- Biomedical Research (2010) Volume 21, Issue 1
Evaluation of clinical outcome of cancer related thrombophilia: A Review
Mohd. Yusuf1, Ashish Gupta1*, Ashutosh Kumar1, Sheeba Afreen1, Khusboo Ambreen2, Bharti Mishra21Department of Pathology C S M Medical University, Uttar Pradesh, Lucknow, India
2Department of Microbiology, C S M Medical University, Uttar Pradesh, Lucknow, India
Accepted Date: August 10 2009
Abstract
Acquired thrombophilic state associated with a significant risk of thrombosis is frequently encountered in malignancy. Venous and arterial thromboembolism is a common complica-tion and patients with cancer, also present with a hypercoagulable state, even in the absence of thrombosis. Furthermore, clotting activation may play a role in tumor progression. The pathogenesis of thrombosis in cancer is multifactorial; however, a relevant role is attributed to the tumor cell capacity to interact with and activate the host haemostatic system. Among other factors, the prothrombotic action of antitumor therapies is also important. New ap-proaches of the prevention and cure of thrombosis in cancer and the hypothesis that the strategies to inhibit clotting mechanism may favorably affect malignant disease are gaining increasing interest.
Keywords
Cancer, Thrombophilia, Pathogenesis, Clinical
Introduction
Patients with cancer are exposed to a significant risk of thrombosis. The interrelationship between hemostasis and cancer although widely accepted but remains poorly understood. However this interrelationship goes beyond the thrombosis. Hemostasis is deeply involved in tumor growth, angiogenesis and metastasis and modulation of these pathways may yield interesting and promising future treatment options. It has been estimated that approximately 15% of all cancer patients develops thrombosis during the course of their disease [1,2]. Thrombotic disorders in cancer patients include venous and arterial thrombosis, migratory thrombophlebitis, thrombotic non-bacterial endocarditis, and systemic syndromes such as thrombotic microangiopathy and disseminated intravascular coagulation (DIC). Severe DIC is generally associated with acute promyelocytic leukemia (AML - M3) and is characterized by life-threatening hemorrhages and concomitant thrombotic complications [3]. Most of the patients with solid tumors and leukemia’s commonly suffer from chronic compensated DIC and have abnormalities in laboratory coagulation tests without manifest thrombosis. This subclinical hypercoagulable condition is characterized by varying degrees of blood clotting activation [4,5]. The results of laboratory tests in these patients demonstrate that a process of fibrin formation and lysis is continuously ongoing during the course of malignancy. The aim of this review was to assess the recent advances in the understanding of the relationship between thrombophilia and cancer particularly the pathophysiology mechanisms of blood clotting activation in malignancy and the current concepts in the prevention and treatment of the thrombosis in cancer.
Cancer associated hypercoagulability
Different tumors of different extent with a different co- morbidities and different inherited hypercoagulable states may promote the development of thrombosis by different combinations of tumor and non - tumor associated procoagulant mechanisms. The interactions between components of the hemostatic system and cancer cells are multifaceted. Strong clinical evidence is accumulating on the prothrombotic tendency of cancer patients, which is enhanced by anticancer therapy, such as surgery and chemotherapy. The mechanisms of thrombus promotion in malignancy include some general responses of the host to the tumor (acute phase, inflammation, angiogenesis) and specific interactions of tumor cells with the clotting/ fibrinolysis systems and with blood (leukocytes, platelets) or vascular cells. Malignancy is an acquired thrombophilic condition associated with a significant risk of thrombosis. Venous and arterial thromboembolism is a common complication for patients with cancer, who also present with a hypercoagulable state, even in the absence of manifest thrombosis. Furthermore, clotting activation may play a role in tumor progression. The pathogenesis of thrombosis in cancer is multifactorial; however, a relevant role is attributed to the tumor cell capacity to interact with and activate the host hemostatic system.
Solid tumor mediated extrinsic vascular compression and invasion can obstruct venous return, resulting in blood flow stasis and promote endothelial cell injury, resulting in coagulation activation [6,7]. Tumor cells promote thrombin generation directly by producing tissue factor; expressing the coagulation factor X activator known as cancer procoagulant; displaying surface sialic acid residues that can support nonenzymatic factor X activation and indirectly by eliciting tissue factor expression from monoctyes and endothelial cells [8,9]. It is widely recognized that the majority of cancer patients present with one or more abnormalities of laboratory coagulation tests. The most frequent abnormalities include increased levels of fibrinogen, factors V, VIII, IX, X, fibrin degradation products (FDPs) and thrombocytosis [4]. Studies of the plasma levels of these parameters have provided a biochemical definition of the hypercoagulable state in humans. However, little or no information is available on the clinical utility of any of them in the single patient. No large prospective studies have been conducted to assess the predictive value of these laboratory tests for thrombosis in cancer patients. In a small series of cancer patients enrolled in a trial of preoperative heparin prophylaxis versus placebo, the study of biological markers such as thrombin- antithrombin III (TAT) complex and prothrombin fragment 1+2 (F 1+2) showed that preoperative thrombin - antithrombin complex (TAT) values > 3.5 ng/ ml were significantly predictive for post – operative deep vein thrombosis (DVT), with a relative risk of 7.5 fold (p<0.05) [10].
Pathogenesis of increased risk of thrombosis in cancer
Activation of blood coagulation and thrombotic diathesis is a complex phenomenon and that it involves different haemostatic pathways in patients with cancer and many factors which contribute includes non - specific factors, tumor-specific factors, and anticancer therapy-related factors [4]. Non - specific causes such as immobilization causing stasis; effects of damaged or necrotic normal or tumor tissues, associated inflammation or infection, entry of mucin coagulation fibrinolysis fibrin (ogen) degradation products into the circulation .and foreign body effects of venous access devices all aids in thrombus formation.
a) Tumor cell Prothrombotic Mechanisms
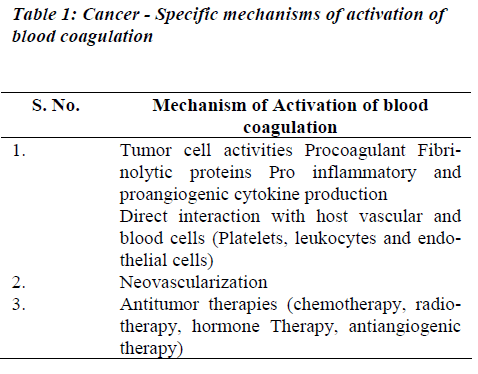
Tumor cells may activate coagulation reactions mainly by the modalities as listed in Table 1.
(i) Procoagulant Activities
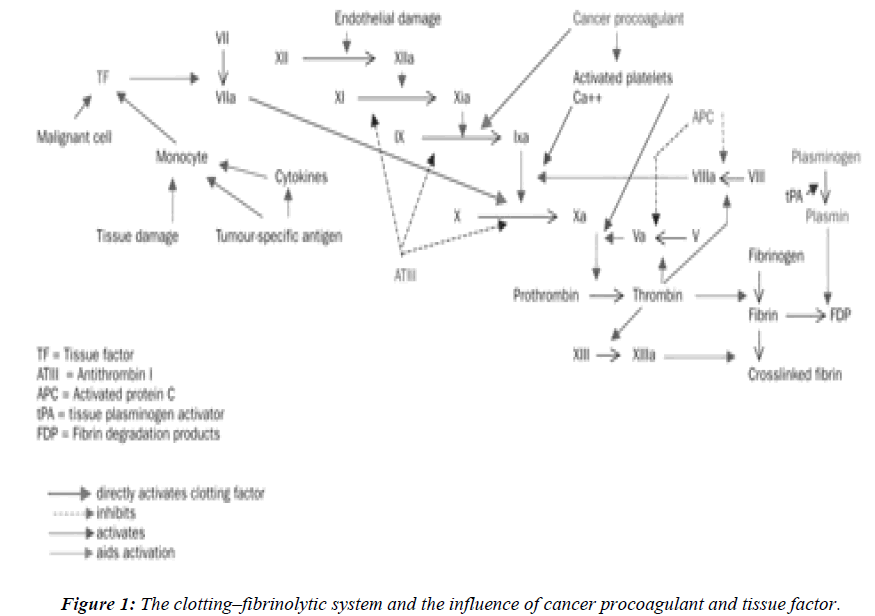
The normal clotting–fibrinolytic system involves a fine balance between activation and inhibition of platelets, procoagulant factors, anticoagulant factors, and fibrinolytic factors (Figure 1). In cancer, tumor cells can activate the coagulation system directly, through interactions with platelets, clotting, and fibrinolytic systems to generate thrombin. The delicate balance between the coagulation and fibrinolytic systems can easily shift to induce a prothrombotic state, perhaps via an excess of procoagulant proteins such as tissue factor, fibrinogen, and plasminogen activator inhibitor, or deficiencies in other molecules, such as antithrombin III, proteins C and S, and tissue plasminogen activator. Tumor cells may express procoagulant, such as tissue factor (TF) and cancer procoagulant (CP) [9].
Tissue factor and cancer procoagulant
Tissue factor (TF) is a membrane glycoprotein that functions as a receptor for factor VII and as the site of activation of factor VII to VII a. The TF - Factor VII a complex and membrane phospholipids further activate factor X. This is a major cellular activator of the coagulation system in cancer patients where tissue injury or cell necrosis is involved. The expression of TF is up regulated by tumor necrosis factor alpha (TNF α), interleukin 1 β (IL - 1β), and other cytokines [11,12]. The activity of the factor VIIa- TF complex can be inhibited by tissue factor path-way inhibitor (TFPI) in conjunction with factor Xa. The procoagulant activity of TF on the cell surface is largely dormant until alterations of the plasma membrane occur. Membrane alterations during cell injury enhance TF procoagulant activity.
Cancer procoagulant is a cysteine proteinase growth factor, consisting of a calcium-dependent, manganese stimulated enzyme. Unlike tissue factor, cancer procoagulant is a direct activator of factor X, without the need for factor VII, and is found in malignant and foetal tissue, but not in normally differentiated tissue. In the presence of factor V, cancer procoagulant may increase thrombin generation by up to three times the amount found in normal tissue.Increased cancer procoagulant concentrations have been reported in patients with acute promyelocytic leu-kaemia, malignant melanoma, and cancers of the colon, breast, lung, and kidney. Antibodies to cancer procoagulant also block the metastatic seeding of lung-cancer colonies in vivo and diminish the viability of tumor cells in vitro [12]. It is expressed in embryonic (amniotic and chorionic cells) tissues and in many malignant cells and tissues including acute promyelocytic leukemia (APML) blasts. As it is not found in normal, differentiated cells, its presence has been used to differentiate normal from malignant tissues. It is used as a tumor marker using enzyme – linked immunosorbent assay (ELISA) [13,14]. CP antigens have been identified in the sera of cancer patients and found to be elevated upto 85% of the study subjects.11 TF and CP have been identified in several human and animal tumor tissues. In recent years, many studies have focused on the procoagulant activities expressed by leukemic cells [3,15]. The rapid resolution of the severe coagulopathy observed in patients with APML receiving all-trans-retinoic acid therapy is strictly associated with the loss of procoagulant activity from bone marrow blast cells [3].
(ii) Fibrinolytic system
The fibrinolytic proteins play a major role in tumor invasion, tumor cell proliferation, and metastasis condition. Fibrinolytic activity depends upon the conversion of the proenzyme plasminogen to plasmin, which in turn degrades fibrinogen and fibrin polymers. Plasminogen is essentially activated via the extrinsic pathway by two immunologically and biochemically distinct serine proteases; tissue plasminogen (t- PA) and urokinase-type plasminogen activator (u-PA). These activators are produced by several tissues. Most of them are based on investigation of their secretion and regulation by endothelial cells. PA released from the endothelium is t-PA, which seems to have a key role in regulating and initiating thrombolysis. This system is control by specific protease inhibitor at different levels of fibrinolytic cascade. Plasmin is initiated by α2-antiplasmin. The activity of PAs is regulated by specific plasminogen activator inhibitors. The presence of inhibitors of fibrinolysis was observed in cancer patients long before the plasminogen activator inhibitor type I (PAI- 1) was identified. Tumor cells can express all proteins of the fibrinolytic system, including the urokinase-type (u-PA) and tissue type (t- PA) plasminogen activators, and their inhibitors PAI-1 and PAI-2 [16].
(iii) Cytokines
Tumor cells synthesize and release inflammatory cytokines, including TNF- and IL-1 which can induce the expression of TF procoagulant activity by endothelial cells and monocytes 17 .These cytokines also down regulate the expression of endothelial cell thrombomodulin (TM), a potent anticoagulant factor. TF upregulation and TM down regulation lead to a prothrombotic condition of the vascular wall. The same cytokines stimulate endothelial cells to produce the fibrinolysis inhibitor PAI-1, which depresses fibrinolysis and further favors a prothrombotic phenomenon 18. In addition, the production of vascular endothelial growth factor (VEGF) by malignant cells may significantly affect the functions of micro vessels in proximity of the tumor and play an important role in tumor neoangiogenesis. Furthermore, VEGF is chemotactic for macrophages and can induce TF procoagulant activity by monocytes and endothelial cells. Interestingly, the expression of TF by tumor cells upregulates the transcription of VEGF in the same cells. Regulation of VEGF synthesis by TF in malignant cells and vascular cells provides an important link between thrombosis, inflammation, and tumor growth and metastasis.
(b) Direct interaction of tumor cells with host cells
The presence of cell adhesion molecules on the surface of tumor cells provides the possibility for direct interactation with host cells. During the hematogenous spread, this interaction occurs with endothelial cells, platelets, and leu-kocytes. The tumor cell capacity to adhere to the endothelium and the underlying matrix is well described and adhesion molecule pathways specific to different tumor cell types have been identified [19,20] Endothelial cells activated by IL-1β or TNF- α increase the exposure on their membranes of counter-receptors for the tumor adhesion molecules. The malignant cells attached to the vessel wall may play a key role in promoting localized clotting activation and thrombus formation by releasing their cytokine content and favoring the adhesion and arrest of other cells, including leukocytes and platelets. The adhesion of tumor cells to leukocytes or to vascular walls may also facilitate cell migration and extravasations.
c) Prothrombotic mechanisms of antitumor drugs
Increasing evidence has accumulated that anticancer therapy may significantly affect the thrombotic risk in cancer. Several mechanisms related to anticancer drugs have been identified. One mechanism relies on the release of procoagulants and cytokines by tumor cells damaged by chemotherapy. The role of these mediators to increase the thrombotic risk in cancer patients has been documented [21]. Another mechanism involves the direct damage exerted by chemotherapy and radiotherapy on vascular endothelium. In animal studies, bleomycin has been demonstrated to cause morphological damage to the vascular endothelium of the lung, resulting in pulmonary thrombosis and fibrosis. A new class of substances with endothelial toxic activity is represented by the antiangiogenic drugs, such as thalidomide and SU5416, an anti-VEGF receptor. In patients with multiple myeloma, an increased rate of venous thromboembolism (VTE) is associated with thalidomide therapy, especially in combination with chemotherapy [22].
Clinical Conditions
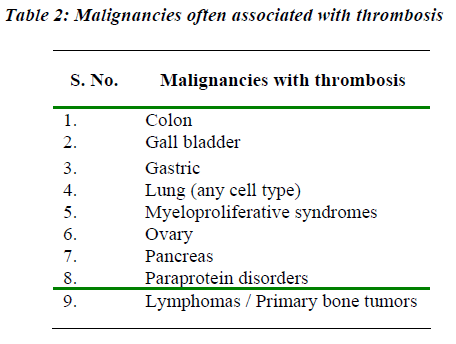
A wide spectrum of clinical manifestations of thrombosis may be observed in cancer, ranging from asymptomatic with laboratory coagulation abnormalities alone, to massive life-threatening venous thrombosis. Patients of cancer presenting with acute VTE detection have a greater likelihood of distant metastases at the time of diagnosis as compared to individuals without concomitant VTE [23]. VTE presentations in the cancer patients include symptomatic DVT, pulmonary embolism, superficial thrombophlebitis, central venous access device thrombosis, arterial thrombosis and non bacterial thrombotic endocarditis. In cancer, syndromes of a systemic involvement of the clotting system, such as DIC or thrombotic microangiopathy have also been described. Some malignancies are associated with a higher incidence of thrombosis than others (Table 2). Although the incidence of thrombosis in malignancy is about 15%, in certain tumors such as pancreatic carcinoma, it may be seen in more than 50% of patients [24]. Mucinous adenocarcinomas have been the most commonly associated with thrombus formation [24]. Evidences have shown that the sialic acid moiety of mucin secreted by these malignancies can initiate coagulation by the nonenzymatic activation of factor X to Xa [25]. Importantly, thrombosis can represent the earliest clinical manifestation of an occult cancer, as shown by a prospective clinical trial [26]. Patients with idiopathic VTE have a 4 to 7-fold increased risk of being diagnosed with cancer in the first year after thrombosis, as compared with patients of VTE. This risk is 10-fold greater in case of idiopathic recurrent VTE. In a report from the Danish Epidemiology Science Center examined registry data from 1977 through 1992 of 15,348 patients with DVT and 11,305 patients with pulmonary embolism. The risk of underlying cancer was approximately 3 times the expected for each cohort examined. This increased risk existed for the first six months which declined to 2.2 fold at one year, and thereafter only marginal at 1.1fold [27].. Edwards et al reported that an extensive screening of patients with DVT with no apparent risk factors is ineffective in identifying an occult malignancy compared with routine clinical practice [28].
VTE: Management strategies in cancer patients
DVT and pulmonary thromboembolism (PTE) warrant prompt institution of antithrombotic therapy in order to effectively ameliorate symptoms; prevent thrombus propagation; embolization; recurrence and allow for thrombus organization, plasmin mediated lysis and restoration of venous patency [29]. Specific therapy and duration of therapy will depend on thrombus location (i.e., ileofemoral DVT versus calf DVT), thrombus extent (i.e., massive PE versus sub segmental PE), and underlying thrombosis “trigger” (i.e., major abdominal surgery versus congenital antithrombin deficiency). The natural history of VTE in the cancer patient differs remarkably from that in the non - cancer patient. Cancer patients are more likely to present with proximal DVT, with a greater initial thrombus burden, experience greater clinical deterioration despite anticoagulant therapy and to have less veno-graphic improvement in response to standard treatment 30. In addition, the risk of recurrences is significantly increased in cancer versus non cancer patients, even during treatment for VTE [31]. The fact that cancer patients have a higher recurrence rate while are reportedly “therapeuti-cally” anti-coagulated suggests that the usual target international normalized ratio (INR) range of 2.0 to 3.0 may not be therapeutic at all. The greatest risk of recurrent VTE was observed in patient with genito - urinary tract, gastrointestinal tract and lung cancers and predominantly during the first month of anticoagulation [32].
Heparin Resistance
Cancer patients, like others with an acute illness and inflammatory processes, have a propensity towards heparin resistance. Requirement of very high dose of heparin (>35,000 units in 24 hours) may reflect this form of heparin resistance. True heparin resistance most likely results from the non specific binding of heparin to mononuclear white cells, vascular endothelial cells and acute phase proteins such as histidine rich glycoprotein, vitronectin, and platelet factor 4, resulting in an inadequate quantity of free or antithrombin bound heparin [32]. Another potential cause of heparin resistance in the cancer patients is compensated DIC - associated antithrombin deficiency. True heparin resistance, can be detected by activated partial thromboplastin test (aPTT) and the anti – factor Xa activity assay.
Cancer patients can also manifest an “apparent” heparin resistance characterized by dissociation between the aPTT and heparin assay [31]. In these patients, the aPTT may be normal or near normal while the anti- factor Xa activity assay reveals a therapeutic heparin activity level between 0.3 and 0.7 IU / ml. Simply escalating the dose of heparin to achieve the desired aPTT without checking a heparin assay may result in a pronounced bleeding risk. Dissociation between the aPTT and heparin concentration likely reflects elevated levels of factor VIII that can shorten the in vitro aPTT without affecting the anti thrombotic actions of the drug.
Warfarin Failure
Warfarin failure is suspected if a patient develops an objectively documented recurrent VTE, despite an apparently stable INR between 2.0 -3.0 and suggests that this degree of anticoagulation was insufficient to neutralize the sum of hypercoagulable stimuli in a given individual. Underlying cancer, because of its potent prothrombotic nature, is often suspected in the setting of warfarin failure [32]. This may reflect cancer associated hypercoagulability in excess of warfarin induced anticoagulation or inability to keep cancer patients within the target therapeutic international normalized ratio.
Conclusion
Cancer patients are at significantly high thrombotic risk. The pathogenesis of thrombosis in cancer is different and reflects the activation of the various components of the hemostatic system, triggered by tumor cell-associated prothrombotic properties. The laboratory coagulation parameters show that patients with cancer very commonly present with a hypercoagulable state, however the clinical utility of laboratory testing for thrombotic markers is not yet established.
Idiopathic VTE can be the earliest manifestation of an occult cancer. The patients with known cancers have an increased risk of secondary VTE. Management of VTE in cancers has shown increased recurrence rates off warfarin, increased VTE recurrence rates on warfarin, increased bleeding rates on warfarin, and greater difficulty maintaining an international normalized ratio.
References
- Rickles FR, Levine MN. Epidemiology of thrombosis in cancer. Acta Haematol 2001; 106: 6-12.
- Donati MB. Cancer and thrombosis: from phlegmasia alba dolens to transgenicmice. Thromb Haemost 1995; 74: 278-281.
- Falanga A, Barbui T. Coagulopathy of acute promyelocytic leukemia. Acta Haematol 2001; 106: 43?51.
- Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patients. Semin Thromb Hemost 1999; 25: 173-182.
- Falanga A, Barbui T, Rickles FR, et al. Guidelines for clotting studies in cancer patients. Thromb Haemost 1993; 70: 540-542.
- Prandoni P, Piccioli A, Girolami A. Cancer and venousthromboembolism: an overwiew. Haematologica 1999; 84: 437-445.
- Ottinger H, Belka C, Kozole G, et al. Deep venous thrombosis and pulmonary embolism in high ? grade non Hodgkin?s lymphoma: incidence, causes and prognostic relevance. Eur J Haematol 1995; 5 4: 186-194.
- Falanga A, Donati MB. Pathogenesis of thrombosis in patients with malignancy. Int J Hematol 2001; 73: 137-144.
- Gordon S. Cancer cell procoagulants and their implications. Hematol Oncol Clin North Am 1992; 6: 1359-1374
- Falanga A, Ofosu FA, Cortelazzo S, et al. Preliminary study to identify cancer patients at high risk of venous thrombosis following major surgery. Br J Haematol 1993; 85: 745-750.
- Carmelilet P, Collen D. Tissue factor. Int J Biochem Cell Biol 1998; 30: 661-667.
- Gale AJ, Gordon S. Update on tumor cell procoagu-lantfactors. Acta Haematol 2001; 106: 25-32.
- Goldenberg N, Kahn SR, Solymoss S. Markers of co-agulationand angiogenesis in cancer-associated venous thromboembolism. J Clin Oncol 2003; 21: 4194-4199.
- Beer JH, Haeberli A, Vogt A, et al. Coagulation markers predict survival in cancer patients. Thromb Haemost 2002; 88: 745-749.
- Falanga A, Consonni R, Marchetti M, et al. Cancer procoagulant and tissue factor are differently modulated by all trans- retinoic acid (ATRA) in acute promyelocytic leukemia cells. Blood 1998; 92: 143?151.
- Zacharski LR, Wojtukiewicz MZ, Costantini V, Orn-stein DL, Memoli VA. Pathways of coagulation/ fibrinolysis activation in malignancy. Semin Thromb He-most 1992; 18: 104-106.
- Look MP, Van Putten WL, Duffy MJ, et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J Natl Cancer Inst 2002; 94: 116-128.
- Falanga A, Marchetti M, Vignoli A, et al. Clotting mechanisms and cancer: implications in thrombus formation and tumor progression. Clin Adv Hematol Oncol 2003; 1:673?8.
- Honn KV, Tang DG, Chen YQ. Adhesion molecules and sitespecific metastases. In: Neri Seneri GG, Ab-bate R, Gensini, G, Prisco D, eds. Thrombosis: An Update. Florence: Scientific. Year /Country?
- Marchetti M, Falanga A, Giovanelli S, et al. All-transretinoic acid increases the adhesion to endothelium of the acute promyelocytic leukemia cell line NB4. Br J Haematol 1996; 93: 360-356.
- Lee AY, Levine MN.The thrombophilic state induced by therapeutic agents in the cancer patients. Semin Thromb Hemost 1999; 25: 137-145.
- Barbui T, Falanga A. Thalidomide and thrombosis in multiple myeloma. J Thromb Haemost 2003; 1: 421-422.
- Sorensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deepvenous thrombosis or pulmonary em-bolism. N Engl J Med 1998; 338: 1169-1173.
- Sproul EF. Carcinoma and venous thrombosis: the frequency of association of carcinoma in the body or tail of the pancreas with multiple venous thrombosis. Am J Cancer 1938; 34: 566-last page number?..
- Pineo GF, Regorczi F, Hatton MWC. The activation of coagulation by extracts of mucin: a possible path-way of intravascular coagulation accompanying ade-nocarcinoma. J Lab Clin Med 1973; 82: 255-last page number?.
- Prandoni P, Lensing AWA, Bu¨ller HR, et al. Deep-vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med 1992; 327: 1128?1133.
- Sorensen HT, Mellemkjaer L, Steffensen FH, et al. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med 1998; 338: 1169-last page number.
- Edwards RI, Silver J, Rickles FR. Human tumor procoagulants. Registry of the subcommittee on Haemostasis and Malignancy of the Scientific and Standardization Committee, International Society of Thrombosis and Haemostasis. Thromb Haemost 1993; 69:205-213.
- Deithcher SR, Carman TL Deep venous thrombosis and pulmonary embolism. Curr Treatment Options Cardiovasc Med 2002; 4: 223-238.
- Breddin HK, Hach ? Wunderle V, Nakow R, Kakkar VV. Effects of a low- molecular ? weight heparin on thrombus regression and recurrent thromboembolism in patients with deep ? vein thrombosis. N Engl J Med 2001
- Falanga A. Thrombophilia in cancer. Sem Thromb Hemost 2005; 31: 104-110.
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anti coagulant treatment in patients with cancer and venous thrombosis. Blood 2002; 100: 3484-3488.