Review Article - Journal of Molecular Oncology Research (2017) Volume 1, Issue 1
Human chorionic gonadotropin (hCG) and hyperglycosylated hCG, seven semi-independent critical molecules: A review.
Laurence A. Cole*
USA hCG Reference Service, 34 Broadmoor Way, P.O. Box 950 Angel Fire, New Mexico
- *Corresponding Author:
- Laurence A. Cole
USA hCG Reference Service, 34 Broadmoor Way
P.O. Box 950Angel Fire, New Mexico
Tel: 575-377-1330
E-mail: larry@hcglab.com
Accepted on 09 July, 2017
Citation: Cole LA. Human chorionic gonadotropin (hCG) and hyperglycosylated hCG, seven semi-independent critical molecules: A review. J Mol Oncol Res. 2017;1(1):22-44.
DOI: 10.35841/molecular-oncology.1.1.22-44
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
hCG exists in two analogous structures, as typified by the hormone hCG which binds an LH/hCG hormone receptor and the TGFß receptor binding autocrine hyperglycosylated hCG. Both share a common α-subunit and ß-subunit amino acid sequence. From the common hormone hCG and autocrine hyperglycosylated hCG structures there are seven common hCG variants with wildly varying hormone hCG or autocrine hyperglycosylated hCG functions. There is placental hormone hCG and placental autocrine hyperglycosylated hCG which together control pregnancy and start hemochorial placentation during pregnancy. There is pituitary sulfated hCG, part of the hormone hCG library which works with LH in driving ovarian steroidogenesis, ovulation and luteogenesis. Fetal hCG a fetal hormone that promotes fetal organ growth during pregnancy, and ovarian hyperglycosylated hCG which drive the final proteolytic enzyme step of ovulation. Cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free ß-subunit drive malignancy in most cancers. The seven variants of hCG drive critical functions during pregnancy, cancer and other bodily functions.
Keywords
Hyperglycosylated hCG, Steroidogenesis, Ovulation, Luteogenesis.
Introduction
The hormone hCG was founded in the early twentieth century by Aschner, Fellner, and Ascheim and Zondek [1]. In 1912, Aschner promoted the genital tract of guinea pigs with injections of water-soluble extracts of human placenta [1-4]. In 1913, Fellner promoted ovulation in immature rabbits with a saline extracts of human placenta [2]. In 1919, Hirose induced ovulation and normal luteal function in immature rabbits by repeated injections of human placental tissue [3]. All of these studies show that there was a clear link between the placenta and the female gonads [1-3]. In 1927, Ascheim and Zondek demonstrated that pregnant women produce a gonadstimulating molecule [4]. They showed that injecting this hormone into intact immature female mice let to follicular maturation, ovulation, and hemorrhaging into the ovarian stroma.
The realization around this time that the placenta was producing a molecule that promoted ovarian progesterone production led researchers to coin the name human chorionic gonadotropin (hCG): Chorion comes from the Latin chordata meaning afterbirth; gonadotropin because the hormone was considered a gonad tropic molecule, or a stimulator of gonad (or ovary) steroid production.
I am far from sure that the name is correct today. Firstly, because the hormone and autocrine dealt with today are produced by placenta cells and cells other than placenta cells. Secondly, because the hormone and autocrine do not act primarily on gonad cells. Considering that it is both a hormone (hCG) and a autocrine (hyperglycosylated hCG), a general name like human pregnancy glycoprotein or human acidic glycoprotein might have been more appropriate.
The hormone hCG and the autocrine hyperglycosylated hCG evolved in primates from a deletion mutation in luteinizing hormone (LH) ß-subunit [5]. The original molecules, anthapoid CG (aCG), was not very acidic, isoelectric point (pI) 6.3. Over 55 million years it evolved through baboon hCG, orangutan hCG and hominid hCG to form acidic human hCG (pI=3.5) [6].
hCG characteristically had a 92 amino acid α-subunit and a 145 amino acid ß-subunit. The α-subunit has two N-linked oligosaccharides and the ß-subunit has two N-linked and four O-linked sugar side structures [7,8]. Through evolution, hCG subunits contains a cystine knot structure inherited from transforming growth factor-ß (TGF-ß). aCG evolved from primate LH, fish LH evolved from fish gonadotropin ancestral hormone (GAH), which directly evolved from fish TGF-ß [9].
Hyperglycosylated hCG was discovered in 1997 by Laurence A. Cole PhD, the author of this review [7]. While looking at the carbohydrate structure of hCG it was very apparent that two separate molecules of different molecular weights that were present in pregnancy urine, molecular weights 36,100 and 39,000 [6,7]. Hyperglycosylated hCG had an identical α and β- subunit amino acid sequence to the hormone hCG, identical Nlinked sugar structures [7,8-10], but at least 3 of 4 very different O-linked carbohydrate side chains [7,8-10]. This is the carbohydrate side chains on the 145 amino acid β-subunit Cterminal peptide at β121, β127, β132 and β138. It is difference in these carbohydrate side chains that causes hyperglycosylated hCG to fold very differently to hCG, so that hCG is a hormone, and hyperglycosylated hCG an autocrine or cytokine binding a transforming growth factor-β-II (TGF-β-II) receptor. hCG has four type 1 O-linked oligosaccharides, all of which are trisaccharides and tetrasaccharides. Hyperglycosylated hCG has 3 or 4 type 2 O-linked oligosaccharides, all of which are pentasaccharides and hexasaccharides [7,8-10].
While the hormone hCG has essential functions in forming the hemochorial placentation fetal feeding apparatus, it also promotes progesterone production by the ovarian corpus luteum, suppresses fetal contractions during pregnancy, and suppresses maternal macrophage attack on fetal and placental tissues, immunologically foreign tissues. The hormone hCG is also found as pituitary placental hCG, and as the fetal hormone hCG. In the pituitary it function to control ovarian steroidogenesis, ovarian follicular growth, oocyte ovulation and luteogenesis [11]. In the fetus during pregnancy it controls fetal organ growth and organ development [12,13].
Pregnancy Hyperglycosylated hCG has critical functions in pregnancy driving blastocyst implantation [14,15], and in deep implanting hemochorial placentation [16], and in promoting growth of the placenta as pregnancy advances [17]. It also has critical functions as cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free ß-subnit in human cancers, where it uses a mechanisms parallel to blastocyst implantation to drive malignancy or viciousness of cancer [18,19]. As described in this review, ovarian hyperglycosylated hCG also has a function in ovarian ovulation, driving a hole in the follicle and in the ovary to permit ovulation to occur [20].
Hyperglycosylated hCG is secreted by trophoblast cells in vesicles by endocytosis. Secreted molecules are autocrines acting on a TGF-β-II receptor on the same cell surface [21-23]. In simple autocrine pathway, all of the minuscule amount secreted acts on the receptor, in complex autocrine pathways, the higher concentrations of hyperglycosylated hCG circulate before acting on the autocrine receptor [24-26].
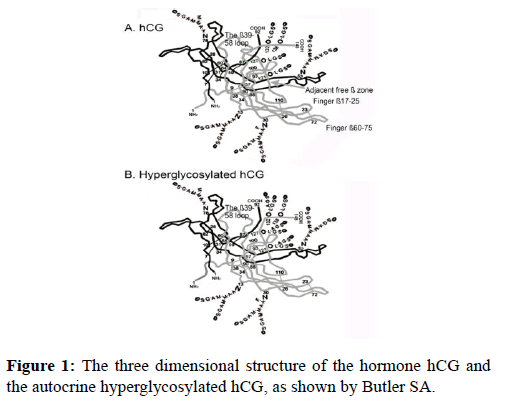
Figure 1 shows the three dimensional structure of the hormone hCG and of the TGF-ß autocrine or autocrine hyperglycosylated hCG. These are unpublished models created by Butler SA from X-ray crystallography data on hCG and thermodynamic computer models of the remaining structure [27-30]. The principal difference between the two different forms of hCG are the ß-subunit C-terminal peptides (ß110-145) (Figure 1). On the hormone hCG the Type 1 O-linked sugar structures cause the C-terminal peptide to fold into loop ß40-58. This blocks nicking or cleavage of this loop. On hyperglycosylated hCG, the Type 2 O-linked O-lined oligosaccharides cause the C-terminal peptide to avoid this ß40-58 loop and to not block nicking or cleavage of this loop.
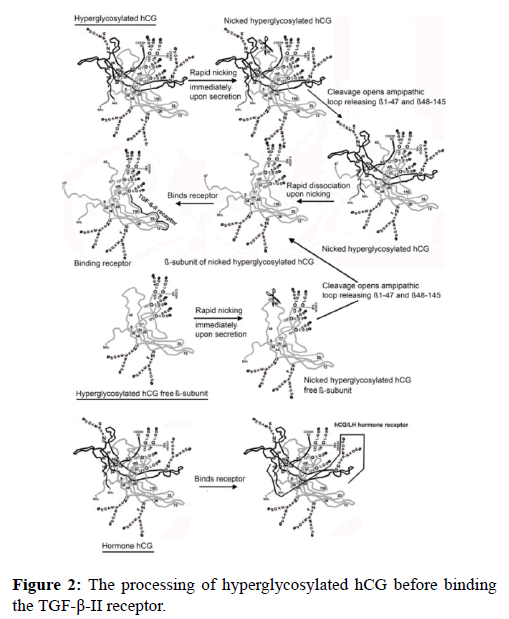
The result of this tiny structural difference between the hormone hCG and the autocrine hyperglycosylated hCG, is that hyperglycosylated hCG, but note the hormone hCG is nicked or cleaved at β47-48 by leukocyte elastase immediately upon secretion. The nicked autocrine is rapidly dissociated because of the cleavage to release a nicked hyperglycosylated hCG separated β-subunit. This is the molecule which acts on the TGF-β-II receptor (Figure 2) [21-23]. Nicked hyperglycosylated hCG ß-subunit binds the TGF-ß-II receptor through the fingers on the dissociated ß-subunit not present on α-ß subunit dimers (Figure 1) [9].
The result is that we have two very separate independent molecules, the hormone hCG, which is an intact αß dimer, and the completely different structure nicked hyperglycosylated hCG ß-subunit. The hormone hCG acts on an hCG/LH joint hormone receptor, while the autocrine nicked hyperglycosylated hCG ß-subunit act on an ancestral TGF-ß-II receptor (Figure 2) [21-23].
All told, there appears to be seven semi-independent variants of the two major forms of hCG molecules, produced by different tissues and acting at different sites in the body (Table 1). Each taking on a hormone hCG or an autocrine hyperglycosylated hCG function. There is the placental hormone hCG, and the placental autocrine hyperglycosylated hCG. These are the primary molecules. There is the pituitary hormone hCG, known structurally as placental sulfated hCG, and the fetal form of hCG known as the fetal hormone hCG. There is the ovarian hyperglycosylated hCG molecule and the cancer hyperglycosylated hCG molecules. Finally there is the hyperglycosylated hCG free ß-subunit produced by cancer cells (Table 1). Here I examine the literature today regarding all these variants of hCG to complete the hCG story.
| Parameter | Placental Hormone hCG | Placental Hyper- glycosylated hCG | Pituitary Sulfated | Cancer hyper- glycosylated hCG | Cancer hyperglycosylated free ß-subunit | Ovarian hyper-glycosylated hCG | Fetal hCG |
|---|---|---|---|---|---|---|---|
| Source cells | Syncytio-trophoblast cells | Cytotrophoblast cells | Pituitary Gonadotrope cells | Trophoblastic malignancy cells | Non-trophoblastic cancer malignancy cells | Ovarian Theca cell | Fetal kidney & liver cells |
| Mode of action | Endocrine | Autocrine | Endocrine | Autocrine | Autocrine | Autocrine | Endocrine |
| Total MW | 36525 | 39149 | 35943 | 40461 | 26271 | Not determined | Not determined |
| Site of action | LH/hCG receptor | TGFß antagonism | LH/hCG receptor | TGFß antagonism | TGFß antagonism | Ovarian follicle | Fetal organ |
| Amino acids α-subunit | 92 | 92 | 92 | 92 | 92 | 92 | 92 |
| Amino acids ß-subunit | 145 | 145 | 145 | 145 | 145 | 145 | 145 |
| Peptide MW | 25813 | 25813 | 25813 | 25813 | 15543 | Not determined | Not determined |
| O-linked sugar units | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Type O-linked sugars sugars | Type 1 | Type 2 | Type 1 + SO4 | Type 2 | Type 2 | Not determined | Not determined |
| N-linked sugar units | 4 | 4 | 4 | 4 | 2 | 4 | 4 |
| Type N-linked sugars | Biantennary | Biantennary | Biantennary + SO4 | Triantennary ß | Triantennary | Not determined | Not determined |
| Sugar side chain MW | 10712 | 13336 | 10130 | 14648 | 10728 | Not determined | Not determined |
| Percentage sugars | 0.29 | 0.34 | 0.28 | 0.36 | 0.41 | Not determined | Not determined |
Table 1: The 7 semi-independent variants of the hormone hyperglycosylated hCG and the autocrine hyper-glycosylated hCG . MW is molecular weight, SO4 is sulfated sugars.
I then examine the hCG assay, the pregnancy test, as used today commercially and what it detects and does not detect, and then examine false positive pregnancy tests or falsepositive hCG tests. I then review the role that the two major pregnancy forms of hCG played in the development and evolution of humans, a very critical function. I also review what this can tell us about our development and the future of humans. Finally, I review the synthesis of hCG molecules, and the degradation of hCG molecules to make this review complete.
Placental Hormone hCG
The placental hormone hCG is the original form of hCG as discovered by Aschner, Fellner, and Ascheim and Zondek at the turn of the twentieth century [1-4]. It is a hormone produced by placental syncytiotrophoblast cells, acting on placental cytotrophoblast cells, maternal macrophages, maternal muscular cells and maternal gonad luteal cells. The size of the hormone hCG including carbohydrate side structures is molecular weight 36,525, carbohydrate comprising 29% of the composition (Table 1).
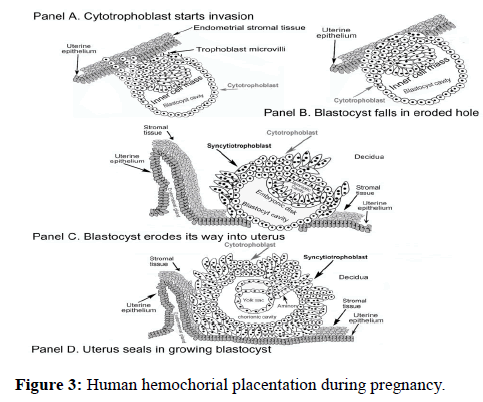
The hormone acts on a joint LH/hCG hormone receptor that functions through promotion of cAMP. The principal function of the hormone hCG is to drive growth and maintenance of hemochorial placentation, the fetal feeding system during pregnancy. (Figure 3) illustrates the human hemochorial placentation system. Human placenta contain 4-6 hemochorial placentation chambers.
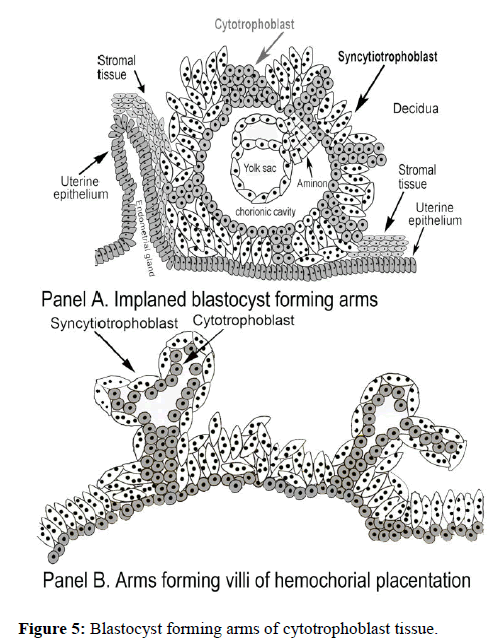
In synthesis of hemochorial placentation chambers, placental hyperglycosylated hCG first drives growth of a tree-like structure of cytotrophoblast cells [17]. These are then fused by the action of placental hormone hCG to form a single cell thick skin of syncytiotrophoblast cells surrounding the cytotrophoblast cells. These are the villus trophoblast cells (Figure 3). Placental hormone hCG then promotes growth of maternal uterine spiral arteries to reach the hemochorial placentation chambers [31-33]. It also promotes growth of the umbilical circulation to link the villus structures with the fetal circulation (Figure 3) [34-36]. Hemochorial placentation chambers are complete around the 10th week of gestation, and then are deep implanted in the uterus.
In functioning, hemochorial placentation chambers fill with maternal blood. Oxygen, glucose and essential nutrients passage from the maternal blood across the single layer of syncytiotrophoblast skin and into the villus chamber (Figure 3). They passage from here into the umbilical circulation and into the fetus.
Other functions of the placental hormone hCG include tempering of blastocyst implantation, the first function in pregnancy [14,15]. Promoting ovarian luteal cells to produce progesterone at 4 to 7 weeks gestation, when menstrual cycle pituitary LH is depleted [37]. The placental hormone hCG also suppresses myometrial muscular contractions during pregnancy [38,39]. It also It prosmotes formation of macrophage migration inhibitory factor to protect fetal and placental tissues from maternal macrophages during pregnancy [40,41].
The placental hormone hCG marks a person as being pregnant, the hCG test being the pregnancy test. Many physicians call hCG “the pregnancy hormone”, placental hormone hCG founding some of the basic commodities of pregnancy (Table 2) shows the concentration of total hCG (placental hormone hCG + placental hyperglycosylated hCG) and placental hyperglycosylated hCG (there is no specific immunoassay for placental hormone hCG) in serum samples during the course of pregnancy.
| Gestation age (weeks since start of menstrual period) | N | Median Total hCG | Range Total hCG | Median hCG-H | Range hCG-H | hCG-H % |
|---|---|---|---|---|---|---|
| 3-weeks | n=42 | 0.26 | 0.04 – 5.5 | 0.2 | 0.01–6.45 (645X) | 87% |
| 4 weeks | n=42 | 3.4 | 0.21–173 (824X) | 2.5 | 0.18–160 (888X) | 51% |
| 5 weeks | n=67 | 65 | 1.86–1308 (704X) | 8.6 | 0.96–698 (731X) | 43% |
| 6-weeks | n=29 | 252 | 3.80–855 (225X) | 86 | 0.76–629 (827X) | 36% |
| 7 weeks | n=30 | 3278 | 203–7,766 (38X) | 359 | 27–931 (34X) | 16% |
| 8 weeks | n=33 | 4331 | 1,064–10,057 (9.4X) | 386 | 67–1050 (15.6X) | 7% |
| 9 weeks | n = 24 | 5832 | 1,031 – 11,586 (11.2X) | 430 | 102–1158 (11.3X) | 5% |
| 10 weeks | n=20 | 10352 | 1,952–19,958 (10.2X) | 521 | 188–1855 (9.9X) | 4% |
| 11 - 13 weeks | n=41 | 5953 | 1,440–15,318 (10.6X) | 137 | 24–330 (13.7X) | 2% |
| 14 - 17 weeks | n=57 | 2934 | 311–4,757 (15.2X) | 26 | 6.7–129 (19.3X) | 1% |
| 18 - 26-weeks | n=62 | 1931 | 210–6,223 (30.3X) | 15.8 | 5.3–95 (17.9X) | 1% |
| 27 - 40 weeks | n=49 | 1911 | 184–8,530 (46.4X) | 2.95 | 0.3–12.2 (40.6X) | 0% |
Table 2: Concentration of total hCG and hyperglycosylated hCG (hCG-H) in 496 serum samples from 310 women with term pregnancies measured using the Siemens Immulite 1000 total hCG assay. Data from 50 pregnancies that failed due to miscarriage were excluded from this table.
Placental Autocrine Hyperglycosylated hCG
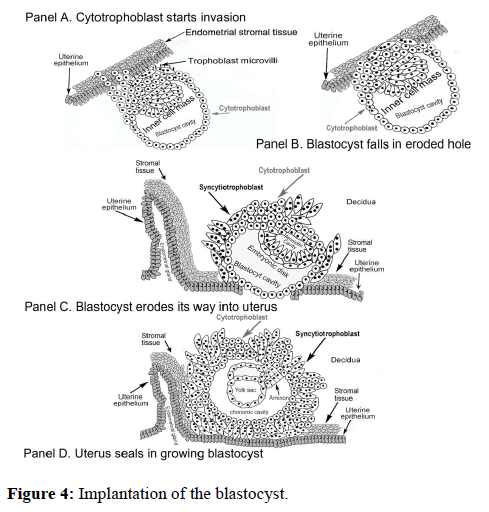
The placental autocrine hyperglycosylated hCG is seemingly the first hCG molecule functioning during pregnancy, driving implantation of the blastocyst of pregnancy. The placental autocrine hyperglycosylated hCG can be detected in serum and urine as early as two days before pregnancy implantation [14,15]. In driving implantation of the blastocyst (Figure 4) cytotrophoblast cells of the blastocyst produce hyperglycosylated hCG, which acts through a TGF-ß-II receptor to promote metalloproteinase and collagenase secretion by the blastocyst cells. This erodes uterine cells permitting implantation to occur (Figure 4). Implantation is tempered by the placental hormone hCG, diminishing implantation [14,15]. Placental hyperglycosylated hCG also promotes growth of the implanting blastocyst (Figure 4). The size of placental hyperglycosylated hCG is molecular weight 39,149, with sugars accounting for 34% of the composition.
Under the promotion of placental hyperglycosylated hCG the growing implanted blastocyst cytotrophoblast cells develop arms. The arms grow out to form the hemochorial placentation tree-shaped villus tissue (Figure 5). These form over multiple weeks the root villus structure of the hemochorial placentation chambers (Figure 3).
Placental hyperglycosylated hCG also deep implants the hemochorial placentation structure, once again using hyperglycosylated hCG driven metalloproteinases and collagenases acion [16]. Placental hyperglycosylated hCG also has the overall responsibility of promoting placental growth as the fetus expands in pregnancy [17].
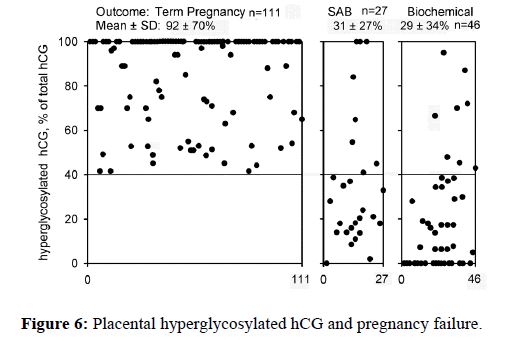
Deficiency of placental hyperglycosylated hCG is a common problem with the autocrine synthesis of placental hyperglycosylated hCG. In the U.S.A. 42% of pregnancies are failures, 25% of pregnancies become biochemical pregnancies, and 17% of pregnancies are spontaneous abortions or miscarriage [42-44]. This is due to failure of pregnancies to implant the blastocyst completely [42-44]. Further examination of this field shows that it actually is due to incomplete action of placental hyperglycosylated hCG (Figure 6) [14,15,45,46].
As shown in Figure 6, term childbirth outcome pregnancies produce a significant proportion hyperglycosylated hCG on the day of implantation, 111 of 111 produced >40% hyperglycosylated hCG. In contrast, 19 of 27 spontaneous abortion or miscarried pregnancies produced <40% hyperglycosylated hCG on the day of implantation, and 38 of 46 biochemical pregnancies produce <40% hyperglycosylated hCG on the day of implantation (Figure 6). The 8+8 pregnancies that did produce >40% hyperglycosylated hCG were later shown to be gross genetic abnormalities. Clearly, a deficiency of hyperglycosylated hCG causes those 42% of pregnancy failures.
As shown, (Figure 7), a deficiency of hyperglycosylated hCG also cause failure of deep implantation at 10-18 weeks gestation. During evolution hyperglycosylated CG correspond drove deep implantation [47-49]. With increase in the acidity of the hormone hCG and the autocrine hyperglycosylated hCG, hemochorial placentation deep evolution implanted at 1% uterine thickness in anthropoid primates, and deeper at 10% in orangutan and 30% in humans [47-50]. Hyperglycosylated hCG drove invasive implantation, as such deep evolution must be driven by placental hyperglycosylated hCG. (Figure 7) shows the hyperglycosylated hCG production at the time of deep implantation, 10-18 weeks of gestation [16-50].
As shown, (Figure 7), the majority of preeclampsia and gestational hypertension cases produce exceptionally low concentrations of pregnancy hyperglycosylated hCG, less than the 3rd percentile or <3%. It is inferred that placental hyperglycosylated hCG deficiency at this time leads to incomplete deep implantation. This leads to failure of fetal feeding and preeclampsia and gestational hypertension [16].
All told, placental hyperglycosylated hCG appears to be the tissue growth and tissue invasion promoter of pregnancy. Hyperglycosylated hCG works alongside the hormone hCG in setting up and regulating pregnancy. (Table 2) shows the concentration of total hCG (placental hormone hCG + placental hyperglycosylated hCG) and placental hyperglycosylated hCG (there is no specific immunoassay for placental hormone hCG) in serum samples during the course of pregnancy.
Pituitary sulfated hCG
hCG evolved from a deletion mutation in the ß-subunit of LH. LH is a pituitary gonadotrope cell hormone. hCG should also therefore be primarily a pituitary gonadotrope hormone. hCG was turned on in pituitary gonadotrope cells by hypothalamic gonadotropin releasing hormone (GnRH), the signal which originally turned on LH. The problem was that placental hormone hCG was also turned on by a placenta form of GnRH [51].
hCG is produced in low concentrations (0.1–10 mIU/ml) by pituitary gonadotrope cells during the menstrual cycle [11,52-55]. It is called pituitary sulfated hCG because the sulfated side chains terminate in a sulfated Nacetylgalactosamine residues [11]. These reduce the acidity of hCG and 50% reduce the biological potency [11].
It is believed that pituitary sulfated hCG supplement and guarantees LH biological activity. Pituitary sulfated hCG taking over enhancing ovulation and enhancing steroidogenesis, ovulation and luteogenesis whenever LH production is insufficient. Placental hCG is approximately 108- fold more potent than LH because of its long circulating halflife. Both hCG and LH act on the same joint LH/hCG receptor [20].
Studies in my laboratory investigated pituitary sulfate hCG function [20]. Table 3 marks 111 women achieving pregnancy. The table shows the concentrations of LH and hormone hCG on the day of LH peak. The urine LH peak concentration and the hormone hCG concentration (total hCG minus hyperglycosylated hCG) were determined at the time of the LH peak in the menstrual cycle immediately preceding a pregnancy. It is inferred that ovulation had to have occurred in this cycle or pregnancy could not occur [20].
| Case | Day of LH peak | Conc. LH mIU/ml | Conc. HCG ng/ml | Case | Day of LH peak | Conc. LH mIU/ml | Conc. HCG ng/ml | Case | Day of LH | Conc. LH mIU/ml | Conc. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | 197 | 0.02 | 38 | 17 | 119 | 0.05 | 75 | 17 | 74 | 0.07 |
| 2 | 14 | 29 | 0.05 | 39 | 14 | 81 | 0.06 | 76 | 14 | 136 | 0.01 |
| 3 | 12 | 78 | 0.03 | 40 | 14 | 272 | 0.03 | 77 | 13 | 164 | 0.02 |
| 4 | 12 | 93 | 0.07 | 41 | 15 | 144 | 0.01 | 78 | 14 | 3.9 | 0.07 |
| 5 | 12 | 110 | 0.02 | 42 | 17 | 275 | 0 | 79 | 16 | 22 | 0.03 |
| 6 | 17 | 6.8 | 0.06 | 43 | 14 | 19 | 0.02 | 80 | 13 | 124 | 0.03 |
| 7 | 14 | 143 | 0.02 | 44 | 17 | 109 | 0.09 | 81 | 17 | 99 | 0.06 |
| 8 | 17 | 56 | 0.06 | 45 | 12 | 180 | 0.03 | 82 | 15 | 122 | 0.07 |
| 9 | 17 | 22 | 0.02 | 46 | 14 | 169 | 0.01 | 83 | 12 | 42 | 0.05 |
| 10 | 14 | 187 | 0.02 | 47 | 14 | 7.1 | 0.07 | 84 | 14 | 82 | 0.06 |
| 11 | 14 | 111 | 0.04 | 48 | 16 | 134 | 0.06 | 85 | 14 | 82 | 0.06 |
| 12 | 13 | 197 | 0.03 | 49 | 14 | 4.7 | 0.07 | 86 | 15 | 109 | 0.05 |
| 13 | 13 | 101 | 0.03 | 50 | 14 | 174 | < | 87 | 15 | 195 | 0.03 |
| 14 | 16 | 336 | 0.04 | 51 | 14 | 117 | 0.02 | 88 | 14 | 112 | 0.02 |
| 15 | 13 | 32 | 0.06 | 52 | 13 | 517 | 0.23 | 89 | 15 | 160 | 0 |
| 16 | 17 | 43 | 0.06 | 53 | 14 | 5.2 | 0.12 | 90 | 14 | 185 | 0.02 |
| 17 | 13 | 112 | 0.15 | 54 | 16 | 242 | 0.01 | 91 | 14 | 187 | 0.02 |
| 18 | 13 | 270 | 0.02 | 55 | 17 | 126 | 0.02 | 92 | 12 | 380 | 0.01 |
| 19 | 14 | 156 | 0 | 56 | 14 | 490 | 0.07 | 93 | 14 | 84 | 0.02 |
| 20 | 13 | 152 | 0.42 | 57 | 14 | 109 | 0.03 | 94 | 14 | 138 | 0.04 |
| 21 | 17 | 99 | 0.01 | 58 | 14 | 122 | 0.06 | 95 | 16 | 137 | 0.01 |
| 22 | 13 | 238 | 0.07 | 59 | 12 | 127 | 0.04 | 96 | 12 | 110 | 0.01 |
| 23 | 12 | 110 | 0.01 | 60 | 15 | 22 | 0.75 | 97 | 14 | 174 | 0,02 |
| 24 | 15 | 64 | 0.06 | 61 | 14 | 121 | 0.05 | 98 | 14 | 459 | 0.01 |
| 25 | 13 | 47 | 0.01 | 62 | 14 | 2.9 | 0.05 | 99 | 14 | 183 | 0.03 |
| 26 | 17 | 76 | 0.06 | 63 | 14 | 258 | 0.08 | 100 | 14 | 61 | 0.08 |
| 27 | 17 | 134 | 0.01 | 64 | 14 | 333 | 0.02 | 101 | 14 | 165 | 0.01 |
| 28 | 14 | 200 | 0.02 | 65 | 13 | 123 | < | 102 | 17 | 376 | 0.03 |
| 29 | 14 | 15 | 0.03 | 66 | 17 | 17 | 0.07 | 103 | 14 | 84 | 0.02 |
| 30 | 16 | 108 | 0.07 | 67 | 14 | 104 | 0.01 | 104 | 17 | 52 | 0.02 |
| 31 | 14 | 111 | 0.05 | 68 | 14 | 43 | 0.04 | 105 | 14 | 160 | 0.03 |
| 32 | 14 | 138 | 0.04 | 69 | 17 | 75 | 0 | 106 | 14 | 90 | 0.06 |
| 33 | 14 | 160 | 0.03 | 70 | 14 | 297 | 0.02 | 107 | 14 | 99 | 0.06 |
| 34 | 14 | 88 | 0.01 | 71 | 14 | 116 | 0.02 | 108 | 14 | 179 | 0.01 |
| 35 | 15 | 120 | 0.04 | 72 | 14 | 84 | 0.04 | 109 | 16 | 331 | 0.01 |
| 36 | 14 | 110 | 0.05 | 73 | 12 | 197 | 0.03 | 110 | 14 | 209 | 0.05 |
| 37 | 13 | 56 | 0.02 | 74 | 15 | 223 | 0.05 | 111 | 14 | 19 | 0.07 |
Table 3: One hundred and eleven women achieving pregnancy, peak LH, and hormone hCG at LH peak in cycle immediately preceding pregnancy
If one considers a low urine LH peak being <23 mIU/ml as deficient LH in urine (serum equivalence of 4.6 mIU/ml LH), too low to have driven ovulation, could pituitary sulfated hCG have substituted, and driven ovulation [20]. Looking at (Table 3) case 6, LH peak=6.8 mIU/ml, pituitary sulfated hCG=0.15 ng/ml (equivalent considering 108 fold x50% difference to 89 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case? Looking at case 9, LH peak=22 mIU/ml, pituitary sulfated hCG=0.10 ng/ml (equivalent to 59 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case? Looking at case 29, LH 15 mIU/ml, pituitary sulfated hCG=0.22 ng/ml (equivalent to 130 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case? Looking at case 43, LH peak=19 mIU/ml, pituitary sulfated hCG=0.10 ng/ml (equivalent to 59 mIU/ml LH) indicating once again that pituitary sulfated substituted for deficient LH.
Looking at case 47, LH peak=7.1 mIU/ml, pituitary sulfated hCG=0.11 ng/ml (equivalent to 65 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case? Looking at case looking at case 49, LH peak=4.7 mIU/ml, pituitary sulfated hCG=0.15 ng/ml (equivalent to 85 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case; looking at case 53, LH peak=5.2 mIU/ml, pituitary sulfated hCG=0.40 ng/ml (equivalent to 238 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case; looking at case 60, LH peak 22 mIU/ml, pituitary sulfated hCG 0.75 ng/ml (equivalent to 446 mIU/ml LH); looking at case 78, LH peak=3.9 mIU/ml, pituitary sulfated hCG=0.07 ng/ml (equivalent to 41 mIU/ml LH) could pituitary sulfated hCG have substituted for deficient LH in this case; looking at case 79, LH peak=22 mIU/ml, pituitary sulfated hCG=0.03 ng/ml (equivalent to18 mIU/ml LH), could pituitary sulfated hCG has substituted for deficient LH in this case; looking at case 62, LH peak=2.9 mIU/ml, pituitary sulfated hCG=0.05 ng/ml (equivalent to 30 mIU/ml LH), could pituitary sulfated hCG have substituted for deficient LH in this case; looking at case 66, LH peak=17 mIU/ml, pituitary sulfated hCG=0.07 ng/ml (equivalent to 41 mIU/ml LH); looking at case 111, LH peak=19 mIU/ml, pituitary sulfated hCG=0.07 ng/ml (equivalent to 41 mIU/ml LH) [20].
Again results show that in a least 13 of 111 (12%) cases that pituitary sulfated hCG substituted for deficient LH, and was seemingly responsible for ovulation [20]. This shows that pituitary sulfated hCG was important, at least as an LH standby (Table 3). It is assumed that in the other 98 cases that pituitary sulfated hCG supplemented LH in achieving ovulation (except in cases 44, 65, 90 and 94 where clearly pituitary sulfated hCG is deficient, <1.0 mIU/ml). It is concluded, that in approximately 12% of cases, pituitary sulfated hCG substitutes for LH in ovulation.
Fetal Hormone hCG
Four independent research groups [12,13,55,56] have demonstrated that a variant of hCG is independently produced in the pregnancy fetus, by fetal liver and fetal kidney cells. It was demonstrated that this variant of hCG controls fetal organ growth and development. It was also demonstrated that production of fetal hormone hCG is halted at parturition [12,13,55,56].
Fetal research is today highly controlled and limited by ethics boards. Today there is no information on the structure and physical properties of fetal hCG, nor whether it is a hCG hormone or an autocrine like hyperglycosylated hCG. The USA hCG reference service discovered recently an inherited disorder of hCG production, Familial hCG Syndrome [57,58]. In this syndrome people produce a mutant forms of hCG free ß-subunit possible produced by the liver or kidney. It is thought that this syndrome may derive from fetal hCG or be the continued production of fetal hCG during a life time.
Ovarian Autocrine Hyperglycosylated hCG
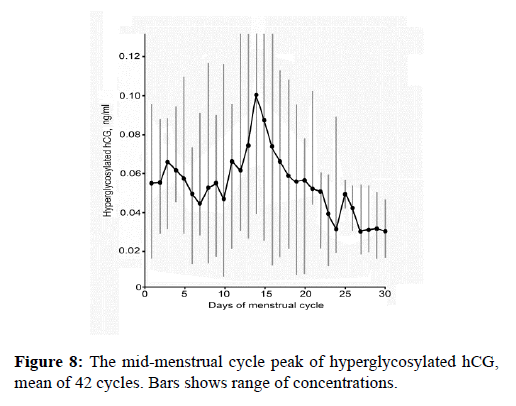
Just my laboratory has discovered ovarian autocrine hyperglycosylated hCG, we await other laboratories to test for it and confirm our findings [20]. Basically a tiny peak of hyperglycosylated hCG was detected (Figure 8), about 1.5 ng/ml at the time of the LH peak in every menstrual cycle. This low concentration may limit discovery by others. The final step of ovulation is that metalloproteinases and collagenase make holes in the ovary and the follicle to permit oocyte ovulation. It is inferred since hyperglycosylated hCG drives such proteolytic actions in implantation and deep implantation, that this is driven by a theca cells hyperglycosylated hCG (Figure 8). This has to be confirmed.
Cancer Autocrine Hyperglycosylated hCG and Cancer Hyperglycosylated hCG free ß-subunit
My original discovery of hyperglycosylated hCG in 1997 was a discovery of cancer hyperglycosylated hCG produced by choriocarcinoma cells [7]. Choriocarcinoma cells produced a form of hCG which promoted cancer cell growth, blocked choriocarcinoma apoptosis and promoted cancer cell invasion of other cells, malignancy processes, that was clearly independent of the hormone hCG [7,18].
As found, trophoblastic cancers (choriocarcinoma, ovarian germ cell malignancy, testicular germ cell malignancy) produced hyperglycosylated hCG αß subunit dimer, while all other cancers make a hyperglycosylated hCG free ß-subunit composed of only the ß-subunit of hyperglycosylated hCG. As shown by Beebe et al. [59], a simple explanation describes why non-trophoblastic cancer only produces a free ß-subunit. A trophoblast cell and pituitary cell disulfide isomerase adds the final two disulfide bonds, ß93-ß100 and ß26-ß110. In the absence of this enzyme and the absence of these disulfide bridges ß-subunit is not recognized by α-subunit for combination and only a free ß-subunit is made [59]. This happens with ectopic production of hCG by non-trophoblastic cancers. The TGF-ß-II receptor is antagonized by either hyperglycosylated hCG or the cleaved hyperglycosylated hCG free ß-subunit [18,60].
Hyperglycosylated hCG and its free ß-subunit are useful tumor markers for cancer. They are best measured as their urinary degradation product, ß-core fragment [61-65]. Table 4 shows a ten year tumor marker study completed by the author at Yale University [61-65]. As shown, the tumor marker urine ß-core fragment detected 44% of a wide range of 2167 very different cancer cases. I asked why did it only detect 44% of cases, did the other 56% of cases not express the hCG ß-subunit gene?
| Source | Cut-off >3 fmol/ml | ||
|---|---|---|---|
| #Cases | #Positive | Sensitivity | |
| A. Trophoblastic malignancies | |||
| Choriocarcinoma | 63 | 63 | 100% |
| Ovarian germ cell cancer | 11 | 11 | 100% |
| Testicular germ cell cancer | 17 | 17 | 100% |
| Total | 110 | 110 | 100% |
| B. Non-trophoblastic malignancy | |||
| Bladder cancer | 140 | 62 | 44% |
| Breast cancer | 456 | 156 | 34% |
| Cervical cancer | 410 | 197 | 48% |
| Colorectal cancer | 80 | 29 | 36% |
| Endometrial cancer | 233 | 103 | 44% |
| Gastric cancer | 205 | 90 | 44% |
| Hepatic cancer | 46 | 21 | 44% |
| Lung cancer | 154 | 38 | 25% |
| Intestinal cancer | 17 | 8 | 47% |
| Lymphoma | 41 | 13 | 32% |
| Ovarian cancer | 207 | 145 | 70% |
| Pancreatic cancer | 29 | 16 | 55% |
| Prostate cancer | 12 | 9 | 75% |
| Renal cancer | 66 | 32 | 48% |
| Uterine cancer | 63 | 26 | 41% |
| Vulvar cancer | 8 | 4 | 50% |
| Total | 2167 | 949 | 44% |
| C. Healthy | |||
| NED, post cancer chemotherapy | 33 | 2 | 6% |
| NED, post cancer surgery | 21 | 1 | 5% |
| Healthy female, no cancer history | 72 | 2 | 3% |
| Healthy male, no cancer history | 28 | 1 | 4% |
| Total | 154 | 6 | 4% |
| D. Benign Disease | |||
| Benign gynecological lesion, tumor | 28 | 0 | 0% |
| Benign lung lesion | 4 | 0 | 0% |
| Follicular ovarian cyst, benign | 67 | 1 | 1% |
| Benign ovarian cyst, non-functional | 26 | 0 | 0% |
| Benign prostate hyperplasia | 8 | 0 | 0% |
| Cervical carcinoma in-situ | 12 | 0 | 0% |
| Cervical dyskaryosis | 66 | 2 | 3% |
| Condyloma | 30 | 0 | 0% |
| Endometriosis | 16 | 1 | 6% |
| Myoma | 27 | 3 | 11% |
| Total | 284 | 7 | 3% |
Table 4: Urine degradation products hyperglycosylated hCG ß-subunit + ß-core fragment (B204 assay) as tumor markers.
To investigate the issue a super-sensitive ß-core fragment assay was developed (sensitivity ≥ 0.1 fmol/ml). ß-core fragment is the terminal degradation product of hyperglycosylated hCG and its ß-subunit. New cancer cases were identified, n=56. As shown, all cancer cases, no exceptions, actually produced ßcore fragment. In 17 cases they just produced miniscule quantities of tumor marker ß-core fragment (<3 fmol/ml).
This showed that in reality 100% of cancer expressed hyperglycosylated hCG or hyperglycosylated hCG free ßsubunit. The studies of Acevedo et al. [66] and Regelson [67] used a double antibody method with flow cytometry techniques to examine the expression of hCG ß-subunit by cancer cells. They detected that 100% of cancer cells express the ß-subunit gene. This very much confirms our combined conclusion.
Seemingly, some cancers produce miniscule amounts (e.g. 0.1- 3.0 fmol/ml) of ß-subunit as simple autocrines (TGF-ß autocrines secreted and act directly on the TGF-ß-II receptor), while other cancer produce higher concentrations (3.0–54 fmol/ml) that act as complex autocrines (TGF-ß autocrines that are secreted, circulate and then acts back on cellular TGF-ß-II receptor, according to TGF-ß simple an complex autocrine models [24-26].
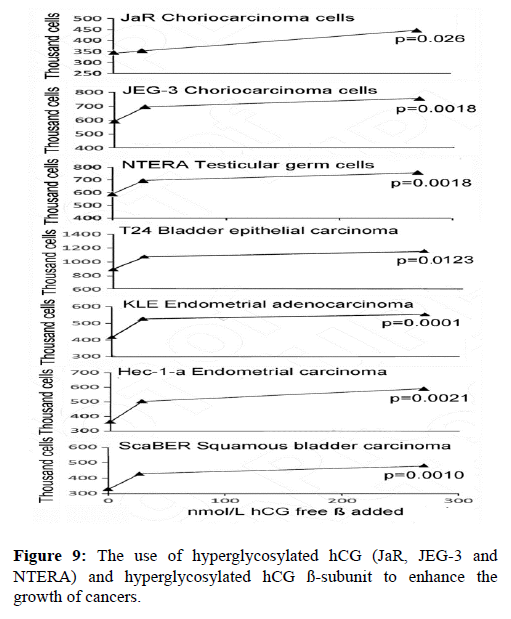
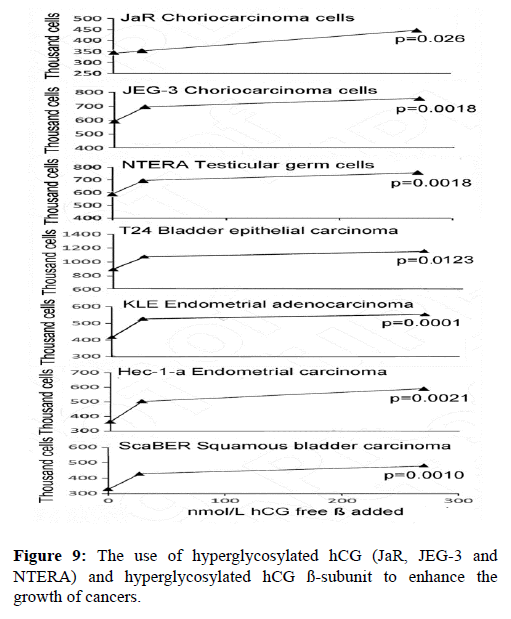
In the last 10 years nine independent groups have each shown that hyperglycosylated hCG and hyperglycosylated hCG free ß-subunit feeds back to the cancer cell and directly promotes cancer cell growth, blocks cancer cell apoptosis and promotes cancer cell invasion, the properties of malignancy [19,21-23,65,68-74]. Shown in Figure 9 is my experience using hyperglycosylated hCG and hyperglycosylated hCG ß-subunit to enhance the growth of cancers. As shown hyperglycosylated hCG and hyperglycosylated hCG ß-subunit significantly enhanced the growth of 7 of 7 cancers, or of all cancer tested.
It is our own belief, studying all this research, that hyperglycosylated hCG and its free ß-subunit are the actual signals in all cancer cells that promotes malignancy in all human cancers [65]. As shown in (Table 4), ß-core fragment is virtually not detected in benign diseases (detection=3.0%), but is probably present in 100% of active cancers. The presence of hyperglycosylated hCG and its free ß-subunit in benign and normal tissues seemingly institutes a malignancy. The exception being pregnancy, where hyperglycosylated hCG has clear normal functions in tissue growth and implantation (Table 5).