396.
- Lee S, Kim J, Cho E, Youn H. A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differentiation 2009; 16: 738-748.