Research Article - Biomedical Research (2017) Volume 28, Issue 3
Expression of matrix Metalloprotein-9 (MMP-9) in rat models brain tissue of experimental Intracerebral Hemorrhage (ICH)
Liu S1, Cai J2, Ge F3 and Song Z1*1Department of Medical Imaging, LinYi People’s Hospital, China
2Department of Neurosurgery, LinYi People's Hospital, China
3Department of Surgery, Linyi Women and Children Hospital, China
- *Corresponding Author:
- Song Z
Department of Medical Imaging
LinYi People’s Hospital, China
Accepted date: September 10, 2016
Abstract
Cerebral edema after cerebral hemorrhage is one of the main reasons for aggravated disease, but the exact mechanism of cerebral edema has been yet completely clarified. The expression and significance of MMP-9 in rat brain after experimental Intracerebral Hemorrhage (ICH) were observed and analyzed. The male adult Wistar rats were randomly divided into three groups, the group with conventional hemorrhage was divided into 7 sub-groups (1 h, 3 h, 6 h, 24 h, 48 h, 72 h and 7 d) with 5 rats in each sub group according to different observation time; the group with different hemorrhage volume was divided into 4 sub-groups with 5 rats in each sub group according to different hemorrhage volume (25 μL, 50 μL, 75 μL and 100 μL); and the sham group (5 rats). The brain edema was observed by dry-wet method, MMP-9 expression was obtained by immunohistochemistry. The results showed that infiltration of inflammatory cells presented at 6 h in perihematoma after ICH, it reached maximum at 48 h, and continued to 7 d. MMP-9 positive cells began to increase at 1 h, it reached maximum at 48 h, and continued to 7 d. In the sham group, there was no hematoma and infiltration of inflammatory cells was found around hematoma, however there was little MMP-9 positive cells detected. In summary, there is the correlation in time period between MMP-9 expression in rat brain and brain edema after ICH. MMP-9 enhanced development of brain edema through degrading of the blood brain barrier component substances.
Keywords
Intracerebral haemorrhage, Brain edema, MMP-9, Immunohistochemistry.
Introduction
Cerebral edema after cerebral hemorrhage is one of the main reasons for aggravated disease, but the exact mechanism of cerebral edema has been yet completely clarified [1-11]. The formation of brain edema is affected by many factors, such as liquid static beat and blood clot retraction, thrombin generation, hemoglobin toxicity, complement activation, inflammatory response to injury and Blood-Brain Barrier (BBB) opening [12-20]. Recently, matrix Metalloproteinase 9 (MMP-9) in the process of brain edema formation has attracted more and more attention [1,3,11,15].
Proteins of the MMP family are involved in the degradation and reconstruction of Extracellular Matrix (ECM) in normal physiological and pathological processes, such as embryonic development, reproduction, bone development, wound healing, arthritis and intracerebral hemorrhage [10,13-18]. MMP-9 is a matrixin, a class of enzymes that belong to the zincmetalloproteinases family involved in the degradation of the ECM. ECM molecules such as type IV collagen, laminin and fibronectin constitute the capillary endothelial cells of the basal membrane - the basement membrane of intracranial BBB, if ECM degradates, BBB will be destroyed to increase the vascular permeability, consequently result in vasogenic brain edema [3,11,15].
Proteins of the MMP family are involved in the degradation and reconstruction of Extracellular Matrix (ECM) in normal physiological and pathological processes, such as embryonic development, reproduction, bone development, wound healing, arthritis and intracerebral hemorrhage [10,13-18]. MMP-9 is a matrixin, a class of enzymes that belong to the zincmetalloproteinases family involved in the degradation of the ECM. ECM molecules such as type IV collagen, laminin and fibronectin constitute the capillary endothelial cells of the basal membrane - the basement membrane of intracranial BBB, if ECM degradates, BBB will be destroyed to increase the vascular permeability, consequently result in vasogenic brain edema [3,11,15].
In this study, the model of cerebral hemorrhage was constructed through injecting fresh autologous blood to the caudate nucleus of Wistar rats. MMP-9 expression pattern and water content in cerebral tissue were investigated at different time after cerebral hemorrhage of rats. The relationship between MMP-9 expression and formation of brain edema after the cerebral hemorrhage was discussed. The current research is potential to develop a new method for the prevention and treatment of cerebral edema.
Materials and Methods
Experimental animals
Sixty SPF grade Wistar rats (age: 8-10 weeks, weight: 350-400 g) were purchased from Animal experimental center of Fundamental Medical College at Shijiazhuang Medical University. Temperature and humidity were controlled at 25 ± 1°C and 55%-60%, respectively. There were no restrictions on drinking water and feeding. The rats were randomly divided into three groups: the group with conventional hemorrhage was further divided into 7 sub-groups (5 rats in each subgroup) according to experimental time points at 1 h, 3 h, 6 h, 24 h, 48 h, 72 h and 7 d; the group with different hemorrhage volume was further divided into 4 sub-groups (5 rats in each subgroup) according to different hemorrhage volume of 25 μL, 50 μL, 75 μL and 100 μL; and the sham group (5 rats).
Reagents and instruments
Anti-rabbit MMP-9 Strept Avidin-Biotin Complex (SABC) method reagent kits were purchased from Beijing Tiandz, Inc. Model CRS-R-2 frameless stereodirected apparatus (Shanghai Tongwei Tech). High precision electronic balance machine (Beijing Kerui); high speed centrifuge (GTR16-2, Beijing Era Beili Centrifuge); UV-vis spectrophotometer (Angilent Cary-300); PCR amplification instrument (Thermo Scientific Piko); electrophoresis tank (Bioduro tech); gel automatic imaging instrument (Gel-Pro_analyzer 4.0).
Model establishment and preparation
A model of rat ICH was prepared according to a published method: the rats were weighed and then anaesthetized by intraperitoneal injection of 15% chloral hydrate. The position of rat caudate nucleus was stereotaxically fixed by frameless stereodirected apparatus. Before taking blood samples, the rat tail was placed in warm water at 50°C for 10 min, and truncated at 30 mm from end after disinfection; the blood sample from tail was then collected. The rat autologous blood (100 μL) was taken with a microinjector, and then slowly and uniformly injected to caudate nucleus under the guidance of the stereodirected apparatus with an injection rate of 5 μL per minute. The needle was kept in the rat body for 10 min after the injection was completed then slow withdrawing the needle, the incision was sutured, all above procedures were preformed according to the aseptic operation. The sham group was also conducted as the same as the hemorrhage group, injected with 100 μL normal saline. The rats in each group were given a deep anesthesia with excessive chloral hydrate at different time after hemorrhage. The rats were killed, lavaged with normal saline, placed and fixed in neutral formalin and stained by immunohistochemical SABC method. The criteria of a success model were estimated by the round, oval or irregular type of presented hematoma in brain slices.
MMP-9 detection
Thick frozen sections (6 μm) were placed at room temperature for 40 min and fixed in cold acetone for 20 min, H2O2 (3%) was used to inactivate endogenous peroxidase for 10 min, followed by dripping with normal goat serum and sealed for 40 min, then placed for overnight at 5°C. The sample was washed with 0.01% PBS, biological prime coupled second antibody (anti rabbit IgG) was added by droplet, and incubation for 40 min at 37°C, oxidase conjugated streptavidin egg white was added by droplet, and incubation for 40 min at 37°C. After haematoxylin staining for 10 min, the sample was sealed for observation of MMP-9 proteins.
Determination of water content in brain tissue
After craniectomy, an incision of brain was made along the pinhole coronal, a slice sample with a thickness of 2 mm was taken, and the blood and cerebrospinal fluid on the surface of sample were carefully removed using filter paper. The wet weight of sample was weighed with an electronic balance (degree of accuracy 100 μg), the samples were placed in an electric oven at constant temperature for overnight at 80°C to weigh the dry weight of sample. Brain water content was determined according to the following equation. Brain water content (%)=(wet weight-dry weight)/wet weight × 100%.
Statistical methods
Statistical analysis of the expression of MMP-9 and brain water content is expressed by x ± s. Statistical method is described as follows. First, database is constructed for the normality test of data, the following test was conducted under the condition of normality; second, each model of the operated side and non-operated side were compared using the paired t test; third, comparison was made between the brain hemorrhage control group, experimental group and shamoperated group for the overall difference, single factor variance analysis was used, if overall difference was found, Least Significant Difference (LSD) method was applied for model pairwise comparison between each of the two groups; fourth, the single factor variance analysis was used for cerebral hemorrhage comparison of the overall difference between the groups, if overall difference was found (P<0.05), LSD method was applied for model pairwise comparison between each of the two groups; fifth, the analysis between MMP-9 expression of surgical or non-surgical side and brain water content was conducted using SPSS 13.0 statistical software, P<0.05 shows that the difference exists, P>0.05 indicates that the difference does not exist.
Results
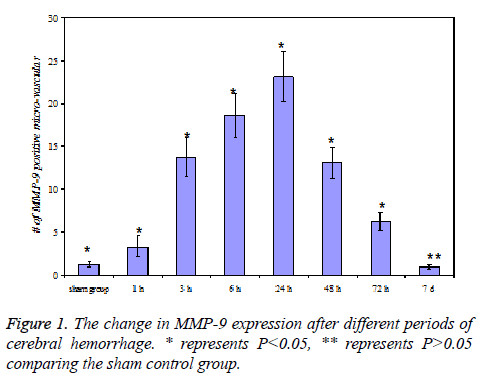
MMP-9 expression after cerebral hemorrhage
No MMP-9 expression was observed in both sides of the brain tissue for sham control groups, and only slight MMP-9 expression was detected in the opposite brain tissue for cerebral hemorrhage group.
At 3 h of cerebral hemorrhage, the number of MMP-9 positive micro-vascular at the edge of hematoma significantly increased (P<0.05), and reached a maximum at 24 h, then decreased after 2-3 d, but the level was still higher than the sham control group (P<0.05), the expression was close to zero at 7 d.
In addition, neutrophil MMP-9 expression was detected at 3 h and 6 h in cerebral hemorrhage group. The results were shown in Figure 1.
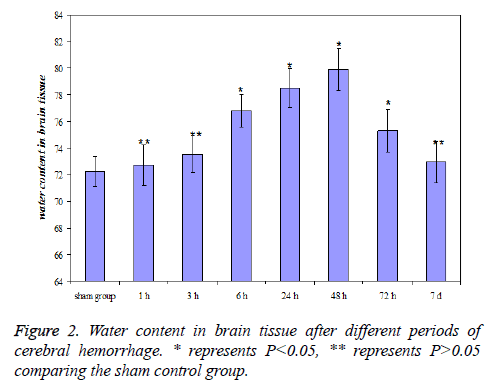
The change of water content in brain tissue after cerebral hemorrhage
The water content in brain tissue after 6 h of cerebral hemorrhage significantly increased (P<0.05) comparing the sham control group, and reached a maximum at 48 h, then gradually decreased. The level was still higher than sham control group (P<0.05) at 3 d and showed no significant difference with sham control group (P>0.05) at 7 d after cerebral hemorrhage. The results were shown in Figure 2.
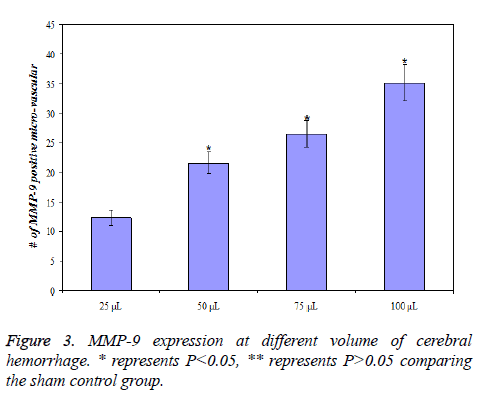
MMP-9 expression at different volume of cerebral hemorrhage
MMP-9 expression of rats with different volume of cerebral hemorrhage after the same time (24 h) was different. MMP-9 expression was lowest for 25 L of cerebral hemorrhage and highest for 100 L of cerebral hemorrhage, that is, the expression of MMP-9 increases with increasing volume of cerebral hemorrhage (P<0.01). The details of data were presented in Figure 3.
The relationship between MMP-9 expression and water content in brain tissue
MMP-9 expression was detected after 3 h of cerebral hemorrhage and peaked after 24 h, then decreased. The water content in brain tissue increased after 6 h of cerebral hemorrhage and peaked after 48 h. Through correlation analysis of MMP-9 expression and water content in brain tissue, it was found that they are positive correlation. Therefore the expression of MMP-9 is related to the increase in water content in brain tissue.
Discussion
MMP-9 is one of the important MMP family members, the primary functional substrates are gelatin, IV- and V-type collagen and elastin. The mechanisms of MMP-9 induced brain edema are mainly related to the degradation of capillary basement membrane collagen composition and increase in permeability of BBB, as well as the secondary brain tissue caused by release of inflammation mediators due to overflow of neutrophils and macrophages from the damaged blood vessels after microvascular damage to aggravate brain edema.
The results in this study showed that MMP-9 has high expression in hemorrhage side of brain tissue after cerebral hemorrhage, the positive cells are micro-vascular endothelial cells and neutrophile granulocytes. After 3 h of cerebral hemorrhage, MMP-9 expression significantly increased in micro-vascular endothelial cells and reached peak at 24 h of cerebral hemorrhage, the expression then dramatically decreased after 48 h, and almost no MMP-9 expression was detected after 1 week. Moreover, MMP-9 expression was enhanced with increased amount of cerebral hemorrhage. There was no or only a small amount of expression in the contralateral brain tissue of cerebral hemorrhage in sham control group. In addition, the changes of water content in brain tissue were measured by dry and wet weight method, it was found that water content significantly increased in brain tissue after 6 h of cerebral hemorrhage and peaked at 48 h, followed by a gradual decrease. After 7 d there were no difference between experimental and sham control groups, which is in agreement with previous results. Our results indicated that the change of MMP-9 expression after cerebral hemorrhage and water content in brain tissue exhibit a similar trend. Statistical analysis has confirmed that there is a positive correlation (r=0.8514, P<0.05) between the two factors in term of time period, that is, after cerebral hemorrhage brain water content increased gradually with significance of MMP-9 expression in brain tissue, intracranial BBB is composed of capillary endothelial cells, intercellular tight junctions and capillary endothelial basement membrane. The activated MMP-9 after cerebral hemorrhage attacked the BBB capillary endothelial basement membrane, and degradated ECM collagen components (such as collagen type IV, V), laminin, elastin and fibronectin to damage the structural integrity, which resulted in an increase in permeability of BBB, water overflowed in blood vessel, leading to an increase in water content of brain tissue at the edge of hematoma, which is vascular brain edema. Furthermore, it was also found in this study that after 6-12 h of intracerebral hemorrhage, neutrophil expressed MMP-9 but the time period of expression was relatively short, there was no expression after 12 h. Neutrophils can promote the expression of MMP-9 to pass through the blood vessel walls and to infiltrate into the brain tissue. Pantoni et al. [21] found that the neutrophils infiltrating into brain tissue can release some vasoactive substances, such as Thromboxane A2 (TXA2), superoxide anion, IL-1, PGI2 and PGH2. These substances changed the reactivity of arterial blood vessel, and simultaneously released cytotoxic enzyme, oxygen free radical, Nitric Oxide (NO) and phospholipid cascade reaction products which can damage the brain tissue, and further aggravate the brain edema. Rosenberg et al. [1] detemined MMP-9 content and water content in brain tissue with the model experiment of collagenase induced intracerebral hemorrhage, the results showed that both factors increased. Also, the brain water content significantly reduced after administration of bb-1101-an inhibitor of MMP-9, indicating the activation of MMP-9 after cerebral hemorrhage was blocked, which might be an effective method for prevention and treatment of brain edema after cerebral hemorrhage.
Conclusions
In this rat model, MMP-9 expression was activated after cerebral hemorrhage. The increase tendency was similar for the amount of hemorrhage and brain water content. It was postulated that MMP-9 enhanced development of brain edema through degrading of the blood brain barrier component substances.
References
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res 2001; 893: 104-112.
- Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp Neurol 2002; 178: 13-20.
- Zozulya A, Weidenfeller CH. Pericyte-endothelial cell interaction increases MMP-9 secretion at the blood-brain barrier in vitro. Brain Res 2008; 1189: 1-11.
- Nessler MB, Puchała J, Chrapusta A, Nessler K, Drukała J. Levels of plasma matrix metalloproteinases (MMP-2 and MMP-9) in response to INTEGRA® dermal regeneration template implantation. Med Sci Monit 2014; 20: 91-96.
- Klebe D, McBride D, Flores JJ, Zhang JH, Tang J. Modulating the Immune Response Towards a Neuroregenerative Peri-injury Milieu After Cerebral Hemorrhage. J Neuroimmune Pharmacol 2015; 6: 1-11.
- Farid K, Hong Y, Aigbirhio F, Fryer T, Menon D, Warburton E, Baron J. Early-Phase 11C-PiB PET in Amyloid Angiopathy-Related Symptomatic Cerebral Hemorrhage: Potential Diagnostic Value? Plos One 2015; 10: 1-11.
- Yang Q, Zhuang X, Peng F, Zheng W. Relationship of plasma matrix metalloproteinase-9 and hematoma expansion in acute hypertensive cerebral hemorrhage. Int J Neurosci 2015; 126: 213-218.
- Jin X, Sun Y, Xu J, Liu W. Caveolin-1 mediates tissue plasminogen activator-induced MMP-9 up-regulation in cultured brain microvascular endothelial cells. J Neurochem 2015; 132:724-730.
- Zhao L, Arbel-Ornath M, Wang X, Betensky RA, Greenberg SM, Frosch MP, Bacskai BJ. Matrix Metalloproteinase 9 mediated intracerebral hemorrhage induced by Cerebral amyloid angiopathy. Neurobiol Aging 2015; 36: 2963-2971.
- Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci 2006; 11: 3121- 3318.
- Zhang FY, Chen XC, Ren HM, Bao WM. Effects of ischemic preconditioning on blood-brain barrier permeability and MMP-9 expression of ischemic brain. Neurol Res 2006; 28: 21-24.
- Hernandez-Guillamon M, Martinez-Saez E, Delgado P, Domingues-Montanari S, Boada C, Penalba A, Boada M, Pagola J, Maisterra O, Rodriguez-Luna D,Molina CA, Rovira A, Alvarez-Sabin J, Ortega-Aznar A, Montaner J. MMP-2/MMP-9 Plasma Level and Brain Expression in Cerebral Amyloid Angiopathy-Associated Hemorrhagic Stroke. Brain Pathol 2012; 22: 133-141.
- Rylski M, Amborska R, Zybura K, Michaluk P, Bielinska B, Konopacki FA, Wilczynski GM, Kaczmarek L. JunB is a repressor of MMP-9 transcription in depolarized rat brain neurons. Mol Cell Neurosci 2009; 40: 98-110.
- Ludewig P, Sedlacik J, Gelderblom M, Bernreuther C, Korkusuz Y, Wagener C, Gerloff C, Fiehler J, Magnus T, Horst AK. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits MMP-9-mediated blood-brain-barrier breakdown in a mouse model for ischemic stroke. Circ Res 2013; 113: 1013-1022.
- Janigro D, Namura S. Frontiers | Bone marrow-derived cells are the major source of MMP-9 contributing to blood-brain barrier dysfunction and infarct formation after ischemic stroke in mice. Brain Res 2009; 1294: 183-192.
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of Stem and Progenitor Cells from the Bone Marrow Niche Requires MMP-9 Mediated Release of Kit-Ligand. Cell 2002; 109: 625-637.
- Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem 1999; 274: 13066-13076.
- Giraudo E, Inoue M, Hanahan AD. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest 2004; 114: 623-633.
- Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 2008; 118: 3012-3024.
- Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 2001; 276: 37258-37265.
- Pantoni L, Sarti C, Inzitari D. Cytokines and cell adhesion molecules in cerebral ischemia: experimental bases and therapeutic perspectives. Arterioscler Thromb Vasc Biol 1998; 18: 503-513.