Research Article - Journal of Biochemistry and Biotechnology (2022) Volume 5, Issue 5
In vitro Antidiabetic assay, Phytochemical Screening, Antioxidant activity and Antimicrobial Potential of Amaranthus viridis Linn.
Rakhi Das*and Bijay Kumar Das
Department of Biochemistry, Universal Engineering and Science Collage, Pokhara University, Chakupat, Lalitpur, Nepal
- Corresponding Author:
- Rakhi Das
Department of Biochemistry
Universal Engineering and Science College
Pokhara University, Lalitpur, Nepal
E-mail: Rakhidas021@gmail.com
Received: 06-Sep-2022, Manuscript No. AABB-22-73813; Editor assigned: 07-Sep-2022, PreQC No. AABB -22-73813(PQ); Reviewed: 21-Sep-2022, QC No AABB-22-73813; Revised: 23-Sep-2022, Manuscript No. AABB-22-73813(R); Published: 30-Sep-2022, DOI:10.35841/aabb-5.5.123
Citation: Das R, Kumar Das B. In vitro Antidiabetic assay, phytochemical screening, antioxidant activity and antimicrobial potential of Amaranthus viridis Linn. J Biochem Biotech 2022;5(5):123
Abstract
Amaranthus viridis L. belongs to family (Amaranthaceae) commonly known as “Chowlai” is a common wild vegetable and weed of cultivation. A. viridis contains several compounds like amino acids lysine, arginine, histidine, cystine, phenylalanine, leucine, isoleucine, valine, threonine, methionine, tyrosine etc. In search of new activities chemical entities, pytochemical screening of the extract from leaves of A. vilidis L. indicates the presence of biologically active constituent: saponins, tannins and phenols, flavonoids, alkaloids, cardiac glycoside, steroid and triterpenoids. A. vilidis L have some chemical constituent that exhibits potent anti-inflammatory, antihepatotoxic, antiulcer antiallergic, antiviral actions. A. viridis used in Indian and Nepalese traditional system to reduce labour pain and act an antipyretic. The Negritos of the Philippines apply the bruised leaves directly to eczema, psoriasis and rashes etc. Other traditional uses range from an anti-inflammatory agent of the urinary tract, venereal diseases vermifuge, diuretic, anti rheumatic, antiulcer, analgesic, antiemetic, laxative, improvement of appetite, antilep-rotic, treatment of respiratory and eye problems , to treatment of asthma. In the present study, various pharmacological activities of Amaranthus viridis were tried to be determined by utilizing in vitro apprpaches. The result showed that the leaves (50 mg/ml) contain highest amount of phenolic content with the value 82.36 ± 2.589. Similarly, flavonoids and antioxidant activity were found to be highest in seed with the approximate value of 74.38 ± 2.317 and 87.28 ± 2.187 respectively at the concentration of 50 mg/ml. The antidiabetic activity was also found to be highest in leaves at the concentration of 40 mg/ml with the value 85.25 ± 1.37. The methanolic extract of whole plant showed higher antibacterial activity.
Keywords
Phytochemical screening, Antioxidant, Total phenolic and flavonoids content, H2O2 scavenging activity, Alpha amylase inhibition activity, Antimicrobial screening and total protein and carbohydrate content in fresh leaves and seeds.
Introduction
Plants, which contain substances that can be used for treatment of diseases, are called medicinal plants. Medicines derived from plants are widely popular due to their safety, easy availability and low cost. Herbal medicines may include whole parts of plant or mostly prepared from leaves, roots, bark, seed and flowers of plants. They are administered orally, inhaled or directly applied to the skin. Medicinal herbs are more significant to the health of individual and community. The medicinal value of these plants lies in bioactive phytochemical constituents that produce definite physiological action in the human body. Some of the most important bioactive phytochemical constituents are alkaloids, essential oils, flavonoids, tannins, terpenoids, saponins, phenolic compounds and many more. These natural compounds formed the foundations of modern prescription drugs as we know today. Use of plants based drugs for curing various ailments is as old as human civilization and is used in all cultures throughout history. The primitive man started to distinguish between useful and harmful are poisonous plants by trail and errors. A well-defined herbal pharmacopoeia was developed by tribal people that were based on information collected from local flora, religion and culture. The knowledge of medicinal plants was gradually developed and passed on from one individual to other, which foundation for traditional medicine throughout the world.
Nepal is rich in term of biodiversity. Biodiversity is the degree of variation of life. This can refer to genetic variation, species variation or ecosystem variation within area, biome or planet. There are high hills, high mountains and plain land of terai region where different species of plants and animals are found. Nepal also is rich in physical diversity. Physical diversity has created diversity in ecosystem and climatic situation. Each region has its typical topography, climate and vegetation. This diversity has helped in wholesome diversity.
Nepal has separated 18% of the total area for natural parks, conservation areas, wildlife reserves and hunting areas. Most of the ecosystem, besides desert and ocean are found in Nepal. Nepal has a total of 118 types of ecosystem, 75 types of vegetation and 35 types of forests. There are different animals and plants found only in Nepal, according to ecological region. So Nepal is rich in biodiversity [1]. Some important climatic factors influencing biodiversity and the composition of flora and fauna in Nepal include rainfall, winter snowfall and temperature [2].
The amaranthus represents a horticulturally important group of annual herbaceous plants represtented by total of 60 species in the world flora under Amaranthus L., which is the fifty largest genus of the family Amarantheceae. Green amaranthus serve as one of the most delicious leafy vegetables and are rich sources of protein (upto 5.6% on fresh weight basis), requisite vitamins (A, B, C, Folic acid), minerals (Ca, Mg, K, P, Na, N, Fe, Mn, Zn) and fibers (5.25%) [3,4]. Besides, they contain other biologically pro-health compounds such as the antioxidant, squalene, and carotenoids [5] and thus, are recommended as a nutritious food with medicinal properties for young children, lactating mothers and for patients with asthma, hemorrhage, anaemia or kidney complaints. A.virdis contains several chemical compounds like amino acids, lysine, tannins, flavonoids, histidine, cysteine, valine, methionine etc. From the previous published research the phytochemical screening of A.virdis leaves had contained biologically active chemical constuitents like terpenoids, steroids, cardiac glycoside, alkaloids, flavonoids, phenols, saponins etc. The Negritos of the Philippines apply the bruished leaves directly to the eczema, psoriasis and rashes. This is the only plant which has the property to cure almost all disease like from the fever, asthama, heart disease, inflammation up to the cancer (Figure 1).
On seeing its ecology, it requires well drained fertile soil in a sunny position. It should not be provided with inorganic fertilizer. It is cultivated as a food in tropical countries. It photosynthesis by C4 carbon-fixation pathway which effects at high temperature. A.viridis is propagated by sowing seeds in spring. Germination is rapid if the soil is warm. A drop in temperature overnight will assist the germination. Apart from seeds the cuttings of growing plants are also used for propagation which roots easily. Some medicinal uses of this plant are listed below:
• The herb is used as astringent, emollient, in dysentery, inflammation, constipation, eczema, bronchitis, antidiabetic, anaemia and leprosy. Plant is also used as sag for cooking and fodder plant.
• Leaves are emollient and anthelmintic. Roots and shoots are used to control excessive menstruation, purification of blood, digesting agent, piles etc.
• The Negros of the Philippines applies the bruised leaves directly to eczema, psoriasis, and rashes with good results.
• The leaves make a good emollient preparation available in some of the Filipino villages for insect bites, sunburn, and regular burns.
• The reddish-brown fiber from the leaves are soaked and used for eye treatments.
• The decoction of young roots is used for the treatment of respiratory complaints and asthma.
• Provides energy: Highly packed with carbohydrates, proteins, vitamin K, folate, riboflavin, vitamin A, vitaminB6, and vitamin C, amaranth leaves boost energy in the body.
• Prevents electrolyte imbalance: Amaranth leaves are terrific source of manganese, iron, copper, calcium, magnesium, potassium and phosphorus necessary for maintaining proper mineral balance in the body.
• Improves digestion: High dietary fiber content (3 times that of wheat) in the greens improve digestive health and reduces constipation. It is easily digestible and good for both young ones and elders.
• Aids in weight management: Protein in the leaves help to reduce insulin levels in the blood and also release a hormone that lessen hunger pranks and prevent "binging catastrophe".
• Reduces bad cholesterol: One of the key benefits of amaranth leaves is cholesterollowering ability. Being fibrous, this leafy vegetable is effective in reducing LDL levels in the blood and promotes weight loss. Presence of tocotrienols (a type of vitamin E) also aids in cholesterol lowering activity.
• Good for anemic patients: Iron-rich (5 times that of wheat) red amaranth leaves promote coagulation and increase hemoglobin content and red blood cell counts.
• Decreases risk of cardiovascular disease: Amaranth leaves are an excellent dietary source of phytosterols that lowers blood pressure and prevents heart ailments including stroke.
• Fight-off cancer: Presence of lysine (an essential amino acid) along with vitamin E, iron, magnesium, phosphorus and potassium and vitamin C helps to fight against free radicals responsible for aging and formation of malignant cells.
• Ayurvedic treatments: Juice extracted from fresh amaranth leaves are prescribed for treating diarrhea, and hemorrhage conditions.
• Stop hair loss and graying: Besides regular consumption, applying juice from the leaves prevent brittle hair falling. This wonderful cosmetic benefit of amaranth leaves also retards the onset of premature graying.
• Prevents calcium-deficiency ailments: Calcium present in amaranth leaves reduce risk of osteoporosis and other calcium deficiencies because it has twice the calcium as milk. Indeed good news for lactose-intolerance.
• Improves eyesight: Vitamin C found in the leaves contribute to towards healthy vision.
• Green amaranth also has clusters of nutty edible seeds, which can be eaten as snacks or used in biscuits. Porridge can be made by boiling the seeds in water. Unlike other amaranths, the seeds can be easily harvested by scraping the ripe spikes of seeds between the fingers [6].
Methods and Methodologies
Collection, identification and authentication of plant material:
The plant of Amaranthus viridis.Linn was collected in the month of August, 2018 from the Terai region of Nepal. The plant material was identified and authenticated by Botanist of National Herbarium Department, Godawari Lalitpur Nepal.
Shade drying, granulation and extraction
The plants leaf, seed and stem were taken and dried in shade. Then the shade dried plant parts were coarsely powdered by means of mix grinder and was filtered through sieve no 60 to get the coarse powder. Then the coarsely powdered materials were weighed, packed in an airtight container and used for extraction with solvent, phytochemical studies and pharmacological studies.
Preparation of plant extract
About 30 gram of leaves, 15 gram of seeds and 60 gram of whole plant i.e. mixture of leaves, seed and stem of Amaranthus viridis.Linn were placed in 300 ml, 150 ml and 600 ml (solvent to sample ratio of 10:1 (v/w) solvent to dry weight has been used) of methanol and ethanol as a solvent respectively and was then stored at room temperature for 72 hours. The extracts were then filtered through Whatmann No.1 filter paper and were evaporated to dryness using Rotary Evaporator at a much reduced pressure. This was followed by the dilution of the crude extract (concentrated using a rotary evaporator) with mother solvent i.e. DMSO (Dimethyl Sulpho-oxide) to obtain a stock solution of 100 mg/ml from which a series of dilutions were made as required.
Preliminary Phytochemical Screening
Test for Carbohydrates
A small quantity of the extract was dissolved separately in 4 ml of distilled water and filtered. The filtrate was subjected to be following tests to detect the absence of carbohydrates and glycosides.
Molisch’s Test: The filtrate was treated with 2-3 drops of 1% alcoholic α-naphthol solution and 2 ml of concentrated sulphuric acid was added along the test tube. Dis-Appearance of brown ring at the junction of two liquids indicated presence of carbohydrates.
Fehling’s Test: The filtrate was treated with 1 ml of Fehling’s solution A and B and heated on the water bath. A reddish precipitate was obtained shows the carbohydrate [7,8].
Test for Alkaloids
In 1ml of sample extract, a few drops of Dragendroff’s reagent were added. Appearances of Orange red color indicated the presence of the alkaloids [9].
Test for Carotenoid
The different extracts were treated with 1 ml conc. H2So4 to examine the presence of carotenoids. Development of orangeyellow color confirms the presence of carotenoids [10].
Test for Flavonoids
10% of dilute ammonia (5 ml) was added to different extracts of plants. Concentrated sulphuric acid (1ml) was added. A yellow coloration that disappears on standing indicates the presence of flavonoids [11].
Test for Phenols and Tannins
Each extract was diluted with water and 3-4 drop of 10% ferric chloride solution was added. Appearance of the blue-green or black color indicated the presence of phenols and tannins [10].
Test for Polyphenols
1ml of different extract of plant was mixed with 1ml of water and 3 drops of 1% (w/v) ferric chloride solution was added. Presence of polyphenols is confirmed by appearance of greenish blue color [10].
Test for Fatty acids
2ml of different extract was concentrated and spotted on filter paper. It is then observed for yellow spot resistance even after the evaporation of solvents [10].
Test for Saponins
2ml of different extracts were taken and treated with hot water and vigorously shaken for 30 sec. Thick forth forming indicated the presence of saponins [10].
Test for Amino acids
3ml of the test solution was heated with 3 drops of 5% Ninhydrin solution in a boiling water bath for 10 min and then the appearance of dark blue color shows the presence of amino acids [7,8].
Test for Proteins
Biuret test: To the extract solution (2 ml), biuret reagents was added, Violet color indicates the presence of protein.
Xanthoprotein test: To 5 ml of extract solution, 1 ml of nitric acid was boiled, yellow precipitate was formed. After cooling it, added 40% sodium hydroxide solution orange color was formed which indicated the presence of proteins [7,8].
Test of Cardiac glycosides
The extract was mixed with picric acid or sodium picrate. Orange color was formed. Indicates the presence of glycosides [7,8].
Test for Anthraquinone glycosides
200 mg of the material was boiled with 2 ml of dilute H2SO4. It was treated with 2 ml of 5% aqueous ferric chloride solution for 5 minutes. It was shaken with equal volume of chloroform. The organic solvent layers were separated and equal volume of dilute ammonia was added, ammonia layer showed pinkish red color which indicates the presence of glycosides [7,8].
Test for Steroids
Each extract was treated with acetic anhydride (2.5 ml) and chloroform (2.5 ml). Then concentrated solution of sulphuric acid was added slowely and green bluish color was observed for steroids [11].
Quantitative Phytochemical Screening
Determination of Total Phenolics
The total amount of phenolic content of different plant extract was determined by Folin cinocalteu method [11]. To 1ml folin cinocalteu’s reagent, previously (1:4) was added to 1ml of standard solution of concentration 1mg/ml. To the mixture, 4ml of sodium carbonate (75 g/L) and 10 ml of distilled water were added and mixed well. The mixture was allowded to stand for 2 hr at room temperature. Contents were then centrifuged at 2000 rpm for 5 min and the absorbance of the supernatant was taken at 760 nm. A standard curve was obtained using various concentrations of Gallic acid. All the determinations were done in triplicate and the total phenolic content were expressed as Gallic acid equivalents (GAE).
Determination of Flavonoids
The total flavonals in the plant extract were estimated using the method of Pokhrel et al. [11]. In this method 1ml if sample from concentration 1mg/ml was added to 2ml of 2% AlCl3 ethanol and 3.0 ml (50 g/L) sodium acetate solutions were added to the standard solution of different concentration. The absorbance was then taken at 440 nm after 2.5 hr incubation at room temperature. All the measurements were done in triplicate and the total flavonoid content were expressed as Rutin equivalent (RE).
Evaluation of Antioxidant assay and Free Radical Scavenging Activity
DPPH Radical Scavenging Activity
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was measured according to the modified method of Pokhrel et al. [11]. 600 μM DPPH was added by dissolving DPPH powder in methanol and used as stock solution. Plant extracts were taken in various concentrations: 10, 20, 30, 40, 50 mg/ml made in methanol as a solvent. An aliquot of 0.5 ml of 600 μM DPPH solution and 1ml of different plant extracts at different concentrations were mixed and incubated at 25ºC for 30 min in dark and absorbance of the test mixture was read at 517nm using a spectrometer against methanol only blank. Absorbance of DPPH control containing only 1ml of methanol in place of the extract was also measured. All experiments were performed thrice and the results were averaged. Ascorbic acid was used as a standard.
% inhibition = (Acontrol – Asample / Acontrol) × 100
Where A control and A sample stand for absorption of the control sample and absorption of tested extract solution respectively. All experiments were performed thrice and the results were averaged.
Principle
When DPPH reacts with an antioxidant compound or extract, which can donate hydrogen, it is then reduced. The change in color (from deep violet to light yellow) can be measured at 517nm on a UV visible light spectrophotometer.
DPPH. + AH → DPPH-H + A.
DPPH. + R. → DPPH-R
Hydrogen peroxide scavenging activity
The effect of plant extracts of Amaranthus viridis Linn to scavenge hydrogen peroxide was determined according to the method of Ilhami et al. [12]. A solution of hydrogen peroxide (40mM) was prepared in phosphate buffer (pH 7.4). Hydrogen peroxide solution (0.6 ml) was added to five different concentrations (10, 20, 30, 40 and 50 mg/ml) of different extracts of A.viridis. The mixture was allowed to stand for 10 min and the absorbance was measured at 230nm. The blank solution contain only phosphate buffer without hydrogen peroxide. The percentage inhibition of hydrogen peroxide of AC and standard compounds was calculated using the following formula:
% inhibition [H2O2] = [(A0 - A1) / A0] × 100
Where A0 was the absorbance of the control and A1 was the absorbance in the presence of the sample of AC and standards.
In-vitro Anti-diabetic Activity
Alpha amylase inhibition assay
α-Amylase inhibition assay was performed using a standard protocol with slight modification of Kusano et al. [13]. The undigested starch due to enzyme inhibition was detected at 630 nm (blue, starch-iodine complex). Substrate was prepared by dissolving 200 mg starch in 25 ml of NaOH (0.4M) by heating at 100ºC for 5 min. After cooling, pH was adjusted to 7.0 and the final volume was made up to 100 ml using distilled water. Acarbose is used as positive control. 400 μL of substrate solution was pre-incubated at 37ºC for 5 min with 200 μL of acarbose or plant extract at varying concentrations (10, 20, 30, 40 and 50 mg/ml), followed by 200 μL of 50 μg/ ml α-amylase (20mM phosphate buffer with 6.7 mM NaCl, pH 6.9), and incubation at 37ºC for 15 min. Termination of the reaction was carried out by adding 800 μL of HCl (0.1). Then, 100 μL of iodine reagent (2.5 mM) was added, and absorbance was measured at 630 nm. The assay was carried out in triplicates using spectrophotometer. Percentage of inhibition was calculated using the formula:
% inhibition = (1- [(Abs2-Abs1) / (Abs4-Abs3)]) × 100
Where, Abs1 = Absorbance of the incubated mixture containing plant sample, starch and alpha amylase
Abs2= Absorbance of the incubated mixture of sample and starch
Abs3= Absorbance of the incubated mixture of starch and α-amylase
Abs4= Absorbance of the incubated solution containing starch
Antimicrobial Screening
The antimicrobial properties of the plants were tested by using disc diffusion methods. For this the Muller Hinton Agar was prepared with the help of manufacture (Hi-media) recommendations. Similarly small size filter paper discs were prepared by punching Whatmann’s filter paper with the help of punching machine. Both agar and discs were then sterilized in autoclave. Then it was cooled and 20ml media was poured to sterilized petri-plates. The cultural inoculums of the organisms to be tested were also prepared in tubes containing sterile liquid media and were incubated for 24 hr for the growth of the bacteria. The inoculum of bacteria was transferred into petri-plates containing solid nutrient media agar using sterile swab. The swab was used to spread the bacteria on the media in a confluent lawn. One swab was used for one species of bacteria and then discarded. All the petriplates were labeled properly. The sterile filter paper a disc was then soaked in plant extracts of different concentration and was then placed over the bacteria on the petri-plate. The mother solvent DMSO was used as the negative control and the standard broad spectrum antibiotic Tetracycline served as the positive control. The plates were then incubated at 37ºC for 24 hours. After the overnight incubation at 37ºC, the antibacterial was processed by measuring the diameter of the zone of inhibition (ZOI) with the help of millimeter ruler. The inhibitory zone indicates absence of bacterial growth [14].
Test organism
Gram negative bacteria
1. Escherichia coli
2. Salmonella typhi
3. Shigella
4. Pseudomonas aeruginosa
Gram positive bacteria
1. Staphylococcus aureus
The total soluble protein contents in leaves of different amaranth species were extracted and assayed by using the method described by Lowry et al., [15]. 0.5g of leaves were washed in distilled water, and crushed and homogenized in 5ml of Tris-HCl buffer (pH 7.0) by using mortar and pestle. The homogenates were centrifuged at 5000 rpm for 20 minutes. Now 5ml of 10% trichloroacetic acid (TCA) was added to the supernatant and boiled in water bath for 3 minutes. After cooling, the treated samples were centrifuged for 20 minutes at 5000 rpm. The pellets were solubilized in 5 ml of 0.1N NaOH. Subsequently, 5 ml of alkaline reagent was added to the test tube containing 0.1ml of protein extract thoroughly mixed. The samples were allowed to cool for 10 minutes at room temperature and then 0.5ml of folin reagent was added to it. The reaction mixture was incubated for 30 minutes in temperature controlled water bath (37oC). After cooling, the intensity of the color developed (blue) was read at 650 nm in a spectrocolorimeter (spectronic-20 Boasch & Lomb, USA). Protein estimation of each extract was performed in triplicate and the total soluble proteins in each sample was determined by using bovine serum albumin (Sigma) as standard and expressed in mg/gm fresh weight in case of leaves.
Extraction and Estimation of Carbohydrate
Total soluble carbohydrate was measured by the Anthrone method. 0.1 g of fresh leaves was hydrolyzing by keeping it in a boiling water bath for three hours with 5 ml of 2.5N HCl and then cools to room temperature. Neutralize it with sodium carbonate until the effervescence ceases. Make up the volume to 100 ml and centrifuge and collect the supernatant. Carbohydrate estimation of each extract was performed in triplicate. Take 1ml aliquots for analysis. Add 4ml of ice-cold anthrone reagent, heat for 10 minutes in a boiling water bath. Cool rapidly in running tap water, read green to dark color at 630 nm. The total soluble carbohydrate in each sample was determined by using glucose as standard and expressed in mg/100 g fresh weight [15].
Results and Discussion
Preliminary phytochemical screening
The preliminary phytochemical screening of the different extract of Amaranthus viridis Linn showed the presence of various secondary metabolites. The sample investigated were screened for presence of alkaloids, carotenoids, flavonoids, phenols and tannins, polyphenols, cardiac glycosides, proteins, amino acids etc.by different methods table 1. Alkaloids, proteins, aminoacids, phenols and tannins, polyphenols, flavonoids were the most prominent in the plant extracts. Present of alkaloids, flavonoids and phenolic compounds are a major group of compound that acts as primary antioxidants or free radical scavangers. Pure isolated alkaloids and their synthetic derivatives have been used as analgesic, antispasmodic and bactericidal agent. Flavonoids and phenols used as antibacterial, ant-inflammatory, anti-allergic, antimutagenic, anti-thrombotic and anti-viral activity. Thus the preliminary screening test indicates the presence of the above mentioned bioactive compounds. Subsequently it may be used for the preparation of drug in a systematic way which may lead to the cure of many ailments in the future. So due to the presence of such secondary metabolites A.viridis Linn may have added to medicinal value of the plant.
| Solvents | Dry weight of plant (g) | Volume of solvents (ml) | Weight of extract after evaporation (mg) | Mother solvents added (ml) | Finial concentration (mg/ml) | |
|---|---|---|---|---|---|---|
| Methanol | Leaves | 30 | 300 | 620 | 6.2 | 100 |
| Seed | 20 | 200 | 440 | 4.4 | 100 | |
| Ethanol | Whole Plant(leaf+seed+stem) | 50 | 500 | 565 | 5.65 | 100 |
| Methanol | Whole Plant(leaf+seed+stem) | 60 | 600 | 540 | 5.4 | 100 |
Table 1. The amount of extract obtained from the extraction of green Amaranthus viridis Linn and final concentration was summarized in table.
The result of phytochemical screening of different extracts of A.viridis is as shown in table 2 below:
| Phytochemicals | ||||
|---|---|---|---|---|
| Test | Methanolic Extract | Ethanolic Extact | Methanolic Extract | |
| Leaves | Seed | Whole plant | Whole plant | |
| Carbohydrate | + | + | + | + |
| Proteins | + | + | + | + |
| Alkaloids | + | + | + | + |
| Carotenoids | + | + | + | + |
| Flavonoids | + | + | + | + |
| Polyphenols | + | + | + | + |
| Aminoacids | + | + | + | + |
| Fatty acids | + | + | + | + |
| Saponins | + | + | + | + |
| Steroids | - | - | - | - |
| Cardiac Glycoside | + | + | + | + |
| Anthraquinone Glycosides | + | + | + | + |
| Phenols and Tannin | + | + | + | + |
Table 2. Qualitative analysis of the phytochemical of different extract of A.viridis L.
Quantitative Phytochemical Screening
Total phenolic content in methanolic and ethanolic extracts of A.viridis L
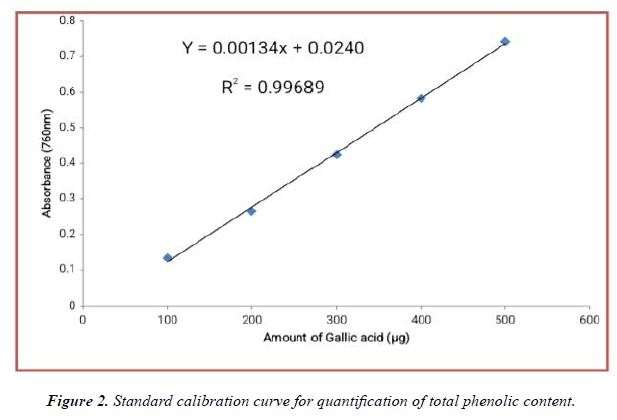
The contents of phenolic compounds in A.viridis L extracts were determined from the regression equation of the calibration curve (y = 0.00134x + 0.02400, R2 = 0.99689). A calibration curve was drawn by using different concentration of Gallic acid and is as shown in (Figure 2). The amount of phenolic compounds was expressed as Gallic acid equivalent (GAE) in mg/g dry weight of plant extract and is shown in Table 3 below. From the experimental data given below, it was found that the total phenolic content in A.viridis L was in the given order:
| Concentrations (mg/ml) |
Methanolic Extract | Ethanolic Extract | Methanolic Extract | |
|---|---|---|---|---|
| Leaves | Seed | Whole Plant | Whole plant | |
| 10 | 53.94 ± 3.273 | 48.87 ± 4.631 | 58.74 ± 2.587 | 63.57 ± 1.540 |
| 20 | 68.52 ± 3.86 | 57.64 ± 2.041 | 65.23 ± 7.493 | 70.12 ± 2.463 |
| 30 | 76.44 ± 3.053 | 63.08 ± 5.874 | 69.51 ± 0.658 | 77.43 ± 1.040 |
| 40 | 80.05 ± 4.261 | 68.59 ± 1.214 | 75.33 ± 4.251 | 83.01 ± 3.860 |
| 50 | 82.36 ± 2.589 | 74.38 ± 2.317 | 80.29 ± 2.924 | 87.09 ± 1.611 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 3. Total amount of phenolic content in methanolic and ethanolic extracts of A.viridis
Leaves > methanolic extract of Whole plant > ethanolic extract of Whole plant > Seed
Total flavonoid content in methanolic and ethanolic extracts of A.viridis L
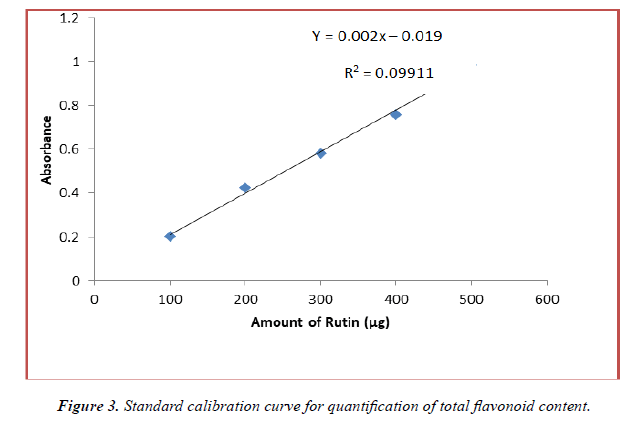
The flavonoid content of the methanolic and ethanolic extracts was determined from the regression equation of the calibration curve (y = 0.002x – 0.019, R2 = 0.991). The calibration curve was drawn by using different concentration of rutin and is as shown in (Figure 3). The amount of flavonoids was expressed as rutin equivalent (RE) in mg/g dry weight of plant extract and is table 4 below. From the experimental data given below, it was found that the total flavonoids content in A.viridis L was in the given order:
| Concentrations | Methanolic Extract | Ethanolic Extract | Methanolic Extract | |
|---|---|---|---|---|
| (mg/ml) | Leaves | Seed | Whole Plant | Whole plant |
| 10 | 54.00 ± 3.983 | 62.87 ± 2.602 | 45.47 ± 3.025 | 48.06 ± 5.274 |
| 20 | 59.64 ± 3.024 | 71.02 ± 1.710 | 52.08 ± 1.142 | 55.62 ± 2.353 |
| 30 | 63.18 ± 2.914 | 83.24 ± 1.729 | 58.25 ± 3.381 | 60.38 ± 0.856 |
| 40 | 71.25 ± 3.650 | 88.19 ± 3.470 | 63.05. ± 4.143 | 64.00 ± 2.517 |
| 50 | 79.43 ± 2.990 | 97.38 ± 2.934 | 73.94 ± 2.001 | 75.56. ± 1.273 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 4. Total amt. of flavonoids content in methanolic and ethanolic extracts of A.viridis.
Seed > Leaves > methanolic extract of Whole plant > ethanolic extract of Whole plant
DPPH radical scavenging activity
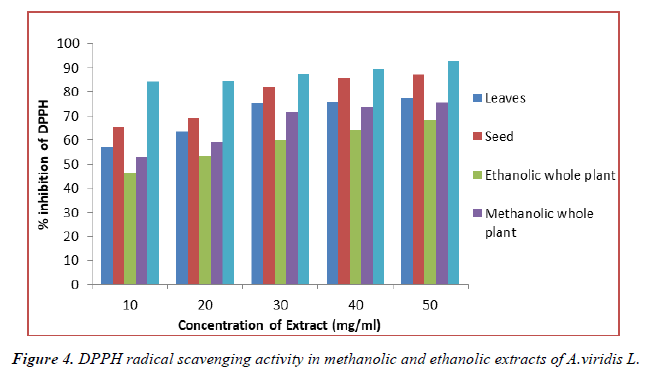
In-vitro antioxidant activity of methanolic and ethanolic extract of A.viridis L was measured on the basis of DPPH radical scavenging activity using ascorbic acid as standaed whis is shown in Table 5. DPPH radical scavenging activity assesses the capacity of extract to donate hydrogen atom or to scavenge the free radicals. The result showed that both methanolic and ethanolic extract of A.viridis L exhibit significant radical scavenging activity in a concentration dependent manner as shown in (Figure 4). The maximum inhibition of DPPH free radical was shown by 50 mg/ml methanolic extract of seed with % inhibition of 87.28% ± 2.187. Similarly, from the experimental data shown in table 5, the DPPH radical scavenging activity shown by leaves, methanolic and ethanolic extract of whole plant was found in the order given below:
| % Inhibition | |||||
|---|---|---|---|---|---|
| Concentrations | Methanolic Extract | Ethanolic Extract | Methanolic Extract | ||
| (mg/ml) | Leaves | Seed | Whole plant | Whole plant | Ascorbic acid |
| 10 | 57.12 ± 3.86 | 65.25 ± 1.025 | 46.29 ± 4.043 | 52.96 ± 2.706 | 84.33 ± 1.083 |
| 20 | 63.37 ± 5.874 | 68.97 ± 4.032 | 53.18 ± 5.614 | 59.03 ± 1.916 | 84.79 ± 1.967 |
| 30 | 75.17 ± 2.056 | 82.19 ± 4.863 | 59.74 ± 2.84 | 71.68 ± 2.731 | 87.63 ± 1.053 |
| 40 | 75.89 ± 2.589 | 85.75 ± 3.421 | 64.08 ± 1.000 | 73.59 ± 2.024 | 89.26 ± 0.957 |
| 50 | 77.51 ± 3.053 | 87.28 ± 2.187 | 68.19 ± 3.917 | 75.61 ± 0.634 | 92.72 ± 1.849 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 5. DPPH radical scavenging activity in methanolic and ethanolic extracts of A.viridis.
Seed > leaves > methanolic extract of whole plant > Ethanolic extract of whole plant
Although the DPPH radical scavenging abilities of methanolic and ethanolic extracts of A.viridis L was lesser than that of ascorbic acid but the result obtained from the study demonstrates that the extracts have protein donating capacity and could serve as free radical inhibitors or scavengers acting possibly as antioxidants.
Hydrogen peroxide scavenging activity
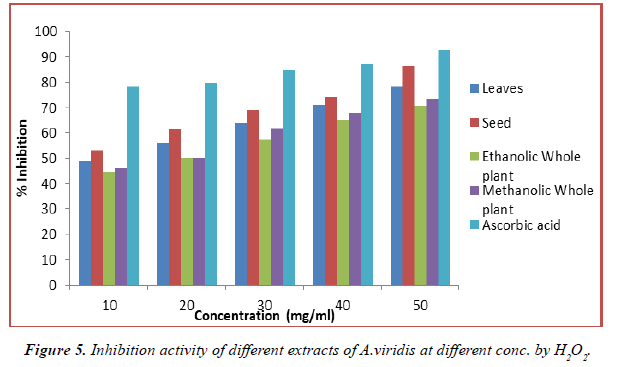
Hydrogen peroxide scavenging activity of the A.viridis L. extract on hydroxyl radical is given in Table 6. And compared with the standards, ascorbic acid. H2O2 is highly important because of its ability to penetrate biological membranes. H2O2 itself is not very reactive, it is a weak oxidizing agent, but it sometimes be toxic to cell because it may give rise to hydroxyl radical in the cells. It can cross cell membranes rapidly, once inside the cell, H2O2 can probably react with Fe2+ and possibly Cu2+ ions to form hydroxyl radical and this may be origin of many of its toxic effects. It can inactive few enzymes directly, usually by oxidation of essential thiol (-SH) groups. It is therefore advantageous for cells to control the amount of H2O2 that is allowed to accumulate. Thus, removal of H2O2 is very important for protection of food systems. A.viridis L. extract also demonstrated hydrogen peroxide decomposition activity in a concentration dependent manner. The decomposition of H2O2 by all extract of A.viridis L. may at least partly result from its antioxidant and free radical scavenging activity. The data obtained in this study reveal that the tested extract was significant free radical scavenger which reacts with H2O2 radical owing to its electron donating ability. Comparision of the antioxidant activity of the different extracts and ascorbic acid is shown in (Figures 5 and 6). In all methanol extract generally, exhibited the higher values of antioxidants. The result clearly shows that the solvent influences the extractability of the phenolic compounds. The antioxidant activity has a positive correlation with a phenolic content of all the solvent extracts of the plant. This confirms the assertion that phenolic content of plants contribute directly to their antioxidant properties. The H2O2 scavenging capacity of the plant extracts may therefore be related to the phenolic compounds present. The greater amount of phenolic compounds leads to more potent radical scavenging effect as shown by A.viridis L. plant extract.
| % Inhibition | |||||
|---|---|---|---|---|---|
| Concentration | Methanolic Extract | ||||
| (mg/ml) | Leaves | Seed | Ethanolic Extract | Methanolic Extract | Ascorbic acid |
| 10 | 48.59 ± 4.32 | 53.06 ± 1.03 | 44.27 ± 2.06 | 46.07 ± 5.85 | 78.16 ± 1.60 |
| 20 | 55.82 ± 1.09 | 61.28 ± 4.97 | 50.31 ± 2.82 | 50.33 ± 3.66 | 79.91 ± 3.26 |
| 30 | 63.91 ± 2.54 | 69.04 ± 4.61 | 57.28 ± 1.93 | 61.84 ± 3.72 | 84.68 ± 2.83 |
| 40 | 71.09 ± 2.78 | 74.19 ± 2.73 | 64.91 ± 3.55 | 67.90 ± 1.38 | 87.38 ± 5.26 |
Table 6. Inhibition activity of different extracts of A.viridis at different concentration by H2O2.
Alpha amylase inhibition activity
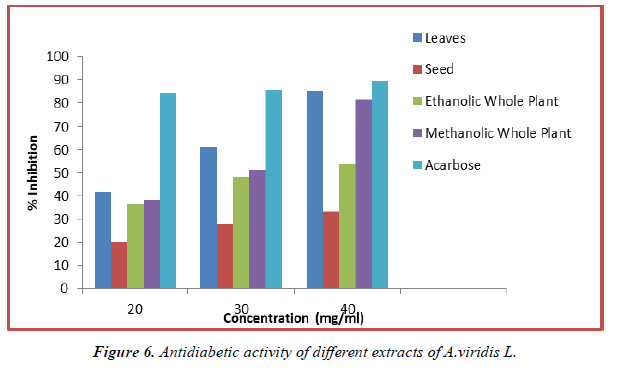
The study of anti-diabetic activity of methanolic and ethanolic extracts of A.viridis L with the standard of anti-diabetic medicine Acarbose was performed. The invitro analysis of samples show the inhibition from 36.23% to 85.25% for 20 mg/ml to 40 mg/ml for leaves, seed, methanolic and ethanolic extract of whole plant respectively.
Diabetes mellitus (DM) is a heterogeneous metabolic disorder majorly affecting global population. The individuals with Type 2 DM have delayed insulin release in response to food intake, contributing to persistent elevated PPG almost all day long. At the same time, the acute phase of diabetes is characterized by fluctuating PPG, which is tightly correlated with oxidative stress.
Postprandial hyperglycemic reduction can be achieved through inhibition of alpha amylase, which allows clearance of undigested carbohydrates thereby slowing down D-glucose absorption into the bloodstream. Drugs such as Voglibose, Acarbose and Miglitol have been found to be effective in the control of Type 2 diabetes by suppressing the hydrolysis of carbohydrates. Several herbal plant extracts have been accounted for the antidiabetic potentials, and extensively used in traditional medicines and ayurvedic treatment of DM for a very long time. The main advantage of these plant extracts is their nontoxicity, but they have not gained global medicinal importance and acceptance due to lack of scientific validation.
Antibacterial screening
The antibacterial activity of methanolic and ethanolic extract of A.viridis L tested against five human pathogenic bacteria is shown in the table 7 below:
| % Inhibition | |||||
|---|---|---|---|---|---|
| Concentration | Methanolic Extract | Ethanolic Extract | Methanolic Extract | ||
| (mg/ml) | Leaves | Seed | Whole Plant | Whole Plant | Acarbose |
| 20 | 41.72 ± 6.51 | 20.07 ± 2.58 | 36.23 ± 6.92 | 38.36 ± 5.83 | 84.36 ± 1.99 |
| 30 | 61.10 ± 3.44 | 27.88 ± 7.01 | 48.31 ± 3.70 | 51.33 ± 7.62 | 85.61 ± 2.08 |
| 40 | 85.25 ± 1.37 | 33.21 ± 5.66 | 53.46 ± 5.48 | 81.52 ± 2.30 | 89.52 ± 2.56 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 7. Alpha-amylase inhibition activity of different extracts of A.viridis.
| Test organism | Diameter of Zone of Inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Control | Tetracyclin | 200 | 300 | 400 | |||
| (DMSO) | (1µg/µl) | (mg/ml) | (mg/ml) | (mg/ml) | ||||
| Gram Negative Bacteria | ||||||||
| Escherichia coli | Leaves | - | 28 | 10 | 11 | 12 | ||
| Seed | - | 32 | 12 | 12 | 13 | |||
| Ethanolic-Whole plant | - | 24 | 9 | 6 | 9 | |||
| Methanolic-Whole plant | - | 33 | 13 | 14 | 17 | |||
| Salmonella typhi | Leaves | - | 27 | 8 | 9 | 12 | ||
| Seed | - | 30 | 10 | 12 | 14 | |||
| Eth-Whole plant | - | 25 | 5 | 8 | 10 | |||
| Meth-Whole plant | - | 28 | 12 | 15 | 16 | |||
| Leaves | - | 26 | 9 | 4 | 8 | |||
| Pseudomonas aeruginosa | Seed | - | 29 | 10 | 8 | 11 | ||
| Eth-Whole plant | - | 24 | 6 | 4 | 7 | |||
| Meth-Whole plant | - | 30 | 13 | 10 | 14 | |||
| Shigella | Leaves | - | 30 | 8 | 7 | 10 | ||
| Seed | - | 32 | 11 | 9 | 12 | |||
| Eth-Whole plant | - | 27 | 6 | 5 | 8 | |||
| Meth-Whole plant | - | 29 | 13 | 14 | 15 | |||
| Gram Positive Bacteria | ||||||||
| Staphylococcus aureus |
Leaves | - | 30 | 7 | 8 | 10 | ||
| Seed | - | 33 | 11 | 10 | 12 | |||
| Eth-Whole plant | - | 27 | 5 | 5 | 9 | |||
| Meth-Whole plant | - | 35 | 12 | 13 | 16 | |||
Table 8. Results of antibacterial activity of methanolic and ethanolic extracts of A.viridis.
From the above table 8 it is clear that, the whole plant methanol extract (400 mg/ml) possessed the highest zone of inhibition against the E.coli (17 mm) where as Pseudomonas aeruginosa has low zone of inhibition (14 mm) in 400 mg/ml concentration of methanolic extract (Whole plant). Methanol extracts were the most potent of the both extracts i.e. ethanol and methanol suggesting that the active components must be highly present in methanolic extracts. Also the active components like phenol, flavonoids, tannins, alkaloids are found in higher amount in methanolic extract in comparision to ethanolic extract. Thus, the methanolic extract of the whole plant showed more ZOI against all of the bacterial strains used in our study.
Result of antibacterial screening of A.viridis L

Total protein content in fresh leaves and seeds extracts of A.viridis L
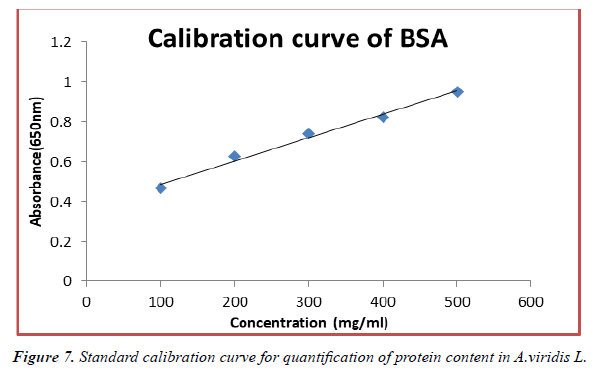
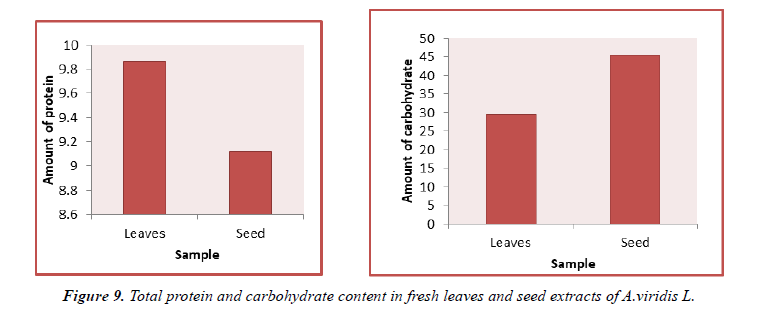
The contents of protein in fresh leaves and seeds of A.viridis L were determined from the regression equation of the calibration curve (y = 0.0021x + 0.21600, R2 = 0.68499). A calibration curve was drawn by using different concentration of Bovine Serum Albumin (BSA) and is as shown in (Figure 7). The amount of protein in leaves and seeds of A.viridis L were expressed as Bovine Serum Albumin (BSA) in mg/g weight of plant extract and is shown in Table 9 below. From the experimental data given below, it was found that the total protein content in A.viridis L was in the given order.

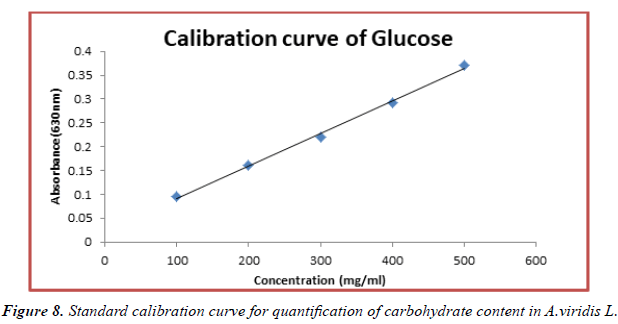
The contents of carbohydrate in fresh leaves and seeds of A.viridis L were determined from the regression equation of the calibration curve (y = 0.000685x + 0.0229, R2 = 0.99650). A calibration curve was drawn by using different concentration of Glucose and is as shown in (Figures 8 and 9). The amount of carbohydrate in leaves and seeds of A.viridis L were expressed as glucose in mg/g weight of plant extract and is shown in Table 10 below. From the experimental data given below, it was found that the total carbohydrate content in A.viridis L was found to be high in seed then leaves:
| Sample | Total protein content |
|---|---|
| Leaves | 9.86 ± 3.621 |
| Seed | 9.123 ± 5.227 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 9. Total protein content in fresh leaves and seed extracts of A.viridis L.
| Sample | Total carbohydrate content |
|---|---|
| Leaves | 29.50 ± 9.42 |
| Seed | 45.56 ± 6.13 |
(All the data are represented in mean ± standard deviation, n = 3)
Table 10. Total carbohydrate content in fresh leaves and seed extracts of A.viridis L.
Discussion
The utilization of herbal medicines and phytonutrients/ nutraceuticals continue to rise as a result of increased acceptance and public interest in both developed and developing nations The soaring interest in traditional medicine is attributed to the failure of modern medicine to alleviate many chronic diseases. Amaranthus viridis Linn, a plant highly venerated for its aromatic
References
- Manandhar, N. P. (2002). Plants and people of Nepal. Timber press.
- Natural Products Foundation. Retrived 2013-12-07. Natural products are represented by a wide array of consumer goods that continue to grow in popularity each year. These products include natural and organic foods, dietary supplements, pet foods, health and beauty products, “green” cleaning supplies and more. Generally, natural products are considered those formulated without artificial ingredients and that are minimally processed.
- Elias, J. Proceedings of First Amaranth seminar. Rodalle Press Inc, Emmaus, 1977: 16.
- Flores HE, Teutonico RA. Amaranths (Amaranthus spp.):Potential grain and vegetable crops. InCrops I. Springer, Berlin, Heidelberg. 1986:568-578.
- Kumari N, Prakash D. Carotenoids: do they defend against cancer and heart diseases. Inov Intel. 2005;40:24-30.
- A Text book of pharmacognosy by M.K.Gupta, P.K.Sharma, page no:120-135
- Rao SK, Andrade C, Reddy K, et al. Memory protective effect of indomethacin against electroconvulsive shock-induced retrograde amnesia in rats. Biol psychiatry. 2002;51(9):770-3.
- Trease GE, Evans WC. Pharmacognosy 13th edition Bailliere Tindall Ltd.
- Pokhrel B, Raut S, Rijal S. Phytochemical screening, antimicrobial and antioxidant activity of Melia azedarach leaves in methanol solvent. World J Pharm Pharm Sci. 2015 May 19;4(07).
- Gülçin ?, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull. 2005;53(3):281-5.
- Kusano R, Ogawa S, Matsuo Y, et al. α-Amylase and lipase inhibitory activity and structural characterization of acacia bark proanthocyanidins. J Nat Prod. 2011;74(2):119-28.
- Olutiola PO, Famurewa O. Sonntag HG. An Introduction to General Microbiology- a Practical Approach. [2nd ed]. 1991.
- H Lowry. Protein Measurement with the Folin phenol reagent. J. biol. Chem. 1951;193:265-75.
- Geetha S, Ram MS, Mongia SS, et al. Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium (VI) induced oxidative stress in albino rats. J Ethnopharmacol. 2003;87(2-3):247-51.
- Fresco P, Borges FI, Diniz C, et al. New insights on the anticancer properties of dietary polyphenols. Med Res Rev. 2006;26(6):747-66.
- Shimoi K, Masuda S, Shen B, et al. Radioprotective effects of antioxidative plant flavonoids in mice. Mutation Research/Fundamental and Molecular mechanisms of mutagenesis. 1996;350(1):153-61.
- Cai Y, Luo Q, Sun M, et al. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157-84.
- Ren W, Qiao Z, Wang H, et al. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23(4):519-34.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref