Review Article - Journal of Molecular Oncology Research (2018) Volume 2, Issue 3
Incivility of passive immunity
Gerald M. Higa* and Katie Murto
Schools of Pharmacy and Medicine, Robert C. Byrd Health Sciences Center, West Virginia University, Morgantown, USA
- *Corresponding Author:
- Gerald M. Higa
Schools of Pharmacy and Medicine Robert C. Byrd Health Sciences Center West Virginia University, Morgantown, USA
E-mail: ghiga@hsc.wvu.edu
Accepted date: September 9, 2018
Citation: Gerald M. Higa. Incivility of passive immunity. J Mol Oncol Res. 2018;2(3):74-86.
DOI: 10.35841/molecular-oncology.2.3.74-86
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
Survival of the fetus and newborn is not possible without vital immune components. The immune repertoire includes a cadre of innate factors sheltered in the intrauterine domicile as well as maternally-sourced humoral elements. The latter, known as passive immunity, refer to maternal antibodies that mediate vigilant immune surveillance during gestation and for a few months after birth. While immunologically beneficial, their therapeutic luster is tarnished somewhat by a number of antibody-associated abnormalities that have been reported in offsprings. And though basic science tenets of the antibody transfer process are well accepted, the immuno-biochemical mechanisms related to the therapeutic and pathogenic effects remain incompletely understood. This opinion-based paper provides reasoned insight regarding the bi-functionality of immunoglobulins by focusing on maternal benefit, and potential in utero detriment, of a recombinant monoclonal antibody used in the treatment of one breast cancer subtype. If valid, this concept may be applicable to other engineered antibodies.
Keywords
Cardiotoxicity, Engineered monoclonal antibody, FcRn, HER2, IgG1, Immunoglobulin, In utero, Passive immunity, Trastuzumab
Introduction
The incipient human maternal-fetal bond is established during the sacrosanct period of gestation. Not exclusively a site for fetal development, the uterine cavity also acts as a haven for protection against potentially life-threatening infections. A number of innate proteins with antimicrobial activity such as α- defensins, lactoferrin, and phospholipase A2 are intrinsic components of the intrauterine environment, though cellular elements such as polymorphonuclear cells, monocytes and macrophages are functionally immature [1]. While these and many other endogenous factors provide a measure of security, fetal viability is also critically dependent on passive immunity sourced by the mother.
Even though transference of maternal-derived humoral immunity begins by the end of the first trimester, fetal immunoglobulin (Ig) levels are usually less than 10% of maternal concentrations early in the second trimester [2]. As the pregnancy progresses, cross placental antibody transfer increases, reaching 50% of the maternal concentration by the middle of the third trimester; acquired passive immunity is greatest during the final four weeks of gestation [3,4]. Interestingly, passive immunity at term pregnancy often exceeds maternal antibody levels by as much as 30% [2]. And not surprising, the acquired humoral immune repertoire in the full term neonate mirrors that of the mother, further solidifying materno-fetal harmony. In essence, the amount and specificities of immunoglobulin transferred are dependent not only on maternal Ig concentrations and immunologic exposure, but also on duration of gestation and placental integrity. Ultimately, acquisition of passive immunity by the fetus is critical for extra-uterine adaptation and protection against infectious diseases during the first year after birth.
Whereas Ig transfer, from an infectious disease perspective, contributes to a state of fetal and neonatal well-being, not all transmitted antibodies have beneficial consequences. Indeed, compelling evidence of maternal antibody-transferred diseases exists. For example, neonatal lupus erythematosus (NLE) has been linked to maternal transference of anti-Ro/SSA or La/SSB antibodies [5]. This association is further supported by the finding that resolution of the cutaneous manifestations occurs with decreasing levels of the antinuclear antibody [6]. Other immune globulins that have been implicated in a number of disorders in the newborn include antiphospholipid antibody (thrombosis) [7], anti-thyroid peroxidase antibody (hypothyroidism) [8], anti-D, anti-E, anti-K, and anti-C antibodies (hemolysis) [9], and human platelet antigen-1a (HPA-1a) antibody (thrombocytopenia) [10]. In most cases the disorders are usually evanescent.
Even more intriguing are epidemiological and laboratory data linking behavioral abnormalities such as autism spectrum disorder (ASD) in children with in utero exposure to maternal antibodies [11,12] and research in animal models provides some of the most tangible evidence supporting this belief. One study showed that offsprings of non-human primates (who were immunized with purified immunoglobulin from mothers of children with autism spectrum disorder) exhibited signs of neurodevelopmental dysfunction similar to human ASD [13]. Another study in mice had similar results [14].
After several neuronal proteins critical for normal brain development were identified one of the most provocative studies was conducted. Having cloned a brain-reactive antibody targeting contactin-associated protein-like 2 (Caspr2 or C6) investigators immunized healthy pregnant C57BL/6 mice with either C6 antibody or control immunoglobulin [15]. Compared to control mice, male offsprings exposed in utero to C6 had severe neuronal defects involving cortical and hippocampal neurons as well as behavior alterations typical of an ASD phenotype [16]. These findings could reconcile the discordance in timing between onset of developmental disorders in humans and the absence of pathogenic maternal antibodies. Furthermore, the incivility of passive immunity could have significant implications in oncology.
The impetus for undertaking this endeavor is to merge our increased, though by no means complete, understanding of neonatal disorders associated with maternal antibodies. While select portions of the published literature were used to support the accuracy of the textual content, unresolved issues provided the opportunity for reasoned author viewpoints. This opinionbased paper extends upon the former concept in a novel manner by examining a potential fetal abnormality borne, in utero, by exposure to trastuzumab, an engineered monoclonal antibody (mAb) that has significantly improved the prognosis in women with Human EGFR-Related 2-positive (HER2+) breast cancer. While the likelihood of fetal harm may be perceived as unlikely because the drug is not given to pregnant women during cancer therapy, the pathological effect could be significant if conception occurs relatively soon after treatment is completed. As such, the dynamics of maternal-fetal Ig transfer and developmental biology of the heart are reviewed. And to further enhance reader appreciation, a structural and clinical overview of trastuzumab is also included. Finally, an attempt is made to provide proof-of-principle insight into the potential pathogenicity of in utero transfer of this therapeutic mAb on the developing heart and humoral immune response.
Immunoglobulin (Ig)
The immunoglobulin is often characterized graphically as a “Y”-shaped molecule consisting of two heavy and two light chains. Because this relative simplicity undermines an immensely complex molecule, various frameworks of the glycoprotein are worthy of discussion.
Structural context
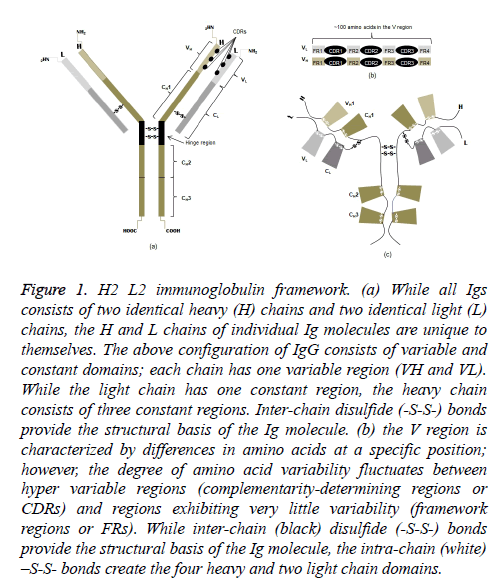
All heavy chain (CH) isotypes and subtypes (CH variants) are composed of four polypeptide chains, identical pairs of light (~20 kDa) and heavy (~50 kDa) chains (Figure 1a). Each chain has an amino-terminal (NH2) in the variable domain and one or more carboxyl-terminals (COOH) in the constant domains. The NH2 domain is notable in that there is a hypervariable region and a region in which the amino acids show little variability. The former consists of three complementarity-determining regions (CDRs); the latter is referred to as framework regions (FRs) (Figure 1b). The terminology should not underestimate their importance because variations in amino acids of the light (VL) and heavy (VH) chains are the structural basis for antibody diversity and specificity.
Figure 1: H2 L2 immunoglobulin framework. (a) While all Igs consists of two identical heavy (H) chains and two identical light (L) chains, the H and L chains of individual Ig molecules are unique to themselves. The above configuration of IgG consists of variable and constant domains; each chain has one variable region (VH and VL). While the light chain has one constant region, the heavy chain consists of three constant regions. Inter-chain disulfide (-S-S-) bonds provide the structural basis of the Ig molecule. (b) the V region is characterized by differences in amino acids at a specific position; however, the degree of amino acid variability fluctuates between hyper variable regions (complementarity-determining regions or CDRs) and regions exhibiting very little variability (framework regions or FRs). While inter-chain (black) disulfide (-S-S-) bonds provide the structural basis of the Ig molecule, the intra-chain (white) –S-S- bonds create the four heavy and two light chain domains.
Inter-chain disulfide bonds linking light and heavy chains in the Fab (fragment antigen binding) portion as well as the two heavy chains in the Fc, or constant, portion result in the monomeric immunoglobulin molecule. Notably, the consequence of two intra-chain disulfide bonds is two domains on all light chains (CL); and depending on isotype, the effect of either four or five intra-chain disulfide bonds is an equal number of CH domains (Figure 1c). The heavy chains of IgG, in particular, have four domains. A small segment enriched with cysteines and prolines between the first and second constant domains of the heavy chains is called the hinge region. More importantly, the intra-chain disulfide bonds not only give the molecule its unique shape but also structural domains that are functional.
Functional context
Immunoglobulin functions are primarily determined by one of the NH2-terminal domains of both light and heavy chains and the latter two or three domains of the heavy chains. The former, characterized by great variability in amino acid sequences, functions as the antigen-binding site; the consequence of antigen-binding enables the Fc region to serve as a conduit for immune-mediated effector functions through engagement of endogenous protective elements.
Although it is beyond the scope to be discussed in detail here, it is important to emphasize that the diverse specificities of the highly variable Fab domains have been predetermined by stochastic recombination of heavy chain V (variable)-D (diversity)-J (joining) gene segments and (variable)-J (joining) light chain gene segments. And while the total repertoire of generated paratopes (antigen-binding sites) is unknown, the number is likely to be extraordinarily large. In contrast, and because of a limited number of Ig classes, the CH and CL domains remain relatively constant though extensive polymorphisms occur in the population (this issue will be discussed later). One apparent contradiction to the general notion that the constant fragment has no specificity is the finding that only IgE binds with high affinity to FcεR1 found on mast cells; and opsonization is carried out most effectively by IgG1 binding to FcγR1 expressed by macrophages.
By binding to an epitope of infectious agents or noxious particles, antibodies can directly neutralize potentially pathogenic organisms or molecules. More frequently, Ig-bound antigens elicit a response from other effectors such as complement, phagocytes, and cytotoxic T cells. While macrophages and CD8+ cells are recruited via binding of Fc to cell surface Fc receptors (FcR), engagement of the complement system is quite unique. In the classical pathway, activation of complement requires an “association” reaction between the CH2 domain of IgG and C1q, the first component of the cascade. In reality, the activation process is much more involved. Crystallographic studies indicate that C1q has six pseudopod-like structures, each containing A, B, and C peptide chains as a triple helix [17]. This multivalent configuration is what enables high-affinity binding of Fc of only immunoglobulin complexed to antigen, which induces the cascading enzymatic reactions. Although not discussed herein, it is worthwhile to note that an alternative pathway for complement activation exists, one that does not require the presence of Ig.
Maternal-fetal context
While the precise mechanism(s) which regulate IgG in the fetal circulation remain somewhat controversial, specie-specific differences regarding transfer of maternal antibody have been well described [18]. It is also important to emphasize that in humans, fetal acquisition of passive immunity is achieved by an active process; and IgG is the only class of antibody transferred [19]. This selective immunity is likely related teleologically to the antibody’s protective role against pathogenic organisms and molecules as well as its predominance in serum.
On a macroscopic level, unidirectional maternal-fetal transfer of IgG proceeds through a dual histological barrier composed of a layer of syncytiotrophoblasts, in direct contact with maternal blood [20], and a layer of fetal capillary endothelial cells. While low molecular weight substances (100-700 Da) such as ions and some amino acids can diffuse through this barrier, very high molecular weight molecules are usually unable to cross the placenta; IgG (~150 kDa) is one of the few exceptions but requires a transporter protein.
When including Ig subclasses, there are actually nine different types of antibodies; IgG consists of four subclasses. The relevance of this finding is the preferential transfer (in descending order) of IgG1>IgG4 and IgG3>IgG2. The largest quantitative difference is between IgG2 and the other subclasses, a finding that appears to be partially based on affinity of the antibody for a novel receptor called FcRn (described below) [21,22].
Microscopically, the transfer of passive immunity to the fetus, all of which occurs preferentially before term, is much more complicated. Initially, the finding of intact IgG in the fetus led to the plausible, though erroneous, belief that antibody transfer was mediated by placental Fcγ receptors (FcγRs) [23]. A quarter century later a unique receptor, homologous to class 1 major histocompatibility complex (MHC) molecules, was identified that appeared to be the mode responsible for the placental transfer of IgG from mother to fetus [24]. Derived from neonatal rat small intestine, the receptor was then referred to as neonatal Fc receptor (FcRn). That fetal acquisition of passive immunity was indeed carried out by FcRn was firmly established in an elegant study comparing a ‘humanized’ IgG1 and an IgG1 variant with negligible affinity for the receptor [25].
One confounding immunological aspect of FcRn relates to its structure and function. Three-dimensional models indicated that the receptor was a single heterodimer of a MHC-class-Ilike heavy chain linked to a β2-microglobulin light chain [26]. However, receptor-IgG binding occurs as a 2:1 complex; that is, two FcRn molecules bind to the Fc fragment of a single immunoglobulin [27]. Because of two distinct contact sites, it would appear that FcRn would have the ability to not only bind IgG but also present intracellular antigens to CD8+ cells. However, the receptor and antibody interaction results in an unusual configuration on the cell membrane, secreting the α1 and α2 domains which typically serve as antigen and T-cell binding sites [28].
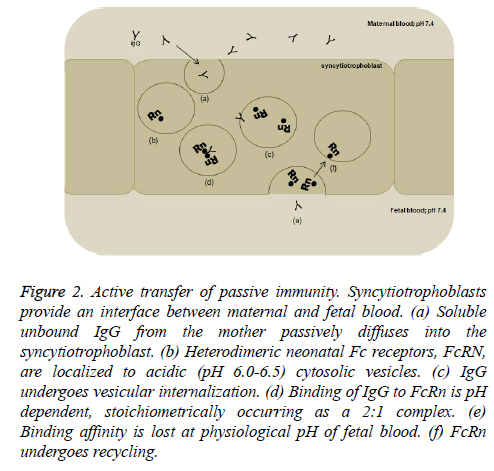
FcRn have been shown to be present in human placenta early in the first trimester of gestation [29]. However, mere binding of IgG to FcRn is a gross oversimplification of the mechanism for transplacental passage of antibodies. It is now well accepted that serum containing unbound maternal IgG undergoes passive diffusion into the syncytiotrophoblast (Figure 2). The antibody is then internalized within a specialized system of small vesicles where FcRn is localized. Interestingly, formation of the IgG-FcRn complex is a pH-dependent phenomenon; binding occurs at a pH of ≤ 6.5 and lost at pH ≥ 7.4 [30]. It has been postulated that the Fc hinge region, which contains several histidine residues, promotes coupling and uncoupling of IgG and FcRn with changes in pH of the milieu [31]. This requisite happenstance serves two multi-faceted purposes. First, acidification increases IgG’s binding affinity to FcRn, prevents degradation of the antibody, and enables vesicular transport to the fetal side of the placental syncytiotrophoblast. Second, the physiological pH of fetal blood causes bound antibody to dissociate from its receptor, facilitates transcytosis of antibody to the fetus, and promotes conservation and recycling of the receptors [32].
Figure 2: Active transfer of passive immunity. Syncytiotrophoblasts provide an interface between maternal and fetal blood. (a) Soluble unbound IgG from the mother passively diffuses into the syncytiotrophoblast. (b) Heterodimeric neonatal Fc receptors, FcRN, are localized to acidic (pH 6.0-6.5) cytosolic vesicles. (c) IgG undergoes vesicular internalization. (d) Binding of IgG to FcRn is pH dependent, stoichiometrically occurring as a 2:1 complex. (e) Binding affinity is lost at physiological pH of fetal blood. (f) FcRn undergoes recycling.
Engineering context
Understanding the dynamics and biological functions of the immunoglobulin has revolutionized the way cancer is treated. The remarkable improvement in disease outcomes should not be attributed only to the antibody’s specificity for the target but also, and perhaps more importantly, the epitope. Indeed, in some instances the use of recombinant human proteins is indicated only for a subset of patients with a specific subtype of cancer. One such engineered antibody is used in the treatment of HER2+ breast cancer.
Trastuzumab is a recombinant humanized mAb (Herceptin; Genentech; South San Francisco, CA) of the IgG class and G1 subclass, hence an IgG1 heavy chain (CH) variant. Simplistically, selection was based largely on the relative abundance of this variant in serum as well as the ability of this isotype to engage cytotoxic T-cells (antibody-dependent cell-mediated cytotoxicity) and activate complement.
Less known about this “designer” molecule are two fundamental principles that guided the structural engineering process. First immunologic dogma predicted that anti-idiotypic antibodies would be generated against any manufactured antibody that was not identical to the natural product (such was the case with a mouse IgG2a antibody used to prevent organ rejection). In order to decrease immunogenicity, scientists at Genentech were able to transfect the exact nucleotides that make up the complementarity-determining regions of a mouse gene into a human immunoglobulin gene. The resulting CDRgrafted antibody, featuring only the murine antigen-binding surface in the framework of the human immunoglobulin molecule, retains epitope-specificity of the mouse mAb for the HER2 protein as well as effector function of the human Fc fragment. Anti-idiotypic responses are minimized though not completely abrogated. Second, optimization of the mAb pharmacokinetic (PK) profile has several clinical and therapeutic benefits including less frequent dosing, reduced patient visits for drug administration, and improved tissuespecific delivery [33,34]. A number of studies demonstrated that binding interactions between IgG and FcRn significantly impacted antibody half-life [35,36]. For example, reducing IgG’s binding ability shortened its presence in serum. While this property may be desirable for diagnostic purposes, it has less appeal when used as a therapeutic agent [37]. Still, the dynamics of prolonging serum antibody concentration is much more intricate than affinity enhancement alone. Recall that FcRn, expressed in endocytic vesicles of the syncytiotrophoblast, mediates the active and selective cross-placental transport of maternal IgG [38]. In vivo, however, the receptor is expressed ubiquitously including epithelial cells of the intestine and glomerulus, endothelial cells, monocytes, macrophages and dendritic cells. The latter three antigen presenting cells have been postulated to be important in determining the biological fate of endogenous and engineered antibodies [39-41]. One mechanism relates to transient sequestration of the antibody, a process by which IgG is internalized, bound to FcRn, and subsequently released into the circulation. No less important is the finding that receptor binding protects the antibody from degradation [42,43].
While engineering of trastuzumab followed these core principles, three additional aspects related to the Fc fragment are of relevance. First, crystalline models indicated that binding of FcRn to IgG occurs in the hinge region between the CH2 and CH3 domains of the immunoglobulin heavy chain.
While binding is pH-dependent, molecular mapping of the interaction sites on IgG revealed that substitutions of one or several amino acids in the binding domain could enhance the antibody’s affinity for FcRn. For example, substitution of alanine for asparagine at residue 434 (N434A) resulted in a variant with a 3.4-fold improvement in binding affinity compared to wild-type trastuzumab; a 12-fold enrichment in receptor binding was achieved with two additional alanine substitutions (N434A/T307A+E380A) [36]. Second, while high- affinity binding to FcRn protects the mAb and engenders a more desirable PK profile, the beneficial effects of trastuzumab (and other IgG1 mAb variants) require tethering of the antibody-antigen complex to endogenous effector systems. One way this is achieved is by binding of the immune complex to other Fc receptors of the Fcγ group such as FcγR1 which is constitutively expressed by monocytes and macrophages [44]; two other receptors include FcγRII and FcγRIII which are expressed on B cells and NK cells, respectively. Although binding involves a number of residues located in the hinge region proximal to the CH2 domain that either interacts directly with the receptors or influences carbohydrate character or position, it should be emphasized that this interaction is immensely more complex. Furthermore, binding affinity varies by receptor class and IgG variant. For example, although FcγR1a can bind any IgG subclass, interactions with IgG1 and IgG3 are much stronger compared to IgG2 and IgG4 [45]. As such, the FcγR1a-trastuzumab complex promotes receptor phosphorylation and activates effector cell-mediated antibody-dependent cellular cytotoxicity (ADCC). In addition, and independent of receptor binding, the hinge region of Fc is composed of specific IgG1 amino acid residues that provides an interface for efficient contact with C1q and raises the binding constant of the C1q-IgG1 interaction [46,47]. As such, IgG1 appears to be the most efficient recruiter of complement-dependent cytotoxicity (CDC) effector functions. And third, while increased binding affinity protects the mAb and prolongs its half-life, persistence of its therapeutic effect also depends on efficient recycling of sequestered trastuzumab, an effect that requires binding (to FcRn) to occur only in a narrow acidic range. Although the embodiment of a naturally occurring molecule, humanized trastuzumab is a drug engineered with enhanced performance characteristics (Table 1).
| Intrinsic characteristics | Relevance |
|---|---|
| IgG1 | Crosses placenta preferentially |
| Amino acid sequence | Alanine substitutions in the hinge region enhance FcRn binding 3-12 fold |
| FcRn | In addition to synciotrophoblasts, expressed in many types of epithelial cells and antigen presenting cells |
| FcRn-mAb complex | Protection from degradation |
| Plasma half-life | ~21 days* (duration does not account for FcRn- bound mAb) [133] |
| Engineered CDRs | Retains target and epitope specificity for HER2 regardless of tissue type |
Table 1: Features associated with trastuzumab and fetal harm risk.
Clinical context
One of the most significant events during the past three decades was the discovery of neu, the proto-oncogene which encodes the HER2 transmembrane receptor [48]. Although normally, but not exclusively, found on mammary epithelial cells, the receptor is overexpressed and/or the gene is amplified in approximately 15% of all new breast cancer diagnoses [49,50]. The importance of HER2-positivity can be highlighted in several ways. First, the relationship between gene amplification and prognosis; second, the predictive value of HER2 overexpression; third, the validated genetic test incorporating HER2 with other genes to identify patients with early hormone-dependent breast cancer who may avoid adjuvant chemotherapy [51], and fourth, the development of trastuzumab, a monoclonal antibody (mAb) which inhibits receptor activation [52,53].
Beneficial effects of trastuzumab have been reported in patients with HER2+ metastatic disease as well as those with HER2+ early breast cancer [52,54]. With regard to the latter, results of three large clinical trials showed that addition of trastuzumab to adjuvant chemotherapy was associated with a significant improvement in disease-free survival (DFS), the primary study endpoint [55-57]. Two studies also reported findings related to overall survival (OS). In the Breast Cancer International Research Group (BCIRG) 006 study, addition of the antibody (compared to no antibody) resulted in a significant improvement in the OS rate 92% vs. 87%, respectively (HR, 0.63; P<0.001) [57]. Similarly, analysis of the OS rate in the Herceptin Adjuvant (HERA) study indicated a 34% lower risk of death with trastuzumab compared with observation alone (HR, 0.66; 95% CI, 0.47 to 0.91; p=0.0115) [56].
The above statistics notwithstanding, numbers in the United States (U.S.) alone continue to be telling. An estimated 250,000 new cases of breast cancer will be diagnosed in 2018; and nearly one-fifth of the new diagnoses will involve premenopausal women [58], an observation consistent with the tenet that breast cancer was, and still is, a disease primarily of older women [59]. Of major concern, however, is the finding that compared to postmenopausal women, younger patients have significantly worse breast cancer outcomes, including mortality, even among those with early stage disease. While many factors contribute to the poorer prognosis, genomic profiling has revealed higher rates of HER2 overexpression among women younger than 45 years of age suggesting tumor biology plays a significant role [60]. Of note also, this finding is not restricted to the U.S. [61].
It should be emphasized that signaling through HER2 is not the only adverse genomic feature underlying tumor biology in younger compared to older women. Nonetheless, targeted inhibition of this receptor has improved the prognosis of patients with this subtype of breast cancer as mentioned previously. Because of the higher risk of dying, the full implications of adjuvant therapies including trastuzumab should be discussed with younger patients. While the goal is cure, treatment can have profound effects on menopausal status, and hence, fertility and pregnancy. Even though premature ovarian failure (POF) is a major concern after chemotherapy, many women continue or resume menses after treatment. However, it should be pointed out that menstruation and ovarian function, in and of themselves, are only passive indicators of fertility [62]. In this same study, a small but significant, association was observed among subjects who received trastuzumab and higher levels of anti-Müllerian hormone which may be a better marker of ovarian reserve [63]. And because the risk of disease relapse does not appear to increase if pregnancy occurs after treatment [64], fertility-preserving options continue to be explored [65,66].
The issue of conception, even in women who do not undergo treatment with chemotherapy, depends on a multitude of factors. And despite even more obstacles, these data do suggest that young breast cancer survivors interested in having a child after breast cancer therapy have a of 5%-27% probability of a successful in vitro fertility-assisted live birth [67].
Cancer and fertility
Quantity and quality of life are usually the most important priorities among those with newly diagnosed cancer, the majority of who will be over 50 years of age. However, younger patients may harbor another concern related to fertility, an issue that affects both males and females. As such, practice guidelines have been promulgated by the American Society of Clinical Oncology [68]. And the latter is no trivial matter as duration of survival has improved significantly for a number of malignancies with poor prognostic features including some subtypes of breast, lung and skin (melanoma) cancers [69-71]. Hence, it is especially important that patients in their reproductive years understand that one of the adverse effects of treatment could be infertility. Once the patient’s feelings are known, options that can increase or improve posttherapy fertility should be incorporated into the overall management and survivorship plans.
An earlier study of males ≤ 40 years of age indicated that at least 50% of those who completed the survey desired having children after completing cancer treatment [72]. Of note, almost two-thirds of those polled had no recollection that infertility was a potential adverse effect of chemotherapy. Interestingly, only a small percentage of subjects actually had sperm preserved, most doing so after discussing available strategies for fertility preservation with their oncologists. Excepting experimental procedures, banking of cryopreserved sperm is the only safe and effective option for males. Harvesting of sperm prior to treatment improves the likelihood that both quantity (sperm count) and quality (treatment-induced DNA damage obviated) of the collected specimen will be attained [73].
For premenopausal women, cryopreservation of in-vitro fertilized eggs or, in some instances, unfertilized oocytes, is an established method [74,75]. Another strategy, though not always successful, is temporary “repositioning” of the ovaries, a procedure called oophoropexy in order to shield it from ionizing radiation involving the pelvis [76]. In women with breast cancer who desire to have children, concomitant use of gonadotrophin-releasing hormone (GnRH) agonists has, more often than not, not been shown to be effective [77-79]. Promising data are emerging regarding an experimental procedure involving cryopreservation of ovarian tissue with subsequent transplantation and restoration of gonadal function [80].
HER2 and cardiomyocytes
The improved survival outcomes in women with HER+ breast cancer can be attributed to not only a better understanding of the molecular (i.e., receptor) aberration but also the structural features of trastuzumab. Target specificity, however, does not impart tumor selectivity.
In reverse manner, results of clinical trials showing trastuzumab-associated cardiotoxicity strongly suggested that the HER2-signaling pathway had an important physiological role, even in the adult heart [55-57]. The latter finding is somewhat surprising because previous research in HER2- knockout mice implicated developmental defects of cardiomyocytes as one of the possible cause of early fetal death and hence, the receptor’s role in embryogenesis [81]. Similar myocardial findings and early mortality were also demonstrated in mice with targeted deficiencies of HER4, which can form heterodimers with HER2, or neuregulin-1, a ligand for the HER-family of receptors [82,83]. Despite the apparent similarities, the role of (and signal transduction through) the receptor could be biologically distinct in cardiac myocytes of the fetus and the adult [84]. For example, HER2- null mice failed to develop trabeculae, a complex structure comprised of muscle strands in the developing myocardium. Not only does the trabecular architecture maintain blood flow in the embryonic heart tube but the structure also appears to be developmentally important for the ventricular conduction system and/or chamber contractility [85].
In adults, normal HER2 signaling in the heart appears to be principally, though not exclusively, related to mechanisms regulating the dynamics of cardiac contraction and muscle relaxation. The receptor has been localized to transverse (t)- tubules of ventricular cardiomycocytes which harbor microscopic channels bridging the adjacent sarcoplasmic reticulum [86]. This relatively seamless contact allows calcium ion exchange and t-tubule regulation of excitation-contraction coupling [87].
Trastuzumab, fertility, and passive immunity
Significant survival benefits have been achieved with trastuzumab in HER2+ breast cancer at a reasonably tolerable (physiological) cost. And because disease outcomes continue to improve, it can be anticipated that fertility will continue to be an important issue among women of child-bearing age.
While a number of diseases in the neonate caused by maternal antibodies have been reported, the focus of this paper is the insidious, though hypothetical, cardiac toxicity mediated by transfer of trastuzumab in women who become pregnant within a few months of completing therapy. While many contingencies exist, the following considerations are biological dogma. First, efficient cross placental antibody transport is an active and selective process mediated by FcRn, which preferentially binds and transports IgG1. Binding to the neonatal Fc receptor also prolongs antibody half-life and prevents its degradation. And depending on maternal levels, initial transfer of the mAb to the fetus may occur before the end of the first trimester. Second, in addition to the selective amino acid modifications engineered into the Fc hinge region of the mAb, humanization of mouse VH and VL (i.e, antigenbinding regions) provided specificity for epitope recognition of the extracellular domain of HER2. Published data from preclinical studies provide clear evidence that the HER2- signaling pathway has a physiological role in cardiac morphogenesis. Third, HER2-/- mice die by embryonic day 11 (E11). The complete absence of ventricular trabeculae suggested that developmental abnormalities of the myocardium were, at least partially, responsible for the early deaths. Correlatively, expression of HER2 in cardiomyocytes and neuregulin in the endocardial lining was also manifested in E10 embryos though earlier expression of the receptor cannot be completely excluded [81,88]. Nonetheless, the lethality observed in mice carrying a HER2-null allele is a disquieting phenomenon. Fourth, in humans derivation of the heart from the mesoderm becomes evident during the third week of embryogenesis, a period of time that corresponds to E7 of the mouse. By extrapolation, it is conceivable that HER2 is also expressed though the extent to which the myocardial developmental process is under the control of the HER2 gene has not been elucidated. What is known is that formation of the ventricular loop is then followed by arteriogenesis from the aortic sac, which occurs around day 25 in humans [89]. Shortly thereafter, the primary myocardium is formed [90]; developing trabeculae then give the ventricles their unique morphology. Interestingly, while the Pitx2 gene partially regulates left and right “sidedness” of the heart [91], this gene does not regulate the process of trabeculation [92].
Another important facet relates to t-tubules found predominantly in ventricular cardiomyocytes; and the structure’s notable absence from pacing, conducting, and most atrial tissue [93]. As mentioned previously, HER2 expression has been localized to this tubular network, which first appears in the third trimester of gestation in humans [94]. The relatively late processing of tubulation appears to be conditioned by development of cardiac myofibrils and corresponds to the rise in ventricular pressure during the perinatal period. Physiologically, the efficient coupling of intracellular calcium and cardiomyocyte contraction is critically dependent on the structural and functional integrity of the t-tubules [95]. In addition, the presence of HER2 in the developing myocardium and later, in t-tubules, provides compelling evidence that the receptor has an ongoing role in the welfare of the heart. Pathologically, destruction or disruption of the t-tubule framework is an integral finding in heart failure, regardless of cause [96]. Moreover, this does not appear to be a case of reverse causality [97]. Extending beyond lore, these biological principles provide more than a hypothetical possibility but rather a strong and rational probability that blockade of the HER2-signaling pathway in utero by trastuzumab could have noxious effects on cardiac morphogenesis as well as function in the fetus.
Re-engineering trastuzumab
Since cross placental transfer is restricted to IgG, isotype switching appears to be a rational way to circumvent development of passive immunity-related disorders in the newborn.
Fc IgM isotype
Not subject to maternal transfer, re-engineering of the Fc portion from monomeric IgG to IgM could result in a functionally superior mAb. For example, because of its higher valence oligomerized IgM possesses much greater complement fixing activity (in the classical pathway) than IgG monomers. Interestingly, IgM is also the only isotype capable of opsonizing foreign particles and does so before engaging components of the alternative complement pathway [98,99]. And though lower in affinity, the 10 antigen-binding sites of the pentameric structure enhances its avidity for antigens [100]. However, it is unlikely that Fc interactions with complement alone could account for all of the IgM-mediated protective effects.
Still, it had been difficult to validate IgM’s ability to recruit other effector systems despite expression of FcμR, a receptor specific of IgM, on a variety of adaptive immune cells. While all of this seems counterintuitive, published reports have only been able to characterize the receptor at the protein level but not at the genomic level. That is, until recently [101]. Even though restricted to cells of the lymphoid lineage including NK cells, there are some subtle differences in cell surface expression levels being relatively higher on CD4+ and α/β T cells compared to CD8+ and γᵹ T cells, respectively. However, the extent to which these slight differences affect the immune response may not be clinically significant [102]. Findings at the nucleic acid level provides substantial new evidence of the interaction between two arms of the adaptive immune response. That FcμR is the only Fc receptor expressed on T cells has also been associated with immunologic protection against various types of infectious and autoimmune diseases [103,104].
Another potentially attractive feature of this isotype relates to its presence. Localized to serum, IgM could effectively bind, agglutinate, and remove circulating tumor cells; the efficacy of which is 2-log to 4-log greater than IgG [105,106]. However, the “upside” of the reconfigured isotype can be offset by its “downside”. Some of the major obstacles relate to expensive growth media, transfection procedures, and yield as production of mAbs is frequently accomplished in mammalian cells such as Chinese Hamster Ovary (CHO), mouse myeloma (NS0), and Human Embryonic Kidney (HEK) [107]. In one recent study, researchers using HEK293F cells, one of the most efficient mammalian protein expression systems [108], reported recombinant production rates for all isotypes with IgM being lower than the yields of IgA and all four IgG variants [109]. In addition, recombinant IgM has solubility and stability issues not characteristic of engineered IgG [110].
Fc IgA isotype
Mentioned earlier, the IgG isotype possesses a number of desirable characteristics. Associated with the Fab fragment, the antigen-antibody complex can inhibit tumor cell proliferation by blocking native ligand binding (i.e., neuregulin/HER2 heterodimer); target cell binding also enables Fc interactions with receptors on immune effector cells and complement. In addition, binding of IgG to FcRn prolongs the half-life and prevents denaturation of the protein. Production, yield and purification procedures for this isotype are also well established.
Is it conceivable then that a mAb of an Fc IgA isotype could be engineered to be even better? Consider the following precepts. First, the functionality of IgA would be similar to IgG in that the humanized paratope contained in the variable region recognizes the same epitope on HER2; the bound antigen complex also induces potent ADCC against tumor cells. Interestingly, induction of the latter can be achieved by Fc binding to FcαR1, a receptor with specificity for IgA, expressed on neutrophils. Because phagocytes also express this receptor, activation of FcαR1 on macrophages can also mediate an antitumor effect by a process called antibody-dependent cellular phagocytosis (ADCP) [111-113].Second, the comparatively shorter half-life of five to six days for IgA is, in large part, due to FcRn, which binds and recycles IgG. With the unlikelihood of bioengineering an IgA mAb with a similar clearance magnitude of 21 days, its pharmacokinetic (PK) profile could still be improved. Since the pH-dependent recycling pathway also mediates the half-life of serum albumin [114], investigators have shown that attaching an albumin-binding domain to either light or heavy chain indirectly targets the functional role of FcRn [115].
Another way is by manipulating Fc sialic acid residues, an important component of the glycosylation site. Increased sialylation of terminal nitrogen-linked (N) glycans increases stability and decreases hepatic clearance of engineered proteins, and appears to enhance the therapeutic efficacy [116-118]. Indeed, improvement in half-life has already been demonstrated with recombinant erythropoietin using these techniques [119]. However, modification of sialic acid is complex and the yield can be highly variable. With CHO cells (expression system used for production of trastuzumab), investigators found that only 20% of the secreted mAb were sialylated [120]. On the other hand, a fully-human system like the myelogenous leukemia-derived GlycoExpress (GEX) cell lines has been shown to be capable of generating mAbs of which 60% of the N-glycans were sialylated [121]. Furthermore, IgA1 and IgA2m(1) allotypes contain four and eight N-glycosylation sites, respectively; IgG1 has only two.
And the efficiency of glycosylation is significantly higher for IgA than IgG [122].
These biopharmaceutical qualities, notwithstanding, a number of critical issues remain. First, the full in-vivo repertoire of the IgA isotype’s antitumor effect is difficult to ascertain. Although a direct cytostatic effect in HER2+ cancer cell lines appears to be attributable to Fab-HER2 binding, therapeutic efficacy is largely dependent on Fc interactions with endogenous effector components, both innate and adaptive [123-125] and mice do not have FcαR1-bearing effector cell [126]. Nonetheless, the anti-HER2 IgA mAb has been shown to be effective in xenograft models of transgenic mice [127].
Notably, IgA has two to six O-glycosylations in the hinge region and two to five pairs of N-glycosylation sites on each heavy chain. O-and N-glycosylations on IgG are zero and one, respectively. Because post-translational glycosylation is critical for antibody function, production of recombinant IgA mAbs is apt to be less efficient, more heterogeneous, and costly. Furthermore, a humanized mAb may also be more immunogenic. On the other hand, increased numbers of sialic acid residues have been shown to be pharmaceutically and therapeutically beneficial. Indeed, decreasing the number of glycosylation sites and increasing carbohydrate sialylation may circumvent some of these issues [128].
Ig polymorphism and immunogenicity
The immunogenicity of recombinant humanized mAbs is indeed less than chimeric products though not totally devoid of immunologic ramifications. In contrast to native human immunoglobulins which are the products of multiple processes that modify the glycoproteins coincidently as well as post-translationally, engineered mAbs are usually produced in xenogeneic tissue systems. As such, recombinant antibodies generated in CHO and NS0 cells may not have the same structural fidelity as natural immunoglobulins due either to lack of appropriate (human) translational modifications or addition of (non-human) components.
The problematic issue of immunogenicity can be further aggravated by profound human genotypic variability. One relevant example is the obtrusive polymorphism inherent in heavy and light chains of innate human IgG. Also referred to as allotypy, exposure to a recombinant mAb with a different polymorphism profile will stimulate an anti-allotypic response. This “allergic” reaction could antagonize the therapeutic effect, intensify systemic clearance, or even produce severe adverse effects.
Currently, human IgG allotypes are defined by unique epitopes expressed on the native immunoglobulin molecule; an extensive array of polymorphisms has been characterized in both CH and CL. Of most interest with regards to engineered antibodies are the allotypes expressed on CH. This particular focus is due largely to the finding that the genes encoding CH are clustered within the immunoglobulin heavy locus and are inherited as a haplotype or a set of DNA alterations. Polymorphisms on the CH of IgG are indicated by genetic marker [Gm], subclass [1m], and number or letter (1 (a), 2 (x), 3 (f), 17 (z)). In addition are two light chains, κ and λ, which comprise the Ig molecule in a 3:2 ratio, respectively. Allotypes for the κ chain have been designated as Km1, Km2, and Km3. Serologically, this information is important clinically in order to reduce the immunogenicity of humanized mAbs. While all of the approved mAbs use the IgG G1m format, engineering of the final construct also included knowledge regarding the prevalence of specific G1m allotypes. Even though specific combinations of alleles are not routinely expressed across different populations, one haplotype, G1m17,1, exhibits crossover between Caucasians, African Americans, and Asians [129].
For trastuzumab, the specific allotype generated for clinical use is designated IgG G1m17; Km3. Notably, replacing lysine with threonine at position 214 of the CH resulted in G1m17, an allotype that appeared to be less immunogenic [130]; alanine and valine on amino acid positions 153 and 191 defines the Km3 allele [131]. Except for the paratope, at least two other mAbs, omalizumab and infliximab, have the identical construct.
Despite incorporation of stringent measures to minimize exposure to non- or altered-self molecules, anti-trastuzumab (or any engineered mAb) antibodies may still be generated. Even “fully-human” mAbs can be immunogenic because of allotypic differences. For those interested in mining the entire set of nomenclature for human immunoglobulin allotypes, the data are available and accessible [132].
Conclusion
The role of passive immunity-borne diseases of the newborn is relatively uncommon, frequently self-limiting, and uncommonly fatal. Still, there are cases where certain conditions remain through adulthood. The possibility of severe neonatal abnormalities mediated by therapeutic anticancer antibodies transferred during gestation is plausible, yet possibly preventable. Engineering additional mAb isotypes should be considered hope, not hubris.
Conflict of Interest Statement
The authors have no financial relationship or other conflict of interest with industry, specifically related to any product referred to in, or could be inferred from, the contents of this paper.
References
- Levy O. Innate immunity of the newborn: basic mechansims and clinical correlates. Nat Rev Immunol. 2007;7(5):379–90.
- Saji F, Samejima Y, Kamiura S,et al. Dynamics of immunoglobulins at the feto-maternal interface. Rev Reproduction. 1999;4(2):81-9
- Gitlin D, Boesman M. Serum alpha-fetoprotein, albumin and gamma-globulin in the human conceptus. J Clin Invest. 1966; 45 (11):1826-38.
- Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248-55.
- Motta M, Chirico G, Rebaioli CB, et al. Anticardiolipin and anti-beta2 glycoprotein I antibodies in infants born to mothers with antiphospholipid antibody-positive autoimmune disease: a follow-up study. Am J Perinatol. 2006;23(4):247-51.
- Lee LA. Neonatal lupus erythematosus: clinical findings and pathogenesis. J Investig Dermatol Symp Proc. 2004;9(1):52-6.
- Paro-Panjan D, Kitanovski L, Avcin T. Neonatal antiphospholipid syndrome associated with heterozygous methylentetrahydrofolate reductase C677T and prothrombin G20210A gene mutations. Rheumatology. 2007;46(4):720-21.
- Ozdemir H, Akman I, Coskun S, et al. Maternal thyroid dysfunction and neonatal thyroid problems. Int J Endocrinol. 2013;2013:987843.
- Koelewijn JM, Vrijkotte TG, van der Schoot CE, et al. Effect of screening for red cell antibodies, other than anti-D, to detect hemolytic disease of the fetus and newborn: a population study in the Netherlands. Transfusion. 2008;48(5):941-52.
- Blanchette VS, Chen L, de Friedberg ZS, et al. Alloimmunization to the PlA1 platelet antigen: results of a prospective study. Br J Haematol.1990;74(2):209-15.
- Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children. Pediatrics. 2011;127(6):1034–1042.
- Braunschweig D, Ashwood P, Krakowiak P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29(2):226-31.
- Bauman MD, Iosif AM, Ashwood P, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3(7):e278.
- Singer HS, Morris C, Gause C, et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol . 2009;211(1-2):39-48.
- Braunschweig D, Krakowiak P, Duncanson P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;3(7):277.
- Silverman JL, Yang M, Lord C, et al. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11(7):490-502.
- Rainey JK, Goh MC. A statistically derived parameterization for the collagen triple-helix. Protein Sci. 2002;11(11):2748-54.
- Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011; 3(4):442-74.
- Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2(7473):1087–93.
- Ellery PM, Cindrova-Davies T, Jauniaux E, et al. Evidence for transcriptional activity in the syncytiotrophoblast of the human placenta. Placenta. 2009;30(4):329-34.
- Carvalho C BT, Vieira HM, Dimantas RB, et al. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res. 1996;29(2):201–04
- Cervenak J, Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet Immunol Immunopathol. 2009;128(1-3):171–77.
- Brambell FW, Hemmings WA, Oakley CL, et al. The relative transmission of the fractions of papain hydrolyzed homologous gamma-globulin from the uterine cavity to the foetal circulation in the rabbit. Proc Royal Soc London B. 1960;151(145):478–82.
- Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15(7):733–38.
- Firan M, Bawdon R, Radu C, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. Int Immunol. 2001;13(8):993–02.
- Gastinel LN, Simister NE, Bjorkman PJ. Expression and crystallization of a soluble and functional form of an Fc receptor related to class I histocompatibility molecules. Proc Natl Acad Sci USA. 1992;89(2):638-42.
- Huber AH, Kelley RF, Gastinel LN, et al. Crystallization and stoichiometry of binding of a complex between a rat intestinal Fc receptor and Fc. J Mol Biol. 1993;230(3):1077-83.
- Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372(6504):379–383.
- Englund JA. The influence of maternal immunization on infant immune response. J Comp Pathol. 2007;137:S16-19.
- Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51(11):2916-27.
- Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71(2):666–69.
- Ober RJ, Martinez C, Lai X, et al. Exocytosis of IgG as mediated by the receptor, FcRn: an analysis at the single-molecule level. Proc Natl Acad Sci USA. 2004;101(30):11076–81.
- Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20(4):460-470.
- Zalevsky J, Chamberlain AK, Horton HM, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157-59.
- Petkova SB, Akilesh S, Sproule TJ, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18(12):1759–69.
- Shields RL, Namenuk AK, Hong K, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J Biol Chem. 2001;276(9):6591–04.
- Fernandez D, Valle I, Llamos R, et al. Rapid detection of rotavirus in faeces using a dipstick system with MAbs and colloidal gold as markers. J Virol Methods. 1994;48(2-3):315–23.
- Simister, NE, Story CM, Chen HL, et al. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26(7):1527–31.
- Israel EJ, Taylor S, Wu Z, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92(1):69-74.
- Haymann JP, Levraud JP, Bouet S, et al. Characterization and localization of the neonatal Fc receptor in adult human kidney. J Am Soc Nephrol. 2000;11(4):632-39.
- Zhu X, Meng G, Dickinson BL, et al. MHC class I‑related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166(5):3266–76.
- Brambell FW, Hemmings WA, Morris IG. A theoretical mode of gammaglobulin catabolism. Nature. 1964;203:1352-54.
- Ghetie V, Hubbard JG, Kim JK, et al. Abnormally short serum half-lives of IgG in b2-microglobulin deficient mice. Eur J Immunol. 1996;26(3):690-6.
- Perussia B, Dayton ET, Lazarus R, et al. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983;158(4):1092-1113.
- Van de Winkel JGJ, Capel PJA. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14(5):215–21.
- Bindon CI, Hale G, Bruggemann M, et al. Human monoclonal IgG isotypes types differ in complement activating function at the leve