Research Article - Biology & Medicine Case Reports (2022) Volume 6, Issue 2
Exploring the degree of retinal involvement in COVID-19 patients: A narrative review.
Noon A. H. Mahgoub1*, Noun E. A. Abdulgader2, Abdelrahman H. Abdelmoneim3, Alia F. B. Mansoor4, Musab M. A. Elhadi5, Raneem A. Ali6, Mahmoud M. A. Mohamed7, Mohammed k. A. Yagoob8, Mohamed S. I. Zaroug9, Rawan K. A. Galil10
1Ophthalmology Resident, Sudan Medical Specialization Board, Khartoum, Sudan
2Human Clinical Anatomy and Education Department, the National University, Khartoum, Sudan
3Clinical Immunology Resident, Sudan Medical Specialization board, Khartoum, Sudan
4Faculty of Medicine, University of Bahri, Khartoum, Sudan
5Faculty of Medicine, National Ribat University, Khartoum, Sudan
6College of Medicine, Almughtaribeen University, Khartoum, Sudan
7Faculty of Medicine, Elrazi University, Khartoum, Sudan
8Opthalmology resident, Sudan Medical Specialization Board, Khartoum, Sudan
9Medical Officer, Imperial Hospital, Khartoum, Sudan
10School of Medicine, Ahfad University for Women (AUW), Khartoum, Sudan
*Corresponding Author:
- Noon A. H. Mahgoub
Ophthalmology Resident
Sudan Medical Specialization Board
Khartoum
Sudan
Tel: 0115661250
E-mail: dr.noonadilhassan@gmail.com
Received: 18-Feb-2022, Manuscript No. AABMCR-22-54813; Editor assigned: 28-Feb-2022, PreQC No. AABMCR-22-54813 (PQ); Reviewed: 14-Mar-2022, QC No AABMCR-22-54813; Published: 21-Mar-2022, DOI:10.35841/aabmcr-6.2.106
Citation: Mahgoub NAH, Abdulgader NEA, Abdelmoneim AH, et al. Exploring the degree of retinal involvement in covid-19 patients: A narrative review. Biol Med Case Rep. 2022;6(2):106
Abstract
Background: In December 2019, Coronavirus disease turned into a global pandemic that is caused by the highly contagious severe acute respiratory syndrome (coronavirus). Above all, eye redness and irritation were found in COVID-19 patients. Therefore true challenge was made by ophthalmologists as they were the first medical professionals to assess a patient with COVID-19 and initiate a concern, as respiratory illness could be transmitted through the eye. Up until that point, only HCoV-NL63 and SARS-COV has been confirmed to cause ocular abnormalities (cotton wool spot, retinal hemorrhage and conjunctivitis). Moreover, Reports continue to emerge on further associations of COVID-19 and the frequency is correlated to the severity of the disease. It has provided an expanding proof that provocative cytokine storm and viral evasion of cellular resistant reactions play an essential part in infection seriousness and progression. As a result, the virus is capable of targeting the endothelium cells in the retina, causing secondary damage to the retinal microvasculature. Aim: This paper was designated to: (i) Summarize the retinal findings and the degree of retinal involvement in COVID-19 patients. (ii) Describe the risk factors relating to retinal landings. (iii) To explain the retinal changes found in COVID-19 patients. Methodology: Firstly, a systemic electronic database search was used to investigate for articles and reviews providing the information on retinal entanglement in COVID-19 patients. Eight papers were found related to our review, finally PubMed and Google Scholar were used as they were easily accessible, and papers related to our topic found were open access. Other data bases such Scopus, Cochrane and web of sciences were involved in the search, but most of the related papers were found to be closed access. Lastly, the quality of each report study was evaluated using Pierson’s 5- component scheme. Result: Data bases search provided a total of eight papers were attached. Four case reports, two cross sectional studies, one cohort study and one case series study with mild to severe COVID-19 were involved in this review. In addition, retinal hemorrhage has been reported in three out of eight papers. Eventually, retinal artery and vein occlusion were observed, in more than two of these studies hypertension and diabetes were common risk factors. Conclusion: As a result, the study infers that the retinal alterations due to COVID-19 have been established through several previous studies, that is to say exaggerated immune response is more likely the mechanism. Adult patients with risk factors are affected by the hypercoagulable state more than the exaggerated immune response, especially in patients with anti-coagulants’ deficiency. The outstanding result that has been observed in various studies was retinal vascular diameter enlargement, which has been mainly linked to impaired venous drainage and reduction of oxygen supply.
Keywords
Vertebrates, COVID-19, Retinal, Ocular, Eye, Hypercoagulable.
Introduction
As a fact that the COVID-19 disease has contributed in developing eye symptoms that appeared on a large scale among COVID-19 patients, this study was intended to summarize most of the eye symptoms to elaborate in early intervention and anticipated risk management as an approach to reach the lowest level of complications following the infection.
Interestingly, ophthalmology is seldom to be involved in other systematic diseases, but in COVID-19 disease, in many cases it has appeared to contribute in early symptoms. The utmost challenge for the healthcare professionals that the disease is highly contagious and eyes represent a rout of transmission, this was the biggest fear that needed to overcome and control as early as possible.
On the 11th of March 2020, the novel Corona Virus, also known as COVID-19, was declared as a pandemic by the World Health Organization and as of the 1st of February 2021, the recorded cases of COVID-19 virus were 102.40 million cases and 2.21 million deaths worldwide [1].
The pathogenesis of COVID-19 remains ineffectively caught on, in spite of the fact that there is expanding proof that provocative cytokine storm and viral evasion of cellular resistant reactions play an essential part in infection seriousness and progression [2]. Coagulopathy and disseminated intravascular coagulation (DIC) have been reviewed as vital complications of serious COVID-19 and may be considered the foremost common causes of death [3,4]. Patients with COVID-19 usually presented with fever and respiratory tract symptoms [5,6]. It also appeared that COVID-19 can influence other organ systems such as cardiovascular [7], neurological, gustatory, olfactory [8], gastrointestinal, hepatic [9], renal, hematological, cutaneous and ocular symptoms.
Since the beginning of the pandemic, there were numbers of theories explaining the pathophysiology of the corona virus disease, however, the exact mechanism is still unknown.
In this review, there are 2 mechanisms suggested in order to give an explanation for the pathogenicity of the disease, expressed in the following:
As the virus invades the cells directly, it could affect the endothelial wall of the cell through infiltration, which will cause a state of cellular vasculitis but not like a true vasculitis; rather a vasculitis that is under the effect of the virus. Therefore, this state was referred to as a pseudo-vasculitis state [2]. Moreover, another suggestive mechanism demonstrating the disease mechanism of the SARS-COV-2 virus reported that the hypercoagulable state caused by the virus has been associated with an increased rate of mortality and morbidity in COVID-19 patients, as this was explained with the elevated inflammatory markers noticed in most of COVID-19 patients [10].
Artificial Intelligence (AI) techniques has important in role in opthalmology and in COVID-19 management as it can help in improvement of the prediction, prevention and detection of the future epidemic diseases [11]. A new framework is introduced by A.S. Albahri and his team to tackle the challenges of prioritization for COVID19 based on multiple laboratory criteria and other Methods [12,13]. By using machine learning to guide the transfusion of Convalescent plasma to patients with severe COVID-19 infection, a rescue framework is introduced based on biological requirements [14].
The link between the on-going SARS-CoV2 and ophthalmology has been started on 30th December 2019, when a Chinese ophthalmologist named Dr. Li Wenliang warned his colleagues about a SARS like outbreak in Wuhan city [15]. Among the seven types of corona viruses that affect the human, only HCoV-NL63 and SARS-CoV have been confirmed to cause ocular abnormalities, especially conjunctivitis [16]. Although the mechanism of ocular manifestations is not fully understood [17], COVID-19 has reported numbers of ocular abnormalities. A study had demonstrated that one out of three patients with COVID-19 disease had ocular abnormalities and the frequency is correlated to the severity of the disease [18].
The ocular manifestations vary from anterior segment involvement such as; ocular pain, discharge, redness [19], dry eye [20], conjunctiva conjection [21], hyperemia, epiphora, visual disturbance, chemosis [22,18], follicular conjunctivitis and hemorrhagic conjunctivitis with pseudomembranous [23], to posterior segment involvement including; cotton-woollike lesions, microhaemorrhages [17,24,25] dilated veins, tortuous vessels in retina [26], CRVO[2], CRAO[10] and papillophlebitis [27].
Considering retinal abnormalities, studies have shown that SARS-CoV2 RNA has been detected in patients’ retinae who underwent autopsy [28]. The virus has the ability to target the endothelium cells in the retina [29], causing secondary damage to the retinal microvasculatures.
As this review article elucidate a relevant concern in a brief and organized manner, which will help feasible and quick access reference for the main objective of this review is to summarize the retinal findings in COVID-19 infection, to contribute as a brief reference for in other scientific papers and help overcome the disease.
Materials and Methods
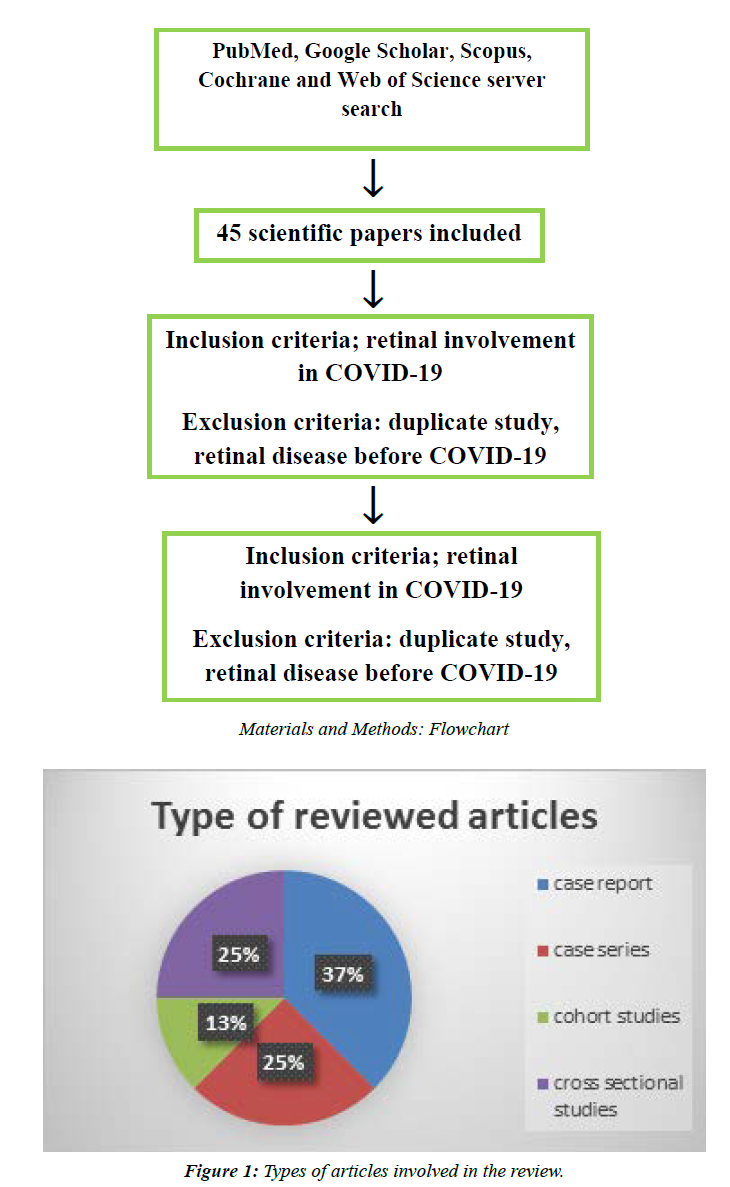
A systemic electronic database search conducted in PubMed and Google Scholar was performed when screening for articles and reviews providing the information on retinal involvement in COVID-19 patients. PubMed and Google Scholar were used as they were easily accessible, and papers related to our topic found were open access. Other data bases such Scopus, Cochrane and web of sciences were involved in the search, but most of the related papers were found to be closed access. The inclusion criteria were any published case reports and cross-sectional reports in English language. We excluded any retinal manifestations before COVID-19 diagnosis in patients, duplicate publications and articles with no significant information. The quality of each report study was evaluated using Pierson’s 5- component scheme, which is composed of the following five components; uniqueness, documentation, interoperation, objectivity and educational value. Each of these components carries a score of zero to one, with scores of 5 or less indicative of insufficient quality. Assessment of the endorsed papers was undertaken precisely by all authors equally. In light of that, the most pertinent information was summarized into different sections by each author, as well as revising and formatting the final version of the text.
Results
There were eight papers in this review, most of which were case reports as illustrated in Figure 1. A total of 223 patients with mild to severe COVID-19. With cotton wool spot and retinal hemorrhage being reported in three out of the eight papers. Furthermore, retinal artery and vein occlusion were noticed in three out of these studies. Most of the involved patients were adults with the age reported to be in a range between 30 and 75 years. Furthermore in accordance with this age, diabetes and hypertension was noticed to be a common comorbidity with a percentage as high as 60% and 32% in the Rafael et al. study.
A complete list of retinal findings and risk factor are illustrated in Table 1A and Table 1B.
|
Authors |
no. of patients |
serology and PCR test |
main retinal findings |
severity of covid-19 disease |
vascular and arterial investigation |
time of retinal symptoms appearing |
age |
preexisting condition |
positive ocular test |
inflammatory markers |
study type |
associated disease |
|
Yahalomi et al. |
1 |
pcr -ve, IgM negative and IgG Positive |
unilateral Central Retinal Vein Occlusion |
none |
none |
two weeks after covid19 symtoms disappers |
33 |
none |
Fluorescein angiography showed marked delay in arteriovenous transit time, staining of dilated tortuous veins and masking by retinal hemorrhages. Fundoscopy showed tortuosity and dilatation of all branches of the central retinal vein, dot, blot and flame-shaped hemorrhages throughout all four quadrants, and optic disc edema |
|
case reports |
|
|
Savastano et al. |
80 patients and 30 controls |
vascular involovment |
6.25% were in ICU |
RPCP-PD was lower in post SARS-CoV-2 patients compared to controls. Patients treated with lopinavir + ritonavir or antiplatelet therapy during admission had lower RPCP-FI and RPCP-PD. patients with systemic arterial hypertension had lower RPCP-FI and age was inversely correlated to both RPCP-FI and RPCP-PD. |
one month after clearing of covid19 |
52.9 ± 13.5 y |
The prevalence of systemic arterial hypertension, diabetes, and autoimmune or inflammatory systemic diseases was 23.8%, 42.5%, and 23.8%, respectively |
Mean IOP at the visit was 16.2 ± 1.5 mmHg,RNFL average thickness was linearly correlated to RPCP-FI and RPCP-PD within post-COVID-19 group. |
|
case serious |
|
|
|
Acharya et al. |
1 |
pcr positives |
central retinal artery occlusion |
admitted to the ICU |
twelph days after beginong covid1i sytoms |
|
hypertension, dyslipidemia, stable coronary artery disease andchronic obstructive pulmonary disease |
D-dimer Assay 42,131 |
case reports |
|
||
|
|
|
|
|
|
|
|
C-Reactive Protein 7.02 |
|
|
Table 1 A: Summary of retinal signs and associated conditions from three studies
|
Base line co-morbidity. |
None |
HTN 22 (51.2%), DM 8, (18.6%), (CAD, COPD 7) ((16.3%), Other CVA,TIA, CHF, AF & dementia all represent range of 6-2, (14.0%-4.7%). |
HTN 15 (60%), DM 8 (32%) |
12 (66.7%) HTN p.t 9 (50 %) DM p.t |
BMI, smoking, Alcohol, HTN, DM, dyslipidemia, history of CAD, stroke. |
|
Pre-existing retinal disease |
None |
None |
None |
No |
NO |
|
Time of retinal symptom appear |
10 days after COVID test |
NA |
on average after 8.6 ± 3.0 days of symptoms onset, ranging between 2 to 14 days |
None |
NO |
|
Vascular investigation |
FFA ,BCVA, vasculitis screen. |
Fundus examination |
Digital fundal examination |
Color fundus photography |
Color fundus photo |
|
Other retinal /ocular finding. |
Foveolitis, retinitis, neuroretinitis, optic neuritis, and panuveitis. |
Conjectival hyperemia:4 (9.3%) |
---------------- |
Retinal sectorial pallor, retinal pigment hyper plasia, choroidal naevus, macular hemorrhage, and hard exudates. |
no |
|
Main retinal finding |
RVO |
Hypertensive retinopathy, 4 (9.3%). |
only three (12%) |
Flame-shaped hemorrhages, cotton wall spots. |
Retinal hemorrhages |
|
Inflammatory marker /laboratory parameter |
Systemic workup for vasculitic and non?vasculitic causes of RVO |
WBCs , LYM , platelets , |
CRP, D-dimer, ferritin |
None |
In E: HTC, WBC, platelets, PT, PTT, D-dimer, fibrinogen, CRP, ferritin. |
|
Ocular test |
Fundus examination |
Ocular fundus examination,eye swab for |
Fundus examination was performed with an Eyer portable retinographer |
Dilated eye examination |
Digital retinography system( DRS) Fundus camera |
|
Time of ocular test |
10 days after |
Median of 21.5 days |
The fundus examination was performed from 10 to 64 |
Median time between COVID-19 test &dilated eye examination 11.5 (8)days |
E. Within 30 days from covid19 onset symptoms. |
|
Severity of COVID-19 p.t |
----- |
Mild: |
Severely ill p.t 13 (52 %) |
17 (94.4%) (admitted |
14% sever COVID-19 |
|
Serology and PCR Test |
Positive |
Positive |
Positive |
|
E. 54 positive |
|
Age |
52 years |
Mean |
35-75 years |
|
E. 49.9 yrs. |
|
No. of p.t |
1 p.t |
43 p.t |
25 p.t |
18 |
E.54 NE.133 |
|
Study type |
Case report |
Cohort study |
Case series |
Cross sectional study |
Cross sectional study |
|
Author |
Jay Umed Sheth |
Maria et al. |
Rafeal et al. |
Leonardo Pereira..etc |
Alessandro Invernizzi…etc |
Table 1 B: Summary of retinal signs and associated conditions from five studies.
Discussion
From the start of the COVID-19 pandemic, there were reported cases of ocular changes, more specifically related to the retinal part. However, based on the literature and to our current knowledge, the direct potential impact of COVID-19 on the retina is still in debate and needs broader studies to be clear.
Ocular thromboembolic events associated with COVID-19
Ocular involvement, specifically retinal circulation changes, was reported to be associated directly with the hypercoagulable condition through the disease stage, or indirectly by a delayed immune response; whether they are the retinal arteries or veins. Regarding that, most of the authors prefer the delayed immune-mediated response as a more reasonable mechanism [10].
In light of the above, this explains the increased incidence rate of the COVID-19 related retinal vascular occlusion diseases in adult patients, where the main risk factors of the retinal changes are absent and those risk factors are hypertension, diabetes and hyperlipidemia. In contrast, contribution of the hypercoagulable state in the process of the disease in adult patients is strongly suggestive and is more adherent to patients who have anticoagulants deficiency, including protein C and S, antithrombin 3 and activated protein C resistance. In view of the above, ruling out thrombophilia in COVID-19 patients less than 50 years old as well as performing angle neovascularization would be highly valuable [2].
Based on the reported cases in the literature, the effect of COVID-19 on the retinal vasculature is suggested despite the presence of controversial opinions. Upon that, a central retinal vein occlusion was reported in a 33 year-old male, healthy with an unremarkable free medical background presented with unilateral blurred vision associated with flashing lights with no other neurological symptoms involved, but he only reported a 3 weeks history of COVID-19 symptoms at the time of presentation. Additionally, COVID-19 antigenantibody test (IgG + IgM) was done, confirming the presence of COVID-19 antibody and stage of disease recovery. Eventually, eye symptoms showed a good prognosis and he was fully recovered after one month [2].
Furthermore, a reported case of a central retinal artery occlusion in a 60 year-old male with a medical background of hypertension, diabetes, stable coronary artery disease and chronic obstructive pulmonary disease (COPD), confirmed to be positive for COVID-19 by PCR, on the 12th day prior to admission he developed unilateral painless loss of vision. An ophthalmology work-up was done and a central retinal artery occlusion was found. Unfortunately, the prognosis was poor and the occlusion did not resolve [10].
On the other hand, a case of a 52 year-old male presented with unilateral decreased vision along with COVID-19 symptoms, for which it was confirmed positive by PCR as well. Ophthalmology work-up also revealed a hemiretinal vein occlusion, macular edema and extensive phlebitis. Surprisingly, there was no clear diagnosis for the eye symptoms, for which a full systemic investigation was conducted and was unremarkable. Given the suggestive theories of the direct invasion of the retinal nerves by the virus, a presumptive diagnosis of retinal vasculitis secondary to COVID-19 was concluded, and fortunately it lead to a full recovery after 1 month.
Incidence and degree of retinal changes in COVID-19 hospitalized/ICU patients
Another study done illustrated that a series of retinal changes was reported in hospitalized COVID-19 patients who have risk factors and others who have no risk factors. Pharmacological support was provided for some patients, in addition to the confined use of a dilated eye examination as a diagnostic tool for which the study was very restricted and not precise in the suggestion of direct involvement of COVID-19 in the retinal changes. Despite that, some changes were reported (like retinal pigment epithelium hypertrophy, choroidal navaeus) which were considered degenerative changes and less likely to be related to COVID-19. Other changes observed were flameshaped hemorrhage, cotton wool spots, and retinal sectorial pallor [17].
A similar study debating the direct effect of COVID-19 on the retina, presented only 12% of the hospitalized COVID-19 patients who developed serious retinal symptoms (including micro-hemorrhage, flame-shaped hemorrhage and nerve fiber layer infarct). More specifically, those patients were in the late COVID-19 phase and genetic factors were associated in some of these patients as well. Similar to a previously mentioned study, this study suggests that the retinal changes are more likely to be related to a hypercoagulable state and the late immune storm, in which they can cause a multi-organ dysfunction which can also be associated with these retinal changes. In contrast, another theory suggested reports that due to the presence of COVID-19 RNA in the CSF (Cerebrospinal Fluid) and the virus protein spikes in the CNS (Central Nervous System), specifically in the retinal vascular endothelium, it is closely related to the viral involvement on the retina. Another hypothesis supporting the previous one reported that the viral genome was identified in a retinal biopsy through a positive PT-PCR, as well as alternative entry host cell route through the receptor CD147 accompanied by the high expression to CD147 gene in the retina [24].
To the best of our knowledge, one of the noticeable changes during COVID-19 active disease is retinal vascular diameter enlargement. Subsequently, an impaired venous drainage and decreased oxygen saturation was documented as a response to the vascular enlargement under the effect of inflammatory mediators, specifically on its highest peak, which eventually showed a good prognosis. Up to date, there is no clear pathophysiology explaining this enlargement [26].
Going further, another study showed signs of retinal toxicity as a side effect of antiviral medications used to treat COVID-19, taking ritonavir as an example. This drug was reported to exert a toxic effect on the outer retinal pigment epithelium (RPE). Upon fundus examination, atrophy of RPE affecting the macula was remarkable, optical coherent tomography investigation showed outer retinal thinning and loss of photoreceptors, intraretinal cyst and cavitation, epiretinal membrane and full thickness macula hole, and finally incomplete RPE showed an outer retinal atrophy with some visible discontinuous hypertransmission [20].
On a different scope study here, we find a post-COVID-19 low radial peripapillary capillary plex (PRCP) and PRC flow index in comparison with healthy people as a result to anticoagulant medications. On the other hand, micro-vascular impairment involved retinal capillary hemostasis and retinal ganglion cell function. Interestingly, it was reported that RPCP density is highly correlated to retinal nerve fiber layer (RNFL) thickness and visual field (VF) index particularly in glaucoma patients. It is worth mentioning that early limited changes of the retina related to COVID-19 describes the presence of a hyper-reflective lesion at the level of ganglion cell and inner plexiform layer [29,30].
Comparison with another study has shed the light on the same scope of this review, as it has shown that the ocular manifestation could possibly start to appear in different disease phases. Interestingly, some cases reported that the ocular symptoms started to develop days before the respiratory symptoms appear, while other cases reported the ocular symptoms developed in the middle of the disease and other cases were reported to have the symptoms 19 days after the disease. This variety was applied as well for the ocular manifestations including viral-induced retinitis, optic neuritis, kerato-conjunctivitis, acute follicular conjunctivitis, hemorrhagic and pseudo-membranous conjunctivitis. Overall, the findings of this study were approximately similar to the different studies discussed in this review [31].
Ultimately, we firmly believe that having solid based evidences related to retinal changes as a response to the direct invasion of COVID-19 (which are broader, more precise and comprehensive) are highly recommended.
Conclusion
Our Study concludes that the retinal changes due to COVID-19 have been confirmed through several previous studies, but the exact mechanism needs further investigation.
Several studies claimed and concluded simultaneously that age and other risk factors for retinal changes are determinant factors for the prognosis as the mechanism of pathogenicity is different. Furthermore, it was claimed that the robust immune response and vasculitis caused by the virus itself has a good prognosis, and in contrast, a poor prognosis is associated with the hypercoagulable state. Some studies found retinal changes in hospitalized patients but it has been linked to risk factors and a hypercoagulable state.
The most noticeable change that has been observed in the various studies was retinal vascular diameter enlargement which has been mainly linked to impaired venous drainage and decreased oxygen supply.
In addition, antivirals like Ritonavir have been associated with a toxic effect as a side effect causing atrophy of RPE. Also low PRCP and PRC flow was noticed and related to usage of anticoagulants which has been linked to RNFL.
Recommendations
We recommend the following:
• Further studies to be done on large scales to determine the exact mechanism of retinal involvement in COVID-19 patients.
• More clinical trials are demanding to test the use of angle neovascularization for patients aged less than 50 years whom have been excluded from thrombophilia.
• Further studies to be conducted regarding immune response towards corona virus and robust immune response.
• Biopsies and specimens from retina, CSF and CNS should be considered for further investigation.
• The effects of COVID-19 medications like antivirals should be consider in any further studies.
Conflicts of Interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
Acknowledgement
This work is dedicated to our families who were the key to our success along the way as well as our ophthalmology specialist Dr. Maha Sir Alkhatim who helped in finish this work; your efforts are highly appreciated.
References
- Edward L, Karen B, Andrew R. Coronavirus Disease 2019 and Influenza 2019-2020. JAMA - J Am Med Assoc. 2020;323(12):1122.
- Tal Y, Joseph P, Roee A, et al. Central retinal vein occlusion in a young healthy COVID-19 patient: A case report. Am J Ophthalmol Case Reports. 2020;20:100992.
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-47.
- Behnood B, Mahesh VM, David M et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-73.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J Med Virol. 2020;92(10):1902-14.
- Hansen HI, Berge T, Tveita A et al. COVID-19: Symptoms, course of illness and use of clinical scoring systems for the first 42 patients admitted to a Norwegian local hospital. Tidsskr Nor Laegeforen. 2020;140(7).
- Azevedo RB, Botelho BG, de Hollanda JVG et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35:4-11.
- Mehraeen E, Behnezhad F, Salehi MA, et al. Olfactory and gustatory dysfunctions due to the coronavirus disease (COVID-19): A review of current evidence. Eur Arch Oto-Rhino-Laryngal. 2021;278:307-12.
- Xu Y, Liu P, Gu J. Gastrointestinal and liver involvement in patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5(9):798-99.
- Acharya S, Diamond M, Anwar S, et al. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867.
- Albahri OS, Zaidan AA, Albahri AS et al. Systematic review of artificial intelligence techniques in the detection and classification of COVID-19 medical images in terms of evaluation and benchmarking?: Taxonomy analysis, challenges, future solutions and methodological aspects. J Infect Public Health. 2020;13(10):1381-96.
- Albahri AS, Hamid RA, Albahri OS, et al. Detection-based prioritisation?: Framework of multi-laboratory characteristics for asymptomatic COVID-19 carriers based on integrated Entropy – TOPSIS methods. Artif Intell Med. 2021;111:101983.
- Al-obaidi JR, Zaidan AA, Albahri AS. Multi-biological Laboratory Examination Framework for the Prioritisation of Patients with COVID-19 Based on Integrated AHP and Group VIKOR Methods. Int J Inf Technol Decis Mak. 2020;19(5):1247-69.
- Mohammed TJ, Albahri AS, Zaidan AA, et al. Convalescent-plasma-transfusion intelligent framework for rescuing COVID-19 patients across centralised / decentralised telemedicine hospitals based on AHP-group TOPSIS and matching component. Appl Intell. 2021;51:2956-87.
- Olivares-De Emparan JP, Sardi-Correa C, López-Ulloa JA, et al. COVID-19 and the eye: how much do we really know? A best evidence review COVID-19 e o olho: quanto sabemos realmente? Uma revisão das melhores evidências. Arq Bras Oftalmol. 2020;83(3):250-61.
- Ceran BB, Ozates S. Ocular manifestations of coronavirus disease 2019. Graefe’s Arch Clin Exp Ophthalmol. 2020;258(9):1959-63.
- Pereira LA, Soares LCM, Nascimento PA, et al. Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol. 2020;106(1):102-05.
- Wu P, Duan F, Luo C, et al. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575-78.
- Aggarwal K, Agarwal A, Jaiswal N, et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One. 2020;15(11):e0241661.
- Savastano A, Crincoli E, Savastano MC, et al. Peripapillary Retinal Vascular Involvement in Early Post-COVID-19 Patients. J Clin Med. 2020;9(9):2895.
- Lim LW, Tan GS, Yong V, et al. Acute Onset of Bilateral Follicular Conjunctivitis in two Patients with Confirmed SARS-CoV-2 Infections. Ocul Immunol Inflamm. 2020;28(8):1280-84.
- Lee YH, Kim YC, Shin JP. Characteristics of ocular manifestations of patients with coronavirus disease 2019 in Daegu province, Korea. J Korean Med Sci. 2020;35(35):e322.
- Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am J Ophthalmol Case Reports. 2020;19:100735.
- Lani-Louzada R, Ramos C do VF, Cordeiro RM, et al. Retinal changes in COVID-19 hospitalized cases. PLoS One. 2020;15(12):e0243346.
- Pirraglia MP, Ceccarelli G, Cerini A, et al. Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci Rep. 2020;10:17419.
- Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020;27:100550.
- Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, et al. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur J Ophthalmol. 2020;32(1): NP168-NP172.
- Casagrande M, Fitzek A, Püschel K, et al. Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients. Ocul Immunol Inflamm. 2020;28(5):721-25.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-18.
- Cozzupoli GM, Savastano MC, Falsini B, et al. Possible Retinal Impairment Secondary to Ritonavir Use in SARS-CoV-2 Patients?: A Narrative Systematic Review. J Ophthalmol. 2020;2020:1-7.
- Douglas KAA, Douglas VP, Moschos MM. Ocular manifestations of COVID-19 (SARS-CoV-2): A critical review of current literature. In Vivo. 2020;34: 1619-28.
Indexed at,Google scholar,Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar,Cross ref
Indexed at,Google scholar, Cross ref
Indexed at,Google scholar, Cross ref
Indexed at,Google scholar, Cross ref
Indexed at, Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar, Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref
Indexed at,Google scholar,Cross ref