Research Article - Biomedical Research (2017) Volume 28, Issue 1
Freezing with a magnetic field prevents frostbite in mouse hind legs
So Koizumi1, Masato Kaku2 and Toshitsugu Kawata1*1Department of Oral Science, Division of Orthodontics, Kanagawa Dental University Graduate School, 82 Inaoka-cho, Yokosuka, Kanagawa, 238-0003, Japan
2Department of Orthodontics and Craniofacial Developmental Biology, Graduate School of Biomedical Science, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan
- *Corresponding Author:
- Toshitsugu Kawata
Department of Oral Science
Division of Orthodontics
Kanagawa Dental University Graduate School
82 Inaoka-cho, Yokosuka
Kanagawa
Japan
Accepted on June 02, 2016
Abstract
Objective: The aim of the present study was to evaluate the effects of frostbite on feet using Cell Alive System (CAS) freezer with an oscillating magnetic field. We recently reported the application of the CAS freezer to the cryopreservation of various tissues, but did not evaluate the effects of frostbite after freezing. In order to characterize how a freezer with a magnetic field affects frostbite, we observed changes using laser Doppler blood flow measurements, X-ray imaging, and tissue specimens from mouse feet.
Methods: Mice were divided into 3 groups: a group without freezing, a group in which a foot was frozen in the bath of the CAS freezer for 10 minutes, with preliminary cooling to -32°C using ethylene glycol, and a group in which a foot was frozen by holding it under dry ice for 10 minutes.
Results: The results obtained showed that the CAS freezer inhibited freezing-induced damage of the cell cytoskeleton and circulatory disorders 10 days after freezing. Furthermore, the CAS freezer reduced bone deformation after freezing.
Conclusions: These results suggest that the CAS freezer prevented frostbite associated with tissue freezing. These rose it’s may potentially lead to the development of novel methods for cryopreservation.
Keywords
Cryopreservation, Magnetic field, Osteoblasts, Hindlimb.
Introduction
In recent years, ABI has succeeded in developing an innovative freezing technology that prevents the crystallization of water molecules by freezing under a magnetic field [1,2]. This freezing technology was developed through the exploration of methods for the storage of frozen foods, and is characterized the by vibration of water molecules using a magnetic field, which prevents the generation of ice crystals and enables cryopreservation of an item in an amorphous state. This freezing technology, called the Cells Alive System (CAS), does not deteriorate the freshness of foods.
When cells are frozen, the formation of ice crystals damages cell membranes and the cytoskeleton. When these cells are thawed, apoptosis or necrosis occurs, which induces frostbite. Furthermore, since the destruction of blood vessel walls disrupts the circulatory system in animal tissues, severe frostbite leads to causes tissue necrosis, ultimately resulting in gangrene [3].
In an effort to freeze cells for preservation without damaging them, the formation of ice crystals needs to be prevented by making the environment inside cells amorphous through the use of cryoprotective agents or adjusting the rate of freezing.
Techniques for cryopreservation have a wide range of applications, and previous studies reported the cryopreservation of not only cells, but also tissues and organs, including arterial valves and ovaries. However, bodily fluids that are in contact with cells must theoretically be replaced with cryoprotective agents in order to preserve organs in a frozen state, and many cryoprotective agents, such as dimethyl sulfoxide (DMSO), are known to be cytotoxic. Thus, there is currently no established technology for the cryopreservation of organs [4].
The CAS has been implemented for the cryopreservation of cells and tissues with satisfactory results. We herein investigated the effects of the CAS freezer on the onset of frostbite after freezing in the feet of mice, without replacing of blood and tissue fluids with cryoprotective agents.
Materials and Methods
We used male C57BL/6 mice aged 8 weeks (Nihon SLC, Shizuoka, Japan) in the experiments reported here. The animals were housed in Plexiglas cages measuring 40 × 25 × 15 cm and were kept in a room with controlled temperature (22 ± 3°C) and lighting (lights on from 8:00 AM to 8:00 PM). The bedding of the cages consisted of wood shavings. Food and water were freely available. This study was approved by the Animal Care and Use Committee of Hiroshima University and conformed to the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health.
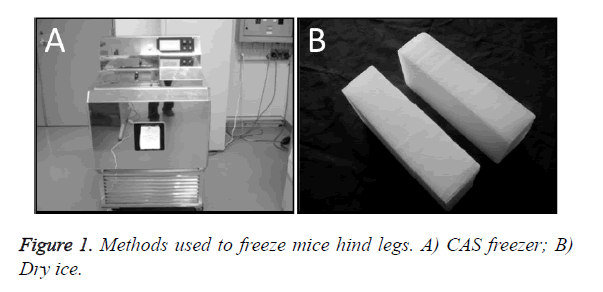
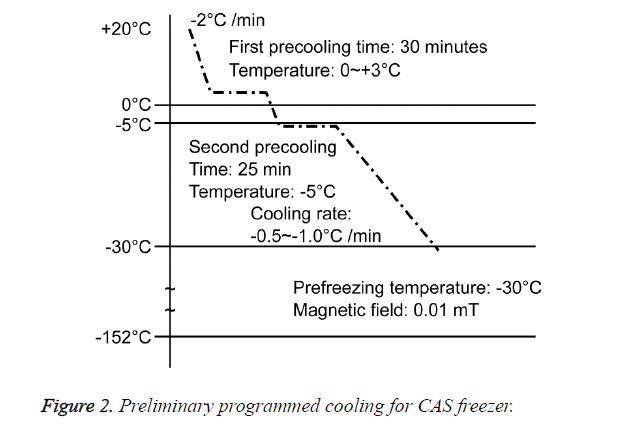
A total of 21 mice were divided into three groups: a group without freezing (CT, n=1); a group in which the right hind foot, beginning at the heel, was frozen by immersion in a bath in a CAS freezer (Cell Alive System, CAS ABI Corporation Ltd., Chiba, Japan) for 10 min (CF, n=10) (Figure 1A), with preliminary programmed cooling to -32°C using 60% w/w ethylene glycol solution (Figure 2); and a group in which the right hind foot, beginning at the heel, was frozen by holding it , on dry ice for 10 min (DF, n=10) (Figure 1B). The CAS provided a 60-Hz oscillating magnetic field and intensity of 0.01 mT.
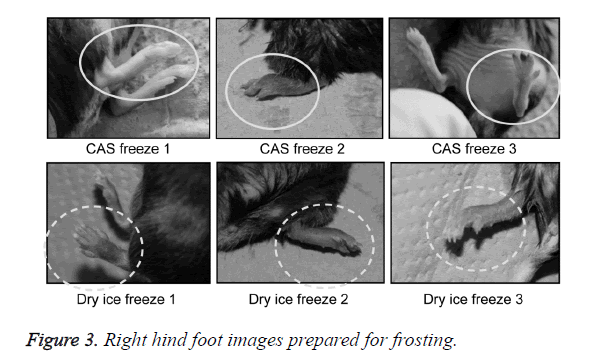
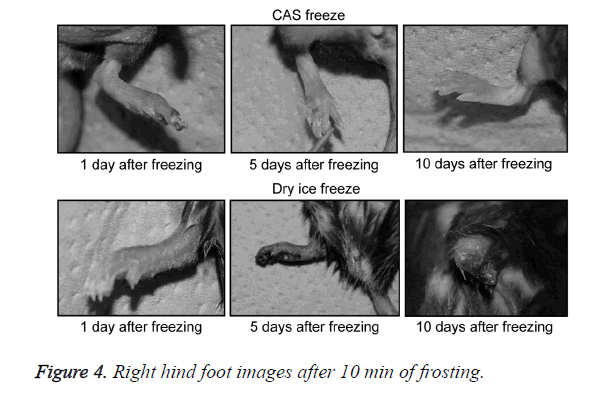
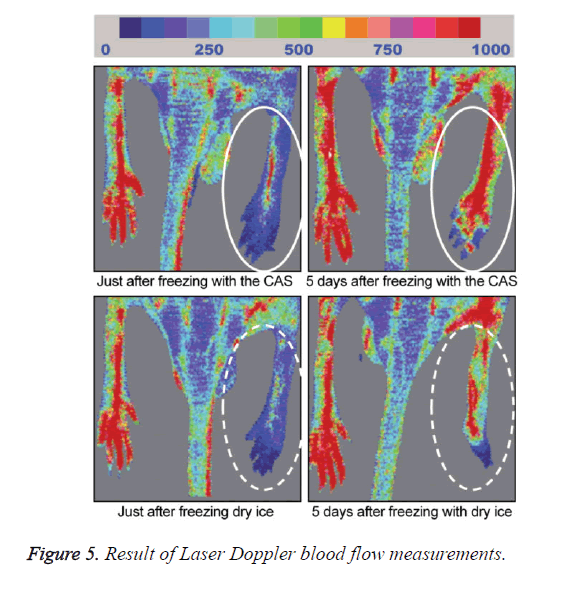
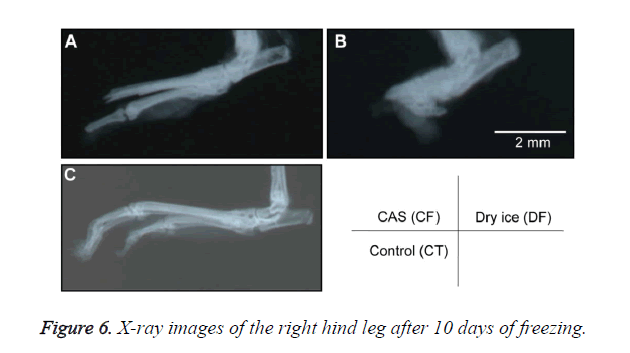
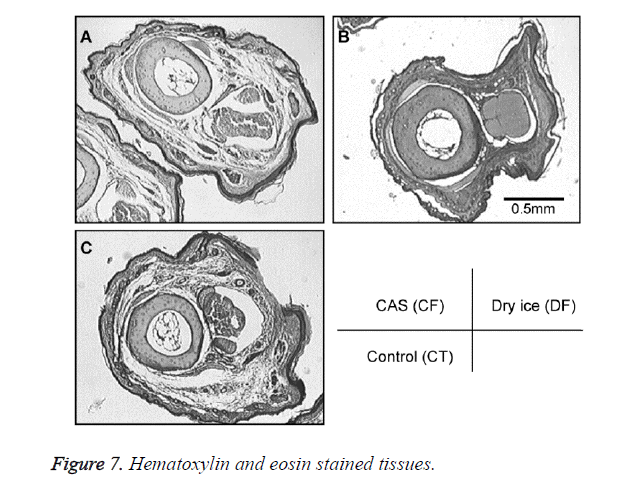
A general anesthetic with pentobarbital sodium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was given to the mice in the CF and DF groups prior to the experiment, and the right hind foot, from the heel back, was fixed in an extended state using a 5-mm plastic tube before it was frozen (Figure 3). The foot of a mouse was defrosted naturally after freezing for 10 min. Mice were then reared in a cage under normal conditions for 10 days (Figure 4). Laser doppler blood flow measurements (PerifluxPF3, Perimed Co., Fukuoka, Japan) were conducted just after freezing with the CAS or dry ice and 5 days later in order to evaluate the circulation (Figure 5). The frozen sections were subjected to X-ray exposure in a micro-FX1000 system (Fuji Film Inc., Tokyo, Japan) after 10 days in order to evaluate the bone tissue (Figures 6A-6C). Perfusion fixation was subsequently performed and, for histological examination, the tissue specimens from the frozen sections were embedded in paraffin and sectioned with hematoxylin and eosin (H&E) staining (Figures 7A-7C).
Results and Discussion
Redness from inflammation and swelling of the frozen section was observed in the CF and DF groups 1 day after freezing. The swelling of the frozen section increased gradually in DF mice and became dark purple in all animals. In half of the animals examined in the DF group, the frozen section desorbed 10 days after freezing. In contrast, all animals in the CF group showed erosion in the frozen section, with increased swelling and a dark purple color; however, these effects were limited to a portion of the terminal part of the foot.
Laser Doppler blood flow measurements revealed blood circulation disorders 10 days after the commencement of the experiment due to frostbite in DF mice; however, circulatory disorders were not observed in CF mice, even after thawing [5,6]. Furthermore, X-ray images showed that bone deformation caused by frostbite was milder in CF mice than in DF mice. In tissue specimens from frozen sections, tissue degeneration due to frostbite was observed in the DF group, whereas normal tissue structures were maintained in the CF group. The CAS freezer did not appear to induce cell destruction due to freezing or circulation disorders because of damage to blood vessel tissues and, thus, the effects of frostbite were restricted to a limited area. The most serious problem during freezing is cell damage induced by ice crystal formation inside the cells as well as mechanical stresses by extracellular ice crystal formation. When cells are frozen, a cluster of ice crystals inside the cells grows and injures the cell membrane. By using a CAS freezer, alternating magnetic fields with an induced electrical field vibrate the cells and water molecules by a non-thermal mechanism. These vibrations were amplified in sympathy with mechanical and thermal vibration (CAS vibration). Such CAS vibration can prevent intracellular ice crystal formation [7]. The miniaturization of intracellular ice crystals through the use of a CAS freezer has been shown with SEM images [8]. The effect of magnetic fields on the freezing process in aqueous solutions and the mechanism are evident in Iwasaka et al. [9]. They showed that when pulsed train magnetic fields were applied, the number of fine ice crystals increased in the early phase of the supercoiled state. Therefore, in this study, we examined the effects of the CAS freezer on the onset of frostbite after freezing in the feet of mice, without replacement of blood and tissue fluids with cryoprotective agents, showing that the CAS freezer did not appear to induce tissue destruction due to freezing or circulation disorders because of damage to blood vessel tissues, thus, the effects of frostbite were restricted to a limited area. Therefore, the use of CAS prevented frostbite associated with tissue freezing, without the replacement of bodily fluids with cryoprotective agents. The results of the present study may potentially lead to the development of novel methods for the cryopreservation of organs, body parts, and the human body in the future.
References
- Nishiyama Y, Iwanami A, Kohyama J, Itakura G, Kawabata S, Sugai K, Nishimura S, Kashiwagi R, Yasutake K, Isoda M, Matsumoto M, Nakamura M, Okano H. Safe and efficient method for cryopreservation of human induced pluripotent stem cell-derived neural stem and progenitor cells by a programmed freezer with a magnetic field. Neurosci Res 2016; S0168-0102: 00296-5.
- Kaku M, Shimasue H, Ohtani J, Kojima S, Sumi H, shikata H, Kojima S, Motokawa M, Tahsin Raquib Abonti, Kawata T, Tanne K, Tanimoto K. A case of tooth autotrnsplantation after long-term cryopreservation using a programmed dreezer with a magnetic field. Angle Orthod. 2015; 85: 518-524.
- Heisig M, Mattessich S, Rembisz A, Acar A, Shapiro M, Booth CJ, Neelakanta G, Fikrig E. Frostbite Protection in Mice Expressing an Antifreeze Glycoprotein. PLoS One 2015; 10: e0116562.
- Kawata T, Abedini S, Kaku M, Koseki H, Kojima S, Sumi H, Motokawa M, Fujita T, Ohtani J, Ohwada N, Tanne K. Effects of DMSO (Dimethyl sulfoxide) free cryopreservation with program freezing using a magnetic field on periodontal ligament cells and dental pulp tissues. Biomed Res 2012; 23: 437-442.
- Finderle Z, Cankar K. Delayed treatment of frostbite injury with hyperbaric oxygen therapy: a case report. Aviat Space Environ Environ Med 2002; 73: 392-394.
- Prommersberger KJ, Mühldorfer-Fodor M, Kalb K. Hand injuries in mountain sports. Unfallchirurg 2015, 118: 515-519.
- Kaku M, Kawata T, Abedini S, Koseki H, Kojima S, Sumi H, Shikata H, Motokawa M, Fujita T, Ohtani J, Ohwada N, Kurita M, Tanne K. Electric and magnetic fields in cryopreservation. A response, Cryobiology 2012; 64: 304-305.
- Aloy-Prósper A, Maestre-Ferrin L, Peñarrocha-Oltra D, Peñarrocha-Diago M. Bone regeneration using particulate grafts: an update. Med Oral Patol Oral Cir Bucal 2011; 16: e210-214.
- Iwasaka M, Onishi M, Kurita S, Owada N. Effects of pulsed magnetic fields on the light scattering property of the freezing process of aqueous solutions. J Appl Phys 2011; 109: e320-323.