Research Article - Biomedical Research (2017) Volume 28, Issue 9
Evaluation of laboratory parameters in predicting ischemic stroke in essential hypertension patients
Yaru Liang, Peisong Chen, Yanhong Sun, Pinning Feng, Bin Huang and Tang Jiang*Department of Laboratory Medicine, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, PR China
- *Corresponding Author:
- Tang Jiang
Department of Laboratory Medicine
The First Affiliated Hospital of Sun Yat-sen University, PR China
Accepted on February 2, 2017
Abstract
Objectives: Essential hypertension is the most common chronic disease which is associated with an array of long-term end-organ diseases. Clinically, it would be helpful to detect high-risk patients early in development process of the disease, in order to avoid or delay the onset of comorbidities. The aim of our study was to evaluate the laboratory parameters which are possibly related to the onset of Ischemic Stroke (IS) in essential hypertension, and identify the strongest predicting laboratory parameters.
Methods: Totally 377 consecutive patients with a primary diagnosis of hypertension were recruited in our study. These patients were categorized according to the presence of IS. Nineteen routine laboratory parameters, such as Alkaline Phosphatase (ALP), Homocysteine (Hcy) and Fibrinogen (Fbg) were evaluated in all subjects.
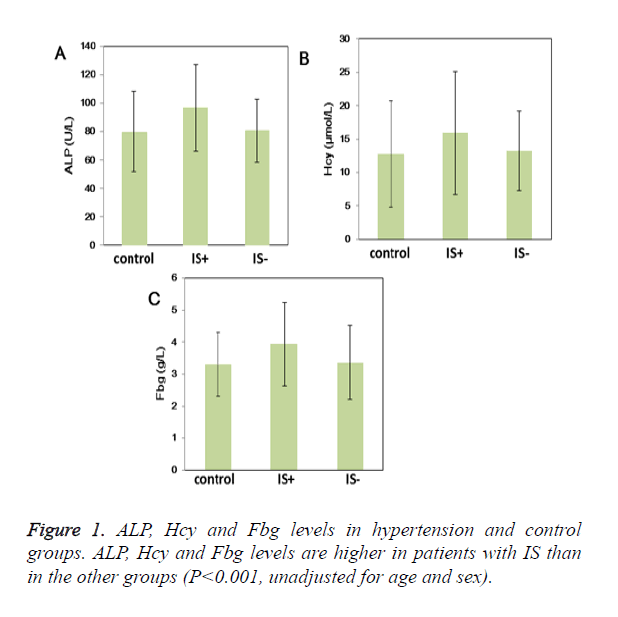
Results: Hypertensive patients have higher serum UA, ACE and HP levels than control patients. More importantly, ALP, Hcy, Fbg, WBC and BUN levels were higher in the group with IS compared with other groups. Three out of 19 laboratory parameters were significantly related with IS in essential hypertension patients on backward elimination including: ALP (1.88; 95% CI: 1.38 to 2.61), Hcy (1.62; 95% CI: 1.17 to 2.25), and Fbg (1.85; 95% CI: 1.35 to 2.52).
Conclusions: According to our results, ALP, Hcy, and Fbg levels were significantly higher in patients with IS, we suggest that the three laboratory parameters were the strongest related to onset of IS in essential hypertension in our patient population.
Keywords
Evaluation, Laboratory parameters, Ischemic stroke, Essential hypertension.
Introduction
At present, cardiovascular diseases are the main cause for disability and mortality worldwide [1]. Hypertension is one of the most prevalent cardiovascular diseases, and it can lead to a variety of target organ damage, such as brain, heart, kidney, retina, and blood vessels. Stroke is regarded as the most devastating neurological disease, and it places a huge burden on healthcare resources because of its high prevalence and recurrence in China [2]. Ischemic Stroke (IS), also called cerebral infarction, accounts for about 80%-85% of stroke cases [3], timely diagnosis is the prerequisite for the IS patients. Recognizing patients with subclinical IS provides the relatively goodtime for preventing progression to obvious complication. Currently, there is no effective method for the identification of every newly diagnosed hypertensive patients with subclinical IS. Moreover, previous studies are generally focused on individual laboratory parameter. As well, it is not known which laboratory parameters are the most strongest associated with hypertension complicated with IS. Accordingly, we aimed to investigate the correlation of routine laboratory parameters in essential hypertension and the relationship with the onset of IS.
Materials and Methods
Patients and methods
The study included 377 consecutive patients who presented to the inpatient department of neurology of the First Affiliated Hospital of Sun Yat-sen University with a diagnosis of hypertension between January 2014 and June 2016. These patients were categorized according to the presence of IS. 114 patients with IS (70 male; mean age, 66.59 ± 11.15 y) and 263 patients without IS (170 male; mean age, 65.00 ± 11.29 y) were enrolled. Hypertensive was defined as systolic blood pressure (BP) more than 140 mm Hg and/or diastolic BP more than 90 mm Hg. Brain MRI with Diffusion-Weighted Imaging (DWI) was performed routinely within 24 h after admission in all subjects, and all patients had no the past history of IS. All patients were diagnosed with IS according to the World Health Organization criteria [4]. 109 healthy people matched for age and gender were assigned to the normal control group (72 male; mean age, 66.13 ± 10.62 y). Those normal cases were from normotensive healthy people who took part in the health examination in our study. Records of potential controls were reviewed by a neurologist to exclude the presence of stroke, and other types of diseases. The systolic and diastolic BPs of the control group were less than 140/90 mm Hg. Exclusion criteria: secondary hypertension, more than 24 h from IS onset to admission, MRI could not be performed, cerebral arteriosclerosis, cerebral haemorrhage, epileptic seizure activity or coma, malignancy, patients with acquired immunodeficiency syndrome, acute or chronic inflammatory disease and any drug use that could potentially influence hematologic disorders, hepatic dysfunction and renal impairment. The Institutional Ethics Committee on Human Research of The First Affiliated Hospital of Sun Yat-sen University approved the study protocol. All subjects received oral and written information concerning the background and procedures of the study, and the subjects or their relatives gave written informed consent prior to participating in the study.
Measurement of BP
In the inpatient department, BP was measured with a mercury sphygmomanometer on the right arm; the first and fifth phases of Korotk off sounds were used for systolic and diastolic BP, respectively. Three BP measurements were taken at 5 minute intervals by a physician with the patient in a seated position after at least a 5 minute rest, using an appropriately sized cuff. The mean of the 3 readings was considered the final BP value.
Laboratory parameters selection and measurement
We investigated 19 laboratory parameters from computerized medical files of the subjects (Table 1), which were selected on the basis of evidence from previous clinical studies. These parameters were measured on baseline blood samples collected in the morning after fasting for at least 8 hours. Participants kept the quiet conditions for 5 to 10 minutes before venipuncture. The parameters of whole blood sample (white blood cell and platelet) were measured immediately with a flow cytometry assay in a calibrated Sysmex XE-5000 analyser (Sysmex Corporation, Hyogo, Japan). Plasma or serum aliquots were collected and centrifuged at 3000 g for 10 min at ambient room temperature and were then frozen at-80˚C until assayed. The concentration of plasma markers (prothrombin time, activated partial thromboplastin time, fibrinogen and ddimer) was measured with a particle-enhanced, immunoturbidimetric assay in a calibrated Sysmex 1500 analyser (Sysmex Corporation, Hyogo, Japan). The concentration of serum markers (glucose, uric acid, fructosamine, free fatty acid, angiotensin converting enzyme, haptoglobin, total cholesterol, low density lipoprotein, blood urea nitrogen, homocysteine, alkaline phosphatase, high sensitive C reactive protein and lipoprotein a) was measured with the continuous monitoring assay in a calibrated Hitachi 7180 automatic analyzer (Hitachi High-Tech Science Systems Corporation, Hitachinakashi, Japan).
| Control | IS (−) | IS (+) | P | |
|---|---|---|---|---|
| GLU | 5.92 ± 2.87 | 6.09 ± 2.38 | 6.12 ± 2.31 | 0.55 |
| UA | 290.82 ± 88.42 | 342.13 ± 103.91* | 354.96 ± 133.04* | <0.05 |
| FRUC | 271.55 ± 113.45 | 287.56 ± 103.04 | 274.64 ± 92.88 | 0.197 |
| FFA | 548.45 ± 252.37 | 584.64 ± 275.15 | 615.94 ± 243.74 | 0.071 |
| ACE | 18.68 ± 7.25 | 22.53 ± 11.94* | 24.66 ± 14.30* | <0.05 |
| HP | 1.32 ± 0.44 | 1.57 ± 0.73* | 1.64 ± 0.72* | <0.05 |
| TC | 4.58 ± 1.25 | 4.76 ± 2.79 | 4.38 ± 1.10 | 0.128 |
| LDL | 2.86 ± 0.95 | 3.04 ± 1.04 | 3.08 ± 1.13 | 0.133 |
| BUN | 5.74 ± 2.75 | 5.57 ± 2.52 | 7.93 ± 5.79*,** | <0.05 |
| Hcy | 12.78 ± 8.00 | 13.24 ± 5.96 | 19.11 ± 9.70*,** | <0.05 |
| ALP | 79.84 ± 28.17 | 80.61 ± 22.02 | 96.77 ± 30.47*,** | <0.05 |
| WBC | 7.65 ± 2.17 | 8.18 ± 2.78 | 9.53 ± 3.55*,** | <0.05 |
| PLT | 240.45 ± 70.93 | 231.85 ± 67.04 | 207.34 ± 69.47*,** | <0.05 |
| PT | 11.91 ± 0.81 | 12.07 ± 1.74 | 12.33 ± 0.81* | <0.05 |
| APTT | 27.23 ± 3.13 | 27.08 ± 4.28 | 27.30 ± 3.90 | 0.621 |
| Fbg | 3.30 ± 0.99 | 3.37 ± 1.17 | 3.94 ± 1.31*,** | <0.05 |
| D-D | 0.31 (0.19-0.99) | 0.55 (0.27-0.99)* | 0.96 (0.39-3.88)*,** | <0.05 |
| HS-CRP | 1.69 (0.61-5.83) | 2.43 (0.969.37)* | 4.55 (2.00-13.36)*,** | <0.05 |
| Lpa | 285 (180-412) | 266 (169-432) | 298 (181-500) | 0.22 |
| Abbreviations: GLU: Glucose; UA: Uric Acid; FRUC: Fructosamine; FFA: Free Fatty Acid; ACE: Angiotensin Converting Enzyme; HP: Haptoglobin; TC: Total Cholesterol; LDL: Low Density Lipoprotein; BUN: Blood Urea Nitrogen; Hcy: Homocysteine; ALP: Alkaline Phosphatase; WBC: White Blood Cell; PLT: Platelet; PT: Prothrombin Time; APTT: Activated Partial Thromboplastin Time; Fbg: Fibrinogen; D-D: D-Dimer; Hs-CRP: High sensitive C Reactive Protein; Lpa: Lipoprotein a. *P<0 .05 vs. control. **P<0.05 vs. IS (-). | ||||
Table 1. Laboratory parameters of IS (+), IS (-), and control groups.
Statistical analysis
Statistical analysis was performed using SPSS software, version 20.0 (IBM SPSS Statistics). Distribution of continuous variables was tested by the Kolmogorov-Smirnov test. Continuous variables were expressed as means ± SD or median and 25th to 75th percentile values as appropriate. Categorical variables were expressed as percentages. Statistical differences among groups were tested by one-way analysis of variance with post hoc Scheffe correction or Kruskal-Wallis test for parametric or nonparametric variables, respectively. We chose a multistage analytical approach to decrease the risk of falsepositive results. Hs-CRP was log transformed, all of the logistic regression models were adjusted for age, sex, systolic and diastolic blood pressure, current smoking. First, we performed multivariable logistic regression analysis to examine the association of the total laboratory parameters with the risk of hypertension with IS. Second, a final informative laboratory parameters were selected by backward elimination, using individual P<0.05 for retention in the model.
Results
The baseline clinical and laboratory characteristics of all groups were presented in Table 1. Age, sex and smoking rate were comparable among the three groups. Presence of hyperlipidemia and Diabetes Mellitus (DM) was comparable in the groups with or without IS. Systolic, diastolic BP and Coronary Artery Disease (CAD) were higher in the hypertension groups compared with the control group (P<0.001). Furthermore, systolic BP (160.13 ± 15.27 mmHg vs. 155.43 ± 20.10 mmHg vs. 117.16 ± 9.87 mmHg, respectively; P<0.05), diastolic BP (96.40 ± 12.40 mmHg vs. 89.84 ± 14.21 mmHg vs. 75.37 ± 11.13 mmHg, respectively; P<0.001) and CAD (24.6% vs. 14.1% vs. 0%, respectively; P<0.001) were prominently higher in the patients with IS.
UA, ACE and HP levels were higher in hypertensive patients than control patients (P<0.05) (Table 2). More importantly, ALP (96.77 ± 30.47U/L vs. 80.61 ± 22.02 U/L vs. 79.84 ± 28.17 U/L; P<0.05), Hcy (19.11 ± 9.70μmol/L vs. 13.24 ± 5.96μmol/L vs. 12.78 ± 8.00 μmol/L; P<0.05) and Fbg levels (3.94 ± 1.31 g/L vs. 3.37 ± 1.17 g/L vs. 3.30 ± 0.99 g/L; P<0.05) were higher in the group with IS than other groups (Table 2 and Figure 1); Moreover, WBC and BUN levels were also higher in the group with IS (Table 2). ButPLT levels were lower in the group with IS than other groups (P<0.05) (Table 2). D-D and Hs-CRP level were higher in the hypertension groups compared with the control group (P<0.05). Furthermore, D-D and Hs-CRP level were prominently higher in the patients with IS (P<0.05). Age- and sex-adjusted Spearman correlations among the laboratory parameters are shown in Table 3. The highest correlations were noted for Hs- CRP and Fbg (r=0.53, P<0.05), HP and Fbg (r=0.49, P<0.05), BUN and Fbg (r=0.38, P<0.05), WBC and Hs-CRP (r=0.35, P<0.05), and Hcy and BUN (r=0.34, P<0.05).
| Control | IS (-) | IS (+) | P | |
|---|---|---|---|---|
| Number of patients, n | 109 | 263 | 114 | |
| Age, y | 66.13 ± 10.62 | 65.00 ± 11.29 | 66.59 ± 11.15 | 0.221 |
| Male/female, n/n | 72/37 | 170/93 | 70/44 | 0.807 |
| SBP, (mm Hg) | 117.16 ± 9.87 | 155.43 ± 20.10* | 160.13 ± 15.27*,** | <0.05 |
| DBP, (mm Hg) | 75.37 ± 11.13 | 89.84 ± 14.21* | 96.40 ± 12.40*,** | <0.05 |
| Current smoker, n (%) | 43 (39.4%) | 96 (36.5%) | 50 (43.9%) | 0.206 |
| Hyperlipidemia, n (%) | 0 (0%) | 45 (17.1%)* | 26 (22.8%)* | <0.05 |
| DM, n (%) | 0 (0%) | 98 (37.3%)* | 47 (41.2%)* | <0.05 |
| CAD, n (%) | 0 (0%) | 37 (14.1%)* | 28 (24.6%)*,** | <0.05 |
| Abbreviations: DM: Diabetes Mellitus; CAD: Coronary Artery Disease. P values represent the comparisons among 3 groups.*P<0.05 vs. control. **P<0.05 vs. IS (-). | ||||
Table 2. Demographic, clinical characteristics of IS (+), IS (-), and control groups.
| Biomarkers | Hcy | UA | Hs-CRP | ACE | HP | BUN | ALP | WBC | PLT | Fbg | D-D |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hcy | 1 | ||||||||||
| UA | 0.14 | 1 | |||||||||
| p<0.05 | |||||||||||
| Hs-CRP | 0.07 | 0.04 | |||||||||
| P=0.13 | P=0.27 | ||||||||||
| ACE | 0.11 | 0.07 | -0.01 | 1 | |||||||
| P<0.05 | P=0.15 | P=0.43 | |||||||||
| HP | -0.03 | -0.01 | 0.34 | -0.01 | 1 | ||||||
| P=0.33 | P=0.44 | P<0.05 | P=0.42 | ||||||||
| BUN | 0.34 | 0.09 | 0.34 | 0.09 | 0.06 | 1 | |||||
| P<0.05 | P=0.08 | P<0.05 | P=0.09 | P=0.16 | |||||||
| ALP | 0.04 | 0.06 | 0.14 | 0.06 | 0.07 | 0.16 | 1 | ||||
| P=0.26 | P=0.17 | P<0.05 | P=0.17 | P=0.15 | P<0.05 | ||||||
| WBC | 0.07 | 0.11 | 0.35 | 0.11 | 0.18 | 0.25 | 0.17 | 1 | |||
| P=0.14 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | |||||
| PLT | -0.11 | -0.06 | 0.02 | -0.05 | 0.19 | -0.07 | 0.08 | 0.1 | 1 | ||
| P<0.05 | P=0.18 | P=0.39 | P=0.22 | P<0.05 | P=0.14 | P=0.12 | P=0.06 | ||||
| Fbg | 0.11 | 0.05 | 0.53 | 0 | 0.49 | 0.38 | 0.21 | 0.28 | 0.16 | 1 | |
| P<0.05 | P=0.23 | P<0.05 | P=0.48 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | |||
| D-D | 0.12 | -0.1 | 0.28 | 0.05 | 0.16 | 0.28 | 0.21 | 0.2 | 0.01 | 0.23 | 1 |
| P<0.05 | P=0.06 | P<0.05 | P=0.24 | P<0.05 | P<0.05 | P<0.05 | P<0.05 | P=0.43 | P<0.05 | ||
| Values are age- and sex-adjusted Spearman correlation coefficients (n=486). | |||||||||||
Table 3. Age- and sex-adjusted correlations among laboratory parameters.
Results of backward elimination models for selecting an intensive set of laboratory parameters (of 19 eligible ones) related to hypertension complicated with IS are shown in Table 4. Three laboratory parameters were retained in the final multivariable model: ALP (1.88; P<0.001), Hcy (1.62; P=0.004), and Fbg (1.85; P<0.001). The magnitude of the association of each laboratory parameter with hypertension complicated with IS was similar, and the three parameters were most strongly related with occurrence of IS in essential hypertension.
| Description of model | Adjusted odds ratio* | Adjusted 95% CI | χ2 statistic | P |
|---|---|---|---|---|
| Global test of all laboratory parameters | 87.68 | <0.001 | ||
| Final model | ||||
| ALP, per SD increment | 1.88 | (1.38 to 2.61) | 14.32 | <0.001 |
| Hcy, per SD increment | 1.62 | (1.17 to 2.25) | 8.27 | 0.004 |
| Fbg, per SD increment | 1.85 | (1.35 to 2.52) | 14.98 | <0.001 |
| *Odds of hypertension with IS associated with a 1-SD increment in the natural logarithm of the biomarker. Results are shown only for variables retained by backward elimination, using P<0.05 as the threshold for retention. Based on likelihood ratio test Odds ratios were adjusted for age, sex, systolic and diastolic blood pressure, cigarette smoking. | ||||
Table 4. Laboratory parameters levels and Hypertension with IS.
Discussion
In the present study, we examined 19 laboratory parameters which represent key biological characteristics implicated in the pathogenesis of hypertension. In the end, we identified 3 laboratory parameters, which were most strongly related with occurrence of hypertension complicated with IS: ALP (vascular calcification), Hcy (oxidative stress), and Fbg (inflammation and thrombosis).
Previous study reported that high levels of ALP were significantly associated with hypertension [5]. Furthermore, some studies showed significant correlation between ALP level and incidence of stroke and mortality [6,7]. However, no study of the relationships between ALP and hypertension complicated with IS has been published in Chinese people. Our findings prove that serum ALP is associated with hypertension complicated with IS. The mechanisms for the positive association of high levels of ALP with occurrence of hypertension complicated with IS may have several aspects. ALP catalyses the hydrolysis of inorganic pyrophosphate [8] which can inhibit vascular calcification [9]. Shioi et al. reported that bone-type ALP expressed in human vascular smooth muscle cells [10], who considered a higher level of bone-type ALP, may accelerate the development of cardio-cerebrovascular events through cardio-cerebrovascular calcification. Another possible mechanism for a higher levels ALP can improve risk of hypertension complicated with IS is impaired vascular homeostasis, because hematopoietic stem cells derived from bone marrow play an important role in vascular homeostasis [11-13]. Some researches thought the activity of osteoblasts can be evaluated by bone-type ALP expression [14], which regulate the production of hematopoietic stem cells in bone marrow [15,16], serum ALP levels may associated with vascular homeostatic activity. Sata et al. reported hematopoietic stem cells participate in the pathogenesis of atherosclerosis [17]. Another study showed that elevated ALP levels were associated with atherosclerosis, and ALP was in dependent of other traditional cardio-cerebrovascular risk factors [18]. Elevated ALP levels may form a risk for hypertension complicated with IS because of progressive atherosclerosis.
Hcy is a non-protein amino acid and is synthesized from methionine, and Hcy can be recycled into methionine. Previous studies found raised Hcy increase oxidative stress, causes oxidative injury of the vascular endothelium, stimulates the proliferation of vascular smooth muscle cells, diminishes vasodilation, and changes the elastic properties of the vascular wall [19-21]. All these are correlation with the occurrence of hypertension complicated with IS. Inflammatory disease such as atherosclerosis plays a crucial role in hypertensive complicated with IS. Previous studies have reported that Hcy may promote synthesis of proinflammatory cytokines.
Furthermore, elevated Hcy conduce to the modification of LDL and HDL particles, and disorders in fibrinolysis and coagulation, these changes enhance the development of hypertension and IS in patients [20,22-24].
Fbg is a coagulation factor, and it belongs also to acute phase proteins. Previous studies [25-27] have shown that plasma Fbg is marker of stroke and atherosclerosis. Further, Fbg was an independent predictor of ischemic stroke. In the present study, we found that Fbg level was significantly higher in patients of hypertension complicated with IS. Moreover, Fbg levels were positively associated with plasma Hs-CRP levels (Table 3). This result may be associated with increased inflammation in hypertension complicated with IS. Several plausible mechanisms could explain the relationship between elevated Fbg levels and hypertension complicated with IS. First, Fbg is markers of inflammation [28,29]. Second, Fbg can increase peripheral vascular resistance and blood viscosity [30,31]. Finally, elevated Fbg level is related with hyperinsulinemia and insulin resistance [32,33].
Similarly, we found that ALP, Hcy, and Fbg levels were higher in the hypertension complicated with IS than the simple hypertensive patients, therefore, we considered that the above three markers were the strongest related laboratory parameters to onset of IS in essential hypertension.
Study Limitations
The study had several limitations. First, it was a retrospective study. It remains unclear if increased ALP, Hcy and Fbg are a cause or a result of essential hypertension complicated with IS. Second, we could not evaluate which type of ALP was implicated with the risk of hypertension complicated with IS. But a significantly positive correlation was observed between ALP levels and occurrence of hypertension complicated with IS. Finally, all hypertension complicated with IS patients were recruited to the study were from 2.5 to 24 h after stroke onset, and the problem of ALP, Hcy, and Fbg levels change by the number of days from the IS development need further research.
Conclusion
In conclusion, ALP, Hcy, and Fbg levels were significantly higher in patients with IS, which were the strongest related laboratory parameters to onset of IS. According to our results, we propose that elevated ALP, Hcy, and Fbg levels might be implicated in the pathogenesis of IS in essential hypertension in our patient population.
Acknowledgments
We are grateful to the department of neurology; the physicians, nurses, and patients who participated in our study.
Conflict of Interests
The authors have declared that no competing interests exist.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
- Kelly BB, Narula J, Fuster V. Recognizing global burden of cardiovascular disease and related chronic diseases. Mount Sinai J Med New York 2012; 79: 632-640.
- Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke J Cerebr Circ 2011; 42: 3651-3654.
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010; 121: e46-e215.
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976; 54: 541-553.
- Schutte R, Huisman HW, Malan L, van Rooyen JM, Smith W. Alkaline phosphatase and arterial structure and function in hypertensive African men: the SABPA study. Int J Cardiol 2013; 167: 1995-2001.
- Shimizu Y, Imano H, Ohira T, Kitamura A. Alkaline phosphatase and risk of stroke among Japanese: the Circulatory Risk in Communities Study (CIRCS). J Stroke Cerebrovascular Dis Off J Nat Stroke Assoc 2013; 22: 1046-1055.
- Tonelli M, Curhan G, Pfeffer M, Sacks F. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 2009; 120: 1784-1792.
- Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 2008; 73: 989-991.
- Harmey D, Hessle L, Narisawa S, Johnson KA. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol 2004; 164: 1199-1209.
- Shioi A, Katagi M, Okuno Y, Mori K. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circulation Res 2002; 91: 9-16.
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell 2000; 102: 199-209.
- Yamada Y, Takakura N. Physiological pathway of differentiation of hematopoietic stem cell population into mural cells. J Exp Med 2006; 203: 1055-1065.
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998; 92: 362-367.
- Lumachi F, Ermani M, Camozzi V, Tombolan V. Changes of bone formation markers osteocalcin and bone-specific alkaline phosphatase in postmenopausal women with osteoporosis. Ann New York Acad Sci 2009; 1173: 60-63.
- Nakamura A, Osonoi T, Terauchi Y. Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig 2010; 1: 208-211.
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425: 841-846.
- Hager S, Lampert FM, Orimo H, Stark GB. Up-regulation of alkaline phosphatase expression in human primary osteoblasts by cocultivation with primary endothelial cells is mediated by p38 mitogen-activated protein kinase-dependent mRNA stabilization. Tissue Eng Part A 2009; 15: 3437-3447.
- Sata M, Saiura A, Kunisato A, Tojo A, Okada S. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med 2002; 8: 403-409.
- Cheung BM, Ong KL, Wong LY. Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999-2004. Int J Cardiol 2009; 135: 156-161.
- van Guldener C, Nanayakkara PW, Stehouwer CD. Homocysteine and blood pressure. Curr Hypertens Rep 2003; 5: 26-31.
- Baszczuk A, Kopczylski Z, Thielemann A. Endothelial dysfunction in patients with primary hypertension and hyperhomocysteinemia. Postepy Hig Med Dosw (Online) 2014; 68: 91-100.
- Wang H, Liu J, Wang Q, Zhao H, Shi H. Descriptive study of possible link between cardioankle vascular index and homocysteine in vascular-related diseases. BMJ Open 2013; 3.
- Wang G, Siow YL, O K. Homocysteine stimulates nuclear factor kappaB activity and monocyte chemoattractant protein-1 expression in vascular smooth-muscle cells: a possible role for protein kinase C. Biochem J 2000; 352: 817-826.
- Poddar R, Sivasubramanian N, DiBello PM, Robinson K. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 2001; 103: 2717-2723.
- Wang G, Woo CW, Sung FL, Siow YL. Increased monocyte adhesion to aortic endothelium in rats with hyperhomocysteinemia: role of chemokine and adhesion molecules. Arterioscl Thromb Vasc Biol 2002; 22: 1777-1783.
- Eidelman RS, Hennekens CH. Fibrinogen: a predictor of stroke and marker of atherosclerosis. Eur Heart J 2003; 24: 499-500.
- Siegerink B, Rosendaal FR, Algra A. Genetic variation in fibrinogen; its relationship to fibrinogen levels and the risk of myocardial infarction and ischemic stroke. J Thromb Haemostasis 2009; 7: 385-390.
- Chuang SY, Bai CH, Chen WH, Lien LM. Fibrinogen independently predicts the development of ischemic stroke in a Taiwanese population: CVDFACTS study. Stroke J Cereb Circulation 2009; 40: 1578-1584.
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012; 34: 43-62.
- Jae SY, Kurl S, Laukkanen JA, Lee CD. Relation of C-reactive protein, fibrinogen, and cardiorespiratory fitness to risk of systemic hypertension in men. Am J Cardiol 2015; 115: 1714-1719.
- Fowkes FG, Lowe GD, Rumley A, Lennie SE. The relationship between blood viscosity and blood pressure in a random sample of the population aged 55 to 74 years. Eur Heart J 1993; 14: 597-601.
- Zannad F, Stoltz JF. Blood rheology in arterial hypertension. J Hypertens Suppl Off J Int Soc Hypertens 1992; 10: 69-78.
- Valek J, Valkova L, Vlasakova Z, Topinka V. Increased fibrinogen levels in the offspring of hypertensive men. Relation with hyperinsulinemia and the metabolic syndrome. Arterioscl Thromb Vasc Biol 1995; 15: 2229-2233.
- Sakkinen PA, Wahl P, Cushman M, Lewis MR. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol 2000; 152: 897-907.