Research Article - Environmental Risk Assessment and Remediation (2025) Volume 9, Issue 1
Is the adsorption of crude oil on clays relevant to free swelling index?.
Kazem Rezaie Sadr*, Ahmad Heidari
Department of Soil Science, University of Tehran, Karaj, Iran
*Corresponding Author:
- Kazem Rezaie Sadr
Department of Soil Science, University of Tehran, Karaj, Iran
E-mail: k1291373@gmail.com
Received: 26-Aug-2023, Manuscript No. AAERAR-23-111301; Editor assigned: 28-Aug-2023, 111301 (PQ); Reviewed: 11-Sept-2023, QC No. 111301; Revised: 20-Jan-2025, Manuscript No. 111301 (R); Published: 27-Jan-2025, DOI: 10.35841/2529-8046.9.1.200
Citation: Sadr KR, Heidari A. Is the adsorption of crude oil on clays relevant to free swelling index?. Environ Risk Assess Remediat. 2025;9(1):1-7.
Keywords
Free swelling index, Crude oil, Bentonite, Electrolytic conductivity, Particle size distribution.
Introduction
Depending on its origin, natural bentonite composed of mineral crystals of >70% smectite, and <30 other minerals such as quartz, cristobalite, feldspar, zeolite, and kaolinite commonly arranged in stacks of several unit layers and contain mixture of several types of exchangeable cation and is mainly high in negative charge and is neutralized by calcium, magnesium, potassium and sodium, which causes bentonite to disperse in water.
Two mechanisms
• The crystalline swelling, increases the distance between the plates inside each particle,
• The diffuse double-layer swelling or osmotic swelling that increases the distance between the particle layer, accounts for Free Swelling Index in montmorillonitic clays exposed to water or electrolytes.
Two scales of the macroscopy and microscopy form swelling, adsorption and repulsion interactions of bentonite. On a macroscopic scale, swelling of bentonite can be described by water adsorption as its apparent volume. whereas, because of multifaceted structure of bentonite and its sensitivity to water and exchangeable intermolecular cation and surface forces such as; van der waals bonds, ion-dipole force, hydrogen bond, covalent bond, bilayer repulsion and ion correlation adsorption, the mechanism of microscopic scale is somewhat complicated [1].
Due to its low hydraulic conductivity and high capability to fix organic and metal contaminants, bentonite is widely applicable as clay liner in landfills to preclude the migration of contaminants to the underground water. The various exchange sites on the surface of minerals and water molecules around ionic sites are active sites that express possible reactions between organic molecules and clay surfaces [2]. Crude oil adsorbed in clay minerals consists of two parts: Polar compounds that bind to clay minerals through hydrogen bonding and compounds that are chemically attached to the mineral surface.
During the gas well drilling and exploitation of oil resources, for various reasons, some crude oil is poured into the site around crude oil wells called the fire pit and incinerated (Figure 1). These wastes pose many environmental hazards; including groundwater pollution, fire hazards, and air pollution in the area. Many efforts have been made to solve this problem via artificial products but their success rate has been relatively limited [3]. To prudently manage and control this environmentally problem, there should be found a naturally solution. Applying bentonite clay is a practical way to curb crude oil from polluting the soil and underground water. Forasmuch as there are various bentonites originated from different places, different physicochemical properties are expected for them. Nevertheless, in this research, this query, “Is the adsorption of crude oil on clays relevant to free swelling index” is answered. Finally the results of this study will be implemented for the design of a natural linear.
Materials and Methods
Materials
Three types of bentonite from different mines located in Tehran, Semnan, and Zanjan provinces (Iran) were selected for experiments [4]. The bentonites were ground and passed through a 2 mm sieve. Table 2 represents the physical and chemical properties of the experimental bentonites. The crude oil was provided from the Maroon 1-6 oil refinery, Khuzestan Province, Iran. Table 1 summarizes some physical and chemical characteristics of the crude oil used [5].
| Specification | Result | Test method |
|---|---|---|
| Specific gravity | 0.8657 | ASTM D 4052 |
| API (American Petroleum Index) | 32.56 | ASTM D 1298 |
| Water content Vol.% | <0.05 | ASTM D 4006 |
| Kinematic viscosity at 10°C mm2/Sec | 16.01 | ASTM D 445 |
| Kinematic viscosity at 20°C mm2/Sec | 10.49 | ASTM D 445 |
| Kinematic viscosity at 40°C mm2/Sec | 6.019 | ASTM D 445 |
| Asphaltenes wt.% | 1.65 | IP 143 |
| Wax content wt.% | 5.4 | BP 237 |
| Drop melting point of wax °C | 56 | IP 133 |
| Carbon residue conradson wt.% | 4.25 | ASTM D 189 |
| Acidity, total mg KOH/gr | 0.15 | UOP 565 |
Table 1. Some characteristics of the crude oil used.
| Physical and chemical properties | Unit | Semnan | Zanjan | Tehran |
|---|---|---|---|---|
| Acidity (pH) | - | 7.61 | 7.63 | 8.01 |
| EC | dS/m | 14.7 | 23 | 4.19 |
| CEC | cmol+/kg | 77.64 | 84.36 | 91.25 |
| Calcium Exchangeable | cmol+/kg | 24.8 | 33.38 | 19.3 |
| Exchangeable magnesium | cmol+/kg | 10.98 | 16.33 | 11.06 |
| Exchangeable sodium | cmol+/kg | 15.85 | 41.83 | 40.44 |
| Exchangeable potassium | cmol+/kg | 13.46 | 5.52 | 44.26 |
| Clay | % | 40 | 43 | 68 |
| Fine silt | % | 23 | 18 | 17 |
| Coarse silt | % | 5 | 9 | 3 |
| Fine sand | % | 27 | 26 | 12 |
| Medium sand | % | 5 | 2 | 0 |
| Coarse sand | % | 0 | 2 | 0 |
| Crude oil adsorption ratio | g/g bentonite | 0.32 | 0.35 | 0.71 |
| Free swelling index | % | 35 | 50 | 125 |
| Sodium adsorption ratio | - | 6.19 | 6.77 | 10.39 |
Table 2. Physical and chemical properties of Tehran, Semnan, Zanjan bentonite samples.
Methods
Physical and chemical measurements: The Electrical Conductivity (EC) of the samples and their reactions (pH) were determined using a combined conductivity and pH meter instrument. Cation Exchange Capacity (CEC) was determined using Bower method [6]. Exchangeable cations were determined in the extracts prepared using ammonium acetate as the extracting agent, and subtraction of the soluble content of cations from the measured values. Calcium and magnesium cations were measured using a complexo-metric titration method and sodium and potassium cations were measured using a flame photometer. Particle Size Distribution (PSD) was determined by hydrometer method in 16 readings over 24 hours. In order to determine the free swelling index of the bentonite samples 20 ml of bentonite was poured into a graded cylinder containing 100 ml of water, and it took one week to reach their maximum expansion in equilibrium with the water [7]. The increase in the volume of the bentonite at equilibrium with water was compared to its primary volume in the cylinder, and then considered free swelling index.
Adsorption of crude oil on bentonite: For determination of crude oil adsorption by each bentonite sample three replicates of 2.5 g of bentonite, were weighed and transferred into centrifuge tubes. Then 12.5 g of crude oil (5 times of bentonite) was added to each tube and after thoroughly mixing, was shaken for 48 hours to reach its maximum adsorption rate. The samples were then centrifuged and the supernatant liquid was discarded. Then the tubes were dried in an oven at 40°C temperature and weighed. The difference between the samples final weight to their primary weights was considered as crude oil adsorption ratio on bentonites [8].
X-Ray Diffraction analysis (XRD): The bentonites mineralogical composition and relative amounts of their components were determined using powder X-Ray Diffraction analysis (XRD) and Cu Kα radiation in 2θ between 4° and 40° [9].
Results and Discussion
Given in Table 1 the primary differences between the bentonite types are differences in EC, CEC, pH, PSD, crude oil adsorption ratio, free swelling index, sodium adsorption ratio and their dominant cations [10]. Bentonite from Zanjan province has the highest EC (23 dS/m), the sample from the Semnan province shows an intermediate EC (14.7 dS/m) and the sample from Tehran province has the lowest EC (4.9 dS/m). The acidity of the samples varies from 7.61 (the Semnan sample) to 8.01 (the Tehran sample). The range of Cation Exchange Capacity (CEC) of the samples varies between 77.64 (Semnan sample) to 91 cmol+/kg (Tehran sample). The difference in the dominant cations at the bentonite exchange sites is another important characteristic of the bentonites demonstrating that Semnan sample is Ca-dominated bentonite, whereas, Tehran sample is Na-dominated bentonite, and Zanjan sample is of calcium and sodium [11].
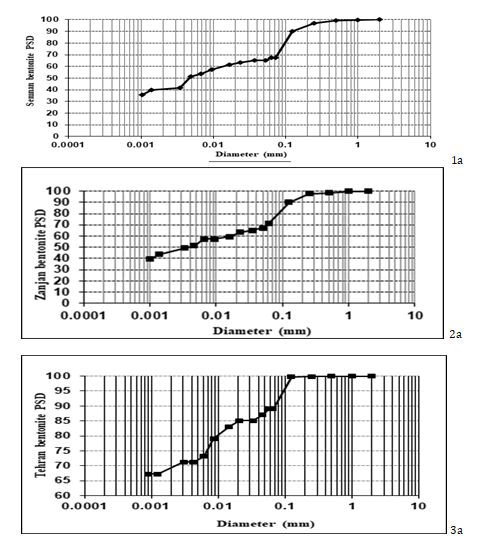
Particle Size Distribution (PSD)
As shown in Figure 2, PSD of the samples obtained from Semnan (Figure 2a) and Zanjan (Figure 2b) mines hold more or less similar size distribution about 35-40% clay-sized (<0.002 mm), 13-23 % fine silt (0.002 to 0.02 mm), and (5-10 %) coarse silt (0.02 to 0.05 mm), 27% fine sand (0.05 to 0.2 mm), and 2-5% medium sand (0.2 to 0.6 mm) particles. however the sample obtained from Tehran mine (Figure 2c) contains (68%) clay-sized (<0.002 mm), (17%) fine silt-sized (0.002 to 0.006 mm), (3 %( coarse silt (0.02 to 0.05 mm) and (12%) fine sand (0.06 to 0.2 mm) particles.
The results showed that in the studied bentonites, clay-sized particles (<0.002 mm) was the main component. There was a slight difference in the percentage of clay-sized particles (<0.002 mm) in the bentonite samples obtained from Zanjan (43%) and Semnan (40%). Clay-sized particles (<0.002 mm) in Tehran sample was 68% which causes significant increase in its surface area compared to the Semnan and Zanjan samples [12].
The Particle Size Distribution (PSD) showed that in Semnan and Zanjan samples the percentage of fine sand-sized particles (0.06 to 0.2 mm), was about 30%, however, fine sand in the Tehran sample comprises 12% of total PSD which was intrinsically effective in increasing the adsorption capacity of Tehran bentonite. Moreover, the percentage of silt-sized particles (0.002 to 0.06 mm) in the Semnan and Zanjan samples was about 27%, however, in the Tehran sample, it was 20. As the size of particles increase, their specific surface area decrease.
Mineralogy of bentonites
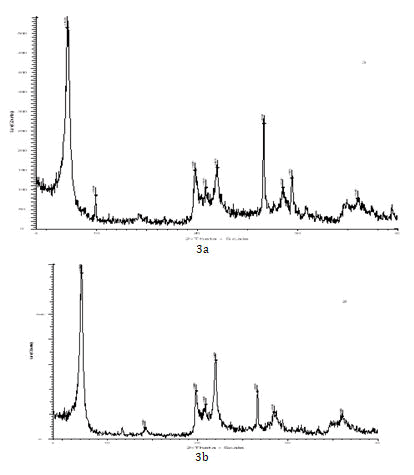
X-ray diffraction analysis was performed to identify the main minerals forming the three bentonites. To ascertain the obtained results, the intensity of the peaks of the identified minerals was checked using the mineralogy database [13].
Given in Figure 3a, there are distinct peaks in the areas of 12.3 Å (160 counts per second) and 3.54 Å (140 counts per second), indicating saponite, with the following chemical composition ((Ca/2,Na)0?3(Mg,Fe++)3(Si,Al)4O10(OH)2•4(H2O)), is the primary expandable mineral in the Semnan bentonite. However, the largest peak in Figure 3a is 4.05 Å, indicating cristobalite (a type of quartz) as the dominant mineral. The presence of a peak of 8.03 Å indicates kuroshunoskite (Mg2Cl (OH)3 3.5-4 (H2O)) or woodallite (Mg6Cr2 (OH) 16Cl2.4 (H2O)), which are magnesium chloride minerals. Some other minerals are also present as small fractions. In the case of the Zanjan and Tehran bentonites (Figure 3a and 3b), the 12.3 Å peak (with (550 and 700 count/s respectively) indicates the presence of saponite, hydrobiotite (K(Mg,Fe)3(Al,Fe)Si3O10(OH,F)2) or zakharovite (Na4Mn+ + 5Si10O24(OH)6.6(H2O)) as possible minerals. Saponite is the most expandable clay mineral existing in all three bentonites. Other peaks in these samples are not regarding to the characteristics of high swelling ability and capability to adsorb water [14].
The relation between PSD and free swell index
Schütz et al. stated that bentonite has exceptional adsorption properties due to its high specific surface area. Free swell index of bentonites augmented by increasing the percentage of clay-sized particles (<0.002 mm). Mahesh et al, Nayak and Singh stated that this phenomenon indicates that the smaller adsorbent size, the higher surface area for adsorption. Hence the Tehran bentonite comprised of the highest percentage (>71%) of clay-sized particles (<0.002 mm), it possessed the highest Free swell index, Table 3. The rate and extent of swelling in bentonite is enhanced by the small particle size (<2 μm), high surface area and porosity [15].
| Bentonite type | Free swell index (%) | Particles < 0.002 mm (%) |
|---|---|---|
| Tehran | 125 | 68 |
| Zanjan | 50 | 43 |
| Semnan | 35 | 40 |
Table 3. Free swell index of bentonites with different PSD.
The relation between pH and free swell index
Given in Table 1, the acidity (pH) in Semnan, Zanjan and Tehran samples were alkaline. Although there was not a sharp difference between the bentonites acidity (pH), nevertheless, Tehran bentonite, which possessed the highest pH, showed the highest Free swell index [16].
The relation between CEC and free swell index
The cation exchange capacity is correlated to layer charge for swelling clays. The location and magnitude of charge on smectite surfaces influence most engineering properties of bentonite. Sorption and attenuation of water is controlled largely by the charge, hydration energy and size of the exchange cation. The clays free swelling index properties are affected by their layer charge. In other words, phyllosilicates free swelling index is closely related to their Cation Exchange Capacity (CEC). Free swelling index often augments as CEC increases. Given in Table 1, as the CEC increased, free swelling index in bentonites augmented. Therefore, hence Tehran bentonite sample has the highest CEC, it shows the highest free swelling index [17].
The relation between monovalent, divalent cations and free swell index
One of the primary differences between clay minerals is the amount, and type of exchangeable cations on their surface which neutralize the negative charge. Al-Rawas also reported that cations are affecting factors in controlling the nature of soil swelling index. For soils that the exchangeable cations are mono-valence, the "index" cation can be Na+ which leads to bigger swelling index. Two mechanisms, the swelling of the crystal and osmotic swelling, are considered for bentonite hydration and free swelling index behavior which depend on the nature of the cation in the interlayer space and the degree of hydration [18]. Given in Table 4, the difference between the amount of monovalent and divalent cations in Tehran, Zanjan and Semnan samples. Evidently the total of monovalent cations in Tehran bentonite is higher than divalent cations, and in Zanjan bentonite both total of monovalent and divalent cations are approximately two units apart, and in Semnan sample total of divalent cations is higher than monovalent ones. Therefore, the size effect of monovalent cations is higher than that of divalent cations.
| Physical and chemical properties | Unit | Semnan | Zanjan | Tehran |
|---|---|---|---|---|
| Calcium exchangeable | cmol+/kg | 24.80 | 33.38 | 19.30 |
| Exchangeable magnesium | cmol+/kg | 10.98 | 16.33 | 11.06 |
| Total of divalent cations | cmol+/kg | 35.78 | 49.71 | 30.36 |
| Exchangeable sodium | cmol+/kg | 15.85 | 41.83 | 40.44 |
| Exchangeable potassium | cmol+/kg | 13.46 | 5.52 | 44.26 |
| Total of monovalent cations | cmol+/kg | 29.31 | 47.35 | 84.7 |
Table 4. Total of monovalent and divalent cations.
The relation between cation type and free swell index
The free Swell index is dictated by the cation type and concentration in solution. For ions such as sodium and lithium, the free Swelling index occurs in two regimes, crystalline and osmotic. In the presence of water, interchangeable cations between clay layers become hydrated, resulting in an increase of the distance between clay layers. Extensive laboratory studies showed that the two active mechanisms of clay swelling are crystalline swelling and osmotic swelling. Crystalline swelling occurs in all types of clay minerals especially in smectite group. As a result of the hydration of cation located between the layers of clay, the distance between the layers of clay increases. Osmotic swelling is due to cation exchange between layers. If the cation concentration in interlayer areas is more than in the water nearby, water molecules enter the area to dilute the concentration of cations and restore the cationic balance [19]. This type of swelling increases the volume more than crystalline swelling. Given in Table 1, the three bentonites hold their specific predominant cations, consequently, specific distances between the plates inside each particle as well as specific distances between the particle layers occupy the structure of bentonites.
Because the predominant cation in Tehran sample was of potassium and sodium type, and Zanjan sample was of sodium and Calcium type, and Semnan sample was merely of calcium type, bentonites free swelling index was Semnan<Zanjan<Tehran, respectively. Ahmed et al., stated that the addition of NaCl, KCl, CaCl2 and MgCl2 separately to different bentonites increased the free swelling index of bentonites with the elements Na>K>Mg>Ca, respectively. Also Krishna Mohan et al., suggested that the valence, dimension and hydration state of the interposed cations determine the distance between different unit layers at the microscopic scale.
The relation between free swell index and oil crude adsorption
The chemical nature and pore structure of bentonite generally determine their adsorption ability. Chemical and physical properties of clays cause significant effects on free swelling index and as a consequence, affect the adsorption of crude oil on clays. As discussed in previous sections, chemical properties such as; cation type, monovalent, divalent cations, pH, CEC and physical properties such as; Particle Size Distribution (PSD) demonstrated strong relation with adsorption and clay swelling. Increase in the specific surface area of the Tehran sample causes an effective physical and chemical properties in absorbing crude oil. Schütz et al. stated that bentonite has exceptional adsorption properties due to its high specific surface area. Viani et al. stated that high specific surface area of clay minerals and common surfaces between solid and liquid exchange phases and the interactions between clays and contaminants lead to contaminants adsorption on clays. As the size of particles increase, their specific surface area decrease [20]. Therefore among the three types of bentonites, the Tehran sample showed highest capability in crude oil absorption. Clay swelling has been widely documented as the primary reason documented as the primary reason leading to oil recovery. Interactions of clay particles with permeating fluid have been recognized as a critical parameter controlling the waste fluids. These interactions are strongly functions of the ionic strength of the permeating fluid. As shown in Table 2, the average oil Viscosity at three temperatures, was 32 mm2/Sec and presumably what could have caused the adsorption of oil to the surface of the clay, were mainly the clay swell ability and to some extent low oil viscosity. Xiong et al., stated viscosity reduction, diffusion and oil swelling are a few of the dominant pore-scale mechanisms governing Enhanced Oil Recovery (EOR). Nevertheless, it can be concluded that low oil viscosity and high clay swell ability lead to higher interaction between oil and clay and, eventually, more oil adsorption on clays.
Conclusion
As there are various bentonites originated from different mines, different physicochemical properties are expected for them. In this research, three bentonites originated from Tehran, Semnan and Zanjan provinces, in Iran, applied to answer this query, “Is the adsorption of crude oil on clays relevant to Free Swelling Index”. The chemical properties such as; cation type, monovalent, divalent cations, pH, CEC and physical properties such as; Particle Size Distribution (PSD) were determined and their relation with clay swelling were compared. Capability of sorbent in sorption and attenuation of fluids and its swelling are highly controlled by the charge, hydration energy and size of the exchange cation. The rate and extent of clay swelling is ameliorated by the small particle size (<2 μm), high surface area and porosity that bentonite provides. The cation exchange capacity is correlated to layer charge for swelling clays. In Tehran bentonite, of potassium and sodium predominant cation type, the smallest particles size, the highest pH and CEC compared to the Semnan and Zanjan bentonite as well as structurally more total of monovalent cations than divalent cations lead to the largest surface area, chemical and mechanical stabilities and layered structure, consequently, high swell ability in clay and low oil viscosity lead to the largest crude oil adsorption. Therefore the clay absorbent is prone to absorb more pollutant.
As a result to prudently manage and control the environmentally problem, oil pollution, applying bentonite clay with unique and distinguishable structural features is potentially amenable as a practical way to curb crude oil from polluting the soil and underground water.
References
- Adamis Z, Williams RB, Fodor J, et al. Bentonite, kaolin, and selected clay minerals. World Health Organization. 2005;231.
- Ahmed AA, Saaid IM, Akhir NAM, et al. Influence of various cation valence, salinity, acidity (pH) and temperature on bentonite swelling behaviour. AIP Conf Proc. 2016;1774(1):040005.
- Al-Asheh S, Banat F, Abu-Aitah L, et al. Adsorption of phenol using different types of activated bentonites. Sep Purif Technol. 2003;33:1-10.
- Al-Rawas AA. The factors controlling the expansive nature of the soils and rocks of northern Oman. Eng Geol. 1998;53(3–4):327-50.
- Barclay LM, Thompson DW. Electron microscopy of sodium montmorillonite. Nature. 1969;222(5190):263-63.
- Browning GRJ. Geosynthetic clay liners: A review and evaluation. Trans Inst Min Metall Sect B. 1998;107:120-29.
- Christidis GE. Physical and chemical properties of some bentonite deposits of Kimolos Island, Greece. Appl Clay Sci. 1998;13(2):79-98.
- Erdem B, Ozcan A, ZOzcan AS, et al. Adsorption and solid phase extraction of 8-hydroxyquinoline from aqueous solutions by using natural bentonite. Appl Surf Sci. 2010;256(17):5422-27.
- Fu-Chuang H, Jiunn-Fwu L, Chung-Kung L, et al. Effects of cation exchange on the pore and surface structure and adsorption characteristics of montmorillonite. Colloids Surf A Physicochem Eng Asp. 2004;239:41-47.
- Gates WP, Bouazza A, Churchman GJ, et al. Bentonite clay keeps pollutants at bay. Elements. 2009;5(2):105-10.
- Gee GW, Bauder JW, Or D. Particle-size analysis. Methods of soil analysis. Part 4. 2002;598:255-93.
- Ghosh S, Mukherjee S, Al-Hamdan AZ, et al. Efficacy of fine-grained soil as landfill liner material for containment of chrome tannery sludge. Geotech Geol Eng. 2013;31(2):493-500.
- Hosseinpour MA, Ghoreishi H, Ghanaat F, et al. Adsorption of hazardous petroleum constituents by ordinary and modified bentonites.
- Jackson ML. Soil chemical analysis: Advanced course. UW-Madison Libraries Parallel Press. 2005
- Kaufhold S, Dohrmann R, Ufer K, et al. Comparison of methods for the quantification of montmorillonite in bentonites. Appl Clay Sci. 2002;22(3):145-51.
- Koch D. Bentonites as a basic material for technical base liners and site encapsulation cut-off walls. Appl Clay Sci. 2002;20:1-11.
- Laird D. Influence of layer charge on swelling of smectites. Appl Clay Sci. 2006;34:74-87.
- Madsen F, Muller-Vonmoos M. The swelling behavior of clay. Appl Clay Sci. 1989;4:143-56.
- Mahesh S, Chitranshi UB, Desh D, et al. Adsorption kinetics of dihydric phenol: Catechol on activated carbon. Indian J Environ Health. 1998;40(2):169-76.
- Min-Yu T, Su-Hsia L. Removal of basic dye from water onto pristine and HCl activated montmorillonite in fixed beds. Desalination. 2006;194:156-65.