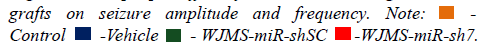Research Article - Journal of Brain and Neurology (2022) Volume 5, Issue 6
Epileptic seizure suppression by xenograft of engineered human Wharton’s jelly mesenchymal stem cells in kindling model.
Hajar Estiri*, Ali Fallah, Bahareh Estiri, Mohammad Estiri, Akbar Farjadfar
Department of Molecular Biology, Iranian Institute of Cell and Gene Therapy, Tehran, Iran
- Corresponding Author:
- Dr. Hajar Estiri
Department of Molecular Biology
Iranian Institute of Cell and Gene Therapy
Tehran
Iran
E-mail: nika@spacemedx.com
Received: 21-Oct-2020, Manuscript No. AAJBN-20-19590; Editor assigned: 25-Oct-2020, PreQC No. AAJBN-20-19590 (PQ); Reviewed: 08-Nov-2020, QC No. Q-AAJBN-20-19590; Revised: 11-Nov-2022, QI No. AAJBN-20-19590 (QI); Manuscript No. AAJBN-20-19590 (R); Published: 15-Nov-2022, DOI:10.35841/ aajbn-5.6.126
Citation: Estiri H, Fallah A, Estiri B, et al. Epileptic seizure suppression by xenograft of engineered human Wharton’s jelly mesenchymal stem cells in kindling model. J Brain Neurol 2022;5(6):126.
Abstract
Epilepsy is a chronic neurological disorder that needs innovative molecular and cellular approaches to address unmet drug resistance epilepsy in 30% of patients. To push preclinical studies forward, we targeted the human Adenosine Kinase gene (ADK), adenosine removing key enzyme, in Human Wharton's Jelly Mesenchymal Stem Cells (hWJMSCs) by a lentiviral anti-ADK miR-shRNA vector. In this study, we enhanced the therapeutic potential of hWJMSCs as adenosine-releasing stem cells by knockdown of ADK, for suppressing seizures in a kindling model of epilepsy among male Wistar rats. After the lentiviral transduction of hWJMSCs with anti-ADK miR-shRNA expression cassette, we implicated the down regulation of ADK up to 95% in RNA and protein level by qRT-PCR and western blot, respectively. Adenosine concentration reached 10 ng per ml of the culture medium when incubating 105 engineered hWJMSCs for 8 hours. Cell transplantation in pentylenetetrazole-induced kindled rats significantly decreased the amplitude, duration, and seizure spike frequency while increased the latency of appearance of the first seizure spike on days 7 and 14 of EEG recording. Behavioral seizure monitoring showed complete protection from convulsive seizures in 100% (n=20) and 83% (n=18) of kindled rats for the first and second weeks after cell graft respectively. An animal showed complete seizure protection (n=16) after 8 weeks. Our findings suggest that adenosine releasing hWJMSC might be a striking source in cell-based gene therapy and may have a therapeutic perspective in epilepsy.
Keywords
Anti-ADK miR-shRNA, ADK knockdown, Adenosine, Epilepsy, Cell-based gene therapy.
Introduction
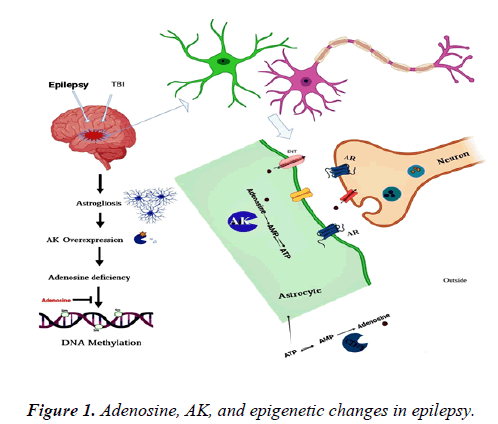
Epilepsy is the third most common serious brain disorder worldwide, which threatens the life of about 1% of different ages, races, gender, and human social class. One-third of epileptic patients are suffering from drug-resistant epilepsy and systemic pharmaceutical side effects. Advanced molecular and cellular strategies are urgent to treat refractory epilepsy. Adenosine is an endogenous metabolite that has neuroprotective and seizure suppression effects [1]. For preventing systemic side effects of adenosine, focal delivery to the brain is needed. Epilepsy as the chronic disease requires lifetime delivery of antiepileptic agents like adenosine. Engineering cells as a releasing biofactory of adenosine is one of the strategies to change the approach in treating epilepsy from controlling the seizures to cure epilepsy. In the present study, the graft of Human Wharton's Jelly Mesenchymal Stem Cells (hWJMSCs) as novel adenosine releasing cells was evaluated in the chronic kindled rat. Adenosine Kinase (ADK) is the key negative regulator of adenosine inside the astrocyte cells. ADK overexpression is a molecular pathologic hallmark in astrogliosis [2]. The up regulation of ADK connects with adenosine failure, seizures, and epigenetic changes. Short hairpin RNA (shRNA) is a promising RNA interference tool in the down regulation of ADK and withdrawing pathological mechanisms involved in epileptogenesis. The knockdown of ADK increases adenosine, terminates seizures, and restores normal epigenetic changes like DNA methylation. In the current study, miRNA-based shRNA was employed for knockdown of ADK in hWJMSCs.
Adult stem cells and induced Pluripotent Stem Cells (iPSC) are an attractive source for ex-vivo gene therapy, cell transplantation, and restoring anticonvulsant molecules like adenosine. Adult mesenchymal stem cells like Bone Marrow (BM) showed some preclinical and clinical. Limitations, for instance, genetic instability, technical issues in BM isolation, and earning adequate numbers of the cells. On the other hand iPSC, with tremendous potential in precise medicine might be hindered by the possibility of tumor formation in some investigations. Epilepsy is the most frequent brain disorder in children. So alternative source for cell transplantation and drug delivery is more urgent. Wharton’s Jelly Mesenchymal Stem Cells (WJMSCs) are a substitute for BM cells [3-7]. hWJMSC has unique features including easy accessibility in limitless numbers without ethical concerns. These cells have a high proliferation rate besides immunomodulatory properties for application in autologous and allogeneic transplantations. This is the first study that shows the genetic engineering potential of hWJMSCs as adenosine releasing cells for preclinical application in a chronic model of epilepsy (Figure 1).
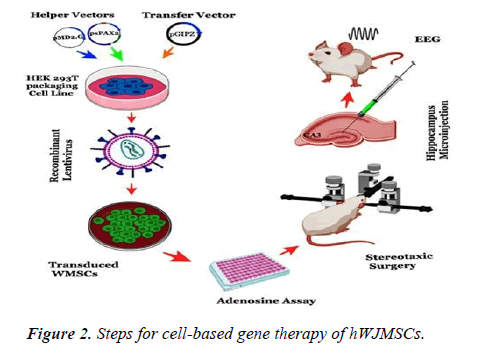
In the present study, we have been using anti-ADK miRshRNA lentiviral systems for ex-vivo gene therapy of hWJMSCs for xenograft transplantation in the PTZ induced model of epilepsy in male rats. In a previous study, we screened eight cassettes of miR-shRNAs that target the human ADK gene to the finding of the most efficient anti-ADK miRshRNA cassette (miR-sh7). hWJMSCs were isolated and cultured after characterizing with flow cytometry for specific mesenchymal markers. The knockdown of the ADK gene in hWJMSCs was confirmed by western blot as well as q-RTPCR after transduction with an anti-ADK miR-sh7 lentiviral vector. Then, Released adenosine from engineered hWJMSCs was measured by the adenosine assay kit. Engineered hWJMSCs were transplanted into the CA3 region of the hippocampi of epileptic rats. The therapeutic application of these cells was investigated by Electroencephalogram (EEG) recording besides following behavioral seizures for 8 weeks (Figure 2).
Materials and Methods
Separation and growth of human Wharton’s jelly mesenchymal stem cells
Human Wharton’s jelly tissues were gained from a newborn umbilical cord under bioethics agreement with the permission of its mother at Taleghani Hospital in Tehran, Iran. Mucoid connective tissue separated from blood vessels (two arteries and one vein of the umbilical cord) then washed twice with PBS contains penicillin/streptomycin and Amphotericin. The cord was. washed with PBS and isolated from the amniotic membrane. Then the jelly part of the cords was cut into fragments and cultured in DME/F12 medium added with 10% FBS and antibiotics. Two weeks later, the tissues were discarded and the isolated growing cells (WJMSCs) were nourished with the same medium. The cells were grown to 60% confluence and passaged by trypsinization. We used the third passage of hWJMSCs for characterization.
Flow cytometry
Cultured hWJMSCs (1 × 105 cells) were washed, immobilized, and incubated for 15 min at 4°C with a 1:9 dilution of normal goat serum in PBS. Then cells were labeled with the following antibodies for 1 H: FITC-conjugated anti-CD44, FITCconjugated anti-CD45. The cells were washed with 2% FBS in PBS and analyzed using a FACSCalibur machine. The control population was stained with matching isotype antibodies (FITC-conjugated and PE-conjugated mouse IgG monoclonal isotype standards) and confirmed by positive fluorescence of the limbal samples. At least 10,000 events were recorded for each sample and data were analyzed using WinMDI software.
Lentiviral constructs for the expression of anti-ADK miR-shRNAs
Different pre-miRNA sequences were homologous to human ADK cDNA and a randomized Scrambled Control (SC) sequence was purchased (GE Healthcare) (supplementary data). All miR- shRNA cassettes were cloned into pGIPZ lentiviral expression vector, which contains a TurboGFP Green Fluorescent Protein (tGFP) as a reporter gene, Internal Ribosome Entry Site (IRES), and a puromycin resistance gene, thus allowing co-expression of the respective miR-shRNA with tGFP and selection of stably transduced cells with puromycin. Expression of tGFP, puromycin, and miR- shRNAs will be under CMV constant promoter [8]. All genes will express as single mRNA. At first, mRNA will be processed in nuclear for producing premature miR-shRNA and bicistronic GFPpuromycin mRNA. Transfer pGIPZ lentiviral expression vector harbored intra LTRs zeocin selection marker for selection of correct intact vector during bacterial proliferation (supplementary data).
Manufacturing of recombinant pseudo lentiviruses
Recombinant lentiviruses were manufactured according to the Prof. Trono lab protocol with some adjustments. Concisely, for miR-shRNA7 and randomized Scrambled Control (SC) sequence, 1×106 HEK 293T cells were incubated in a 10 cm plate in DMEM medium with 10% FBS one day beforehand transfection. The medium was changed 2 hours before transfection with the new medium. The transfection mixture was equipped to contain 21 μg of pGIPZ-miR-sh/SC, 21 μg of psPAX2, 10.5 μg of pMD2, 33 μl of TE 1X, 105 μl of 2.5 M CaCl2, and 1064 μl of 2X HEPES-Buffered Saline (HeBS) rest to 2100 μl with buffered water. All were mixed, and then HeBS 2X added during the solution was vortexing robustly. The transfection mix for any 10 cm2 plate was 2100 μl. The HEK 293T cells with transfection solutions were retained in an incubator for 14 hours. The transfection medium was replaced with fresh medium 14 hours after transfection and cells evaluated for GFP expression with a fluorescent microscope. GFP expression showed transfection efficiency [9]. Five fields under the fluorescent microscope were selected haphazardly and the transfection rate was offered as a percentage of GFPpositive cell number to total cell number. The supernatant of cells containing recombinant viruses was collected three times (24, 48, and 72 hours after transfection) after 14 h posttransfection. These collected supernatants were centrifuged at 1500 rpm and filtered through a 0.25 μm filter before concentration and titration. For concentration, we used PEG 6000 and 9000 g centrifuge. The lentiviral titer from the different miR-shRNA construct due tGFP gene. Vectors were determined by the flow cytometry technique. The determination of the lentiviral titer allowed us to estimate the Multiplicity of Infection (MOI) and thus to diminish the infectious activity of the viral stocks.
Determination of lentivirus titration
Since the stocks of vector carry the GFP transgene that can certainly be censored by flow cytometer, we used this method for titration of lentivectors. Vector titration was implemented based on lab protocols with some alterations. To determine vector titers (TU), 293T cells were incubated in a 12-well plate at a density of 105 cells per well in one ml DMEM added with 10% FBS. The medium was removed and cells were transduced in 500 μl of new DMEM-10% FBS with serial dilutions of the vector that relate to the last amount of 1 μl, 10-1, 10-2, 10-3, and 10-4 μl of the vector. After 24 h, the medium was discarded and one ml of fresh medium was added to each well. 72 h after transduction, cells were administered for Fluorescence-Activated Cell Sorting (FACS) analysis.
Quantitative analysis of ADK knockdown by anti-ADK miR-sh7 in Wharton’s jelly mesenchymal stem cells
A QuantiFast SYBR Green RT-PCR Kit (Qiagen) was utilized to display knockdown of ADK after the transduction of hWJMSCs by the most effective recombinant lentiviral construct that expresses anti-ADK miR-sh7. After puromycin selection of hWJMSCs with 2 μg/ml for 5-7 days, Total RNA from 1 × 106 cells was extracted and cDNA was produced by QuantiTect Reverse Transcription Kit (Qiagen) using random hexamer primers and M-MLV reverse transcriptase. In the second step, cDNA was employed for standard real-time PCR. Specific primer pairs were used for ADK and TBP [10]. The expression of ADK mRNA was assessed in the lentiviral engineered. HWJMSCs cells. TBP expression was measured for the endogenous reference gene for computing the relative expression levels of targeted mRNA.
Preparation of overall protein extracts
Stably transduced hWJMSCs were lysed via ReadyPrep™ mammalian cell lysis reagent (Bio-Rad) including a complete protease inhibitor mixture. Subcellular portions of proteins were done consistent with the manufacturer's instructions. The samples were kept at -80°C until desired for western blot analysis. The protein content of the sample lysate was determined by Bradford’s method with the manufacturer’s instructions.
Animals
All experimentation with animals was performed according to the guidelines of the Iranian institute of cell and gene therapy. Male Wistar rats being housed in pairs under 12 h light 12 h dark conditions with free access to water and food. The weight of animals was between 230 g-270 g at the time of the experiment.
Seizure model
An epilepsy model was induced by kindling with Pentylenetetrazole (PTZ, sigma) with a dose of 35 mg/kg every other day for 15 intraperitoneal injections While the control group received only an intraperitoneal injection of the Phosphate Buffer Saline vehicle (PBS; pH 7.4) in place of PTZ. A majority of rats (85%, n=60) after injection with PTZ developed grade V to VI were defined as fully kindled. 40 kindled male Wistar rats with equally and randomly divided into four groups: Control (n=10), vehicle (n=10), WJMSCsmiR- shSC (n=10), WJMSCs-miR-sh7 (n=10). Animals in the control group were influenced by stereotaxic and electrode surgery without any injection of medium or cells [11]. On the other hand, animal groups in the vehicle or the transplantation group receiving medium and cells respectively at the time of surgery.
Transplantation of hWJMSCs
Under full anesthesia with ketamine and xylose, adult male rats received bilateral stereotaxic microinjection of 2 × 105 engineered hWJMSCs (1 × 105 in each lateral) via a 10 μl Hamilton syringe with a 26-gauge needle into the CA3 region of hippocampal on stereotactic coordinates relative to bregma: AP= -3.84 mm, ML= ± 2.4 mm, DV= -3.6 mm. Animals received cells that were engineered with recombinant lentiviruses that express anti-ADK miR-shRNA or scrambled control sequence in WJMSCs-miR-sh7 (n=10) and WJMSCs-miR-shSC (n=10) groups respectively. The vehicle group was injected slowly with 5 μl of culture medium in each lateral. The cell suspension was transplanted at a speed of 0.5 μL/min for a total of 10 minutes using microinjection. The needle was maintained for 5 minutes and slowly removed. After implanting two electrodes on the parietal cortex and a monopolar electrode as reference placed onto the nasal bone, the transplantation hole was closed with dental acrylate.
Electroencephalographic recording
One week after transplantation and recovery of surgery, the rats underwent EEG monitoring in Plexiglas cages where they could move liberally. EEG recording was done one and two weeks post Xenograft. The recording duration for each rat was 10 min before induction with PTZ and 30 min following it. EEG signals were digitalized at 250 Hz. All of the EEG analog data were collected via a multi-channel neurophysiology system.
Statistical analysis
A viewer not aware of the animal’s characteristics investigated every EEG file manually. Differences between groups were analyzed using one-way ANOVA with a post hoc Tukey test. Statistical analyses were done with the Statistical Package for the Social Sciences (SPSS, version 19). Data were expressed as the mean ± SEM for each group.
Results
Morphological features and marker expression of hWJMSCs
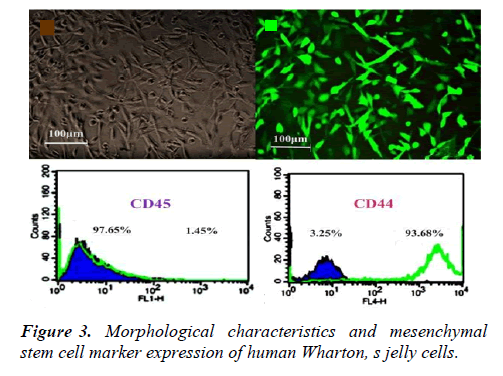
Umbilical cord tissue was grown by an explant culture method. Several days later, hWJMSCs migrated away from the tissues. After two weeks, fibroblast-like cells seemed in culture dishes. The cells grew fast and quickly covered the surface. Flow cytometry analyses showed that the cultured WJMSCs do not express hematopoietic marker CD45; nevertheless, they express mesenchymal stem cell marker CD44 (Figure 3).
Transfection efficiency of lentiviral anti-ADK miRshRNAs vectors in packaging 293T cell line
Independently transfer vectors (anti-ADK miR-sh7 or scrambled control) with helper vectors (psPAX2 and pMD2) were co-transfected in the HEK293T cell line through calcium phosphate transfection protocol. The transfection productivity due to the GFP marker beneath the fluorescent microscope was more than 90%-95% in anti-ADK miR-sh7 as well as a scrambled control (supplementary data). The titer of the recombinant lentivirus was approximately 1.5-2 × 106 vg/ml.
Genetic engineering of hWJMSCs by anti-ADK miR-sh7 and quantitative analysis of ADK knockdown
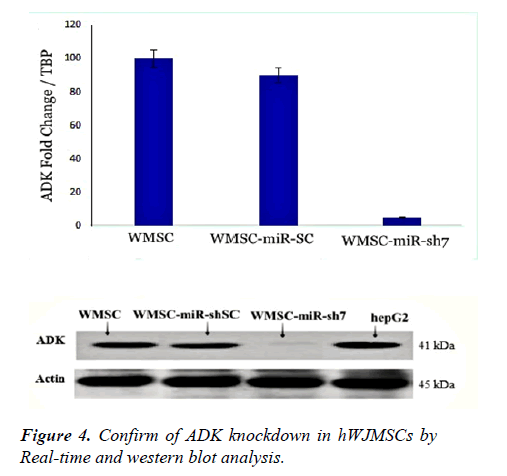
Analysis of real-time data in our previous study indicated that anti-ADK miR-sh7 is the most proficient anti-ADK miRshRNA for knockdown of this gene. For this reason, we used pseudo lentiviruses of miR-sh7 for the transduction of hWJMSCs. The high efficiency of transduction was observed under the fluorescent microscope. After selection with puromycin (1.5 μg/ml) in the culture medium, semiquantitative real-time PCR was employed for assessing ADK expression in the mRNA level. Data exhibited knockdown of the ADK gene in hWJMSCs up 95%.
After puromycin selection of transduced hWJMSCs with anti- ADK miR-sh7 lentiviral vector, cell lysate was used for the performance of western blot. Other samples include Cell lysate from transduced hWJMSCs with scrambled control viruses, hWJMSCs, and hepG2 as control was employed. To measure and normalizing ADK immunoreactivity, the β-actin antibody was used. Analysis of data did not display any drop of ADK in hWJMSC, hWJMSC-miR-shSC, and hepG2 while downregulation of ADK was detected after transducing cells with the human-specific anti- ADK miR-sh7 (Figure 4).
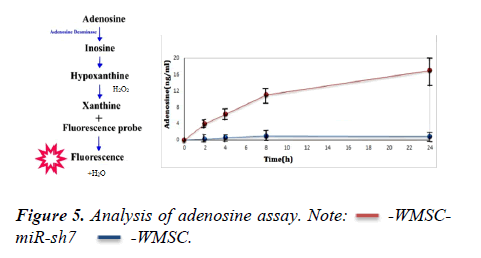
Adenosine assay in engineered hWMSCs
The principle in adenosine assay is illustrated. For quantitative analysis of releasing adenosine in the culture medium, 1 × 105 cells of transduced hWJMSC with anti-ADK miR-sh7 along with untransduced hWJMSC were incubated in serum-free medium for 2,4,8 and 24 hours. The culture medium was collected and adenosine concentration with the adenosine assay kit at different times was assessed. The adenosine level in hWJMSC was not comparable with hWJMSC-miR-sh7 and after 8 h does not show any variation. While adenosine concentration in hWJMSC-miR-sh7 reached 10 ng/ml ± 0.05 ng/ml after 8 h. The level of adenosine continues to raise 17 ng/ml ± 0.15 ng/ml after 24 h. The data showed knockdown of ADK by anti-ADK miR-sh7 induce adenosine releasing in the supernatant of engineered hWJMSC (Figure 5).
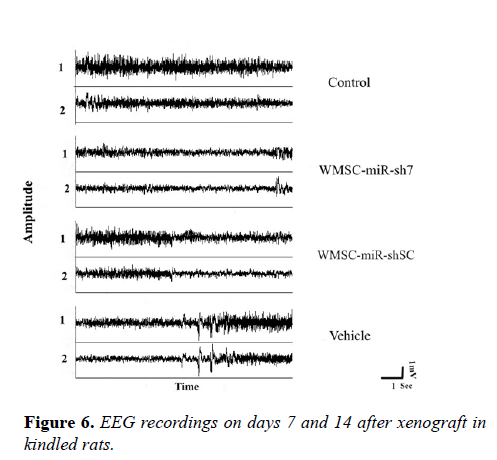
EEG recording and data analysis
A week after cell graft and electrode implantation, the animals showed acceptable recovery. On days 7 and 14 after stereotaxic surgery, bilateral cortical EEG was recorded. Kindled rats that received an adenosine releasing graft of hWJMSC-miR-sh7 demonstrated antiepileptic effects in EEG activity without convulsive seizures. While the control group and vehicle group showed convulsive seizures and epileptic after discharges in EEG activity after injection of PTZ. The animal that received hWMSC-miR-SC graft displayed a moderate seizure activity in comparison to the control and vehicle group (Figure 6).
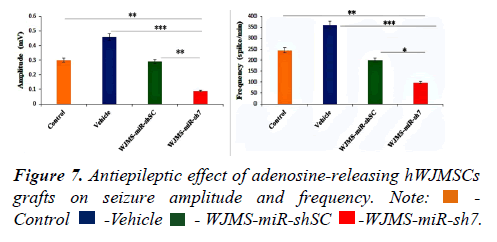
Reduction of PTZ-induced seizures by adenosinereleasing hWJMSCs grafts
To assess seizure control, EEG recording of animals in the control and the vehicle group, as well as cell transplanted groups, was employed. Three previous implanted electrodes made possible cortical EEG recording 10 min before and 30 min following PTZ injection. After the recording of EEG, data were analyzed and amplitudes, frequencies, durations, and latency of seizure spikes. Analysis of data shows that the amplitude (0.09 mv ± 0.006 mv) and duration (0.01 mSec ± 0.002 mSec) of seizure spikes diminished significantly in the WJMSC-miR-sh7 group compare to the control, vehicle, and WJMSC-miR-shSC group. Out reads demonstrates a significant reduction of seizure spike frequency (98 spike/min ± 3 spike/min) in the WJMSC-miR-sh7 group in comparison to other groups (245 ± 5 in control, 361 ± 7 in the vehicle, and 200 ± 4 in WJMSC-miR-shSC group). Results also implicate significantly increasing the latency of seizure spikes (46.6 sec ± 3.2 sec) after induction with PTZ in the WJMSC-miR-sh7 group in comparison to control (2.6 sec ± 0.5 sec), vehicle (8.3 sec ± 1 sec), and WJMSC-miR-shSC (29.1 sec ± 1.5 sec) group (Figures 7 and 8).
Behavioural seizure analysing
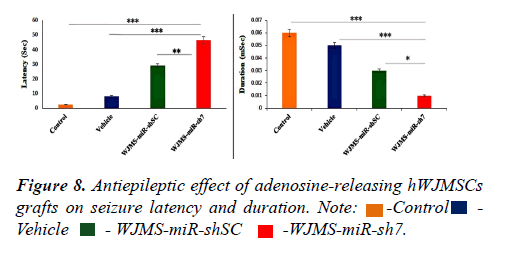
To demonstrate whether the concentration of releasing adenosine is sufficient for the suppression of seizure for a long term in kindled animals, behavioural seizure during 8 weeks was monitored. Before adenosine releasing cell graft all rats (n=20) showed grade V of seizures. One week after cell transplantation a 100% of animals showed complete protection from convulsive seizure. 2 weeks after cell graft, about 83% of animals (n=18) demonstrated full protection from epileptic seizures. Antiepileptic effects were conserved in 55% and 50% of rats (n=18) for the third and fourth weeks. Two rats revealed no seizures after 7 weeks (n=16) while one rat showed complete protection after 8 weeks (n=16) (Figure 9).
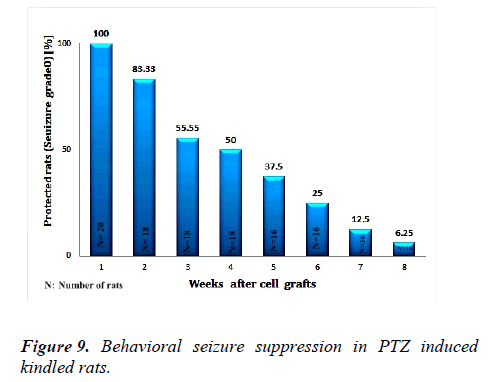
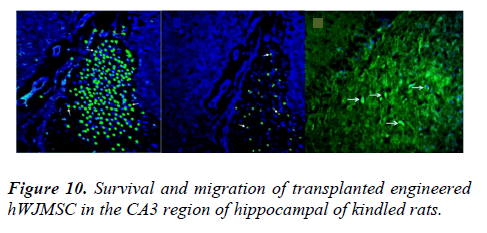
After establishing the epileptic model, animals in cell groups received engineered hWJMSCs in the CA3 region of hippocampal. To assess the therapeutic effect of adenosine released by hWJMSC-anti-ADK miR-sh7, we used the chronic model of epilepsy-induced by PTZ. Male rats received an intrahippocampal implant of 2 × 105 (1 × 105 cells on each side) hWJMSC-miR-sh7 (n=10) or hWJMSC-miR-shSC (n=10) in cell groups respectively. The animal in the vehicle group was injected with the same volume of culture medium while the control group did not receive any cell or culture medium. To investigate the survival and migration of implanted cells in hippocampi, one, 4, and 8 weeks post cell transplantation animals were sacrificed. The histological survey showed that transplanted cells were alive and presented in the margins of the injection zone (Figure 10).
Discussion
In the present study, we examined the therapeutic effects of engineered hWJMSC in PTZ- induced kindling model in rats. Transplanted adenosine releasing cells of hWJMSC in the chronic phase of epilepsy suppressed the seizures and showed antiepileptic properties. In epilepsy, many genes have been implicated in idiopathic epilepsies code for ion channels, cortical development, mitochondrial function, and cell metabolism. For this reason, most advanced epilepsy treatments such as cell and gene therapy focus on the manipulation of excitatory and inhibitory targets in the brain. Attenuate of seizure can earn by overexpression of existing inhibitory receptors or molecules as well as knockdown of excitatory targets [12-16]. Antisense-based gene therapy against N-Methyl-D-Aspartic acid (NMDA) receptor and ADK gene or overexpression of GABA and GABA receptors, neuropeptides and their receptors like Neuropeptide Y (NPY), galanin, somatostatin, and also an expression of Neurotrophic Factors (NTFs) and their receptors, are molecular strategies in the treatment of epilepsy. Adenosine is an endogenous anticonvulsant that has known neuromodulator and neuroprotective effects via G protein-coupled adenosine A Receptors (ARs) since 1980. Despite this discovery in recent years, molecular tools have still uncovered the pathological mechanism of adenosine deficiency in epilepsy. As mentioned above, adenosine is an endogenous anticonvulsant and thereby it is under the physiological clearance. Augmentation of adenosine is non-toxic and focal delivery of this molecule has no side effects.
In the current study, we enriched the therapeutic value of hWJMSCs by knockdown of ADK gene and creating adenosine releasing hWJMSCs to suppress seizures in a related model of human absence epilepsy induced by PTZ in rats. hWJMSCs are an attractive substitute source for cell- based gene therapy. They are achievable stem cell sources, lacking moral and technical concerns, available in infinite amount, amenable to genetic manipulation, and have a therapeutic perspective. The therapeutic potential of these cells by generating neuroprotective and anti-inflammatory cytokines in Pilocarpine-treated rats. Based on these deliberations, we nominated these cells for engineering with lentiviral anti-ADK miR-shRNA expressing systems [17]. miRNA-mediated downregulation of ADK in hBMSC formerly They demonstrated the potential of engineered hBMSC for ameliorating acute seizures in a short term study, but in the current study, we evaluated the therapeutic effects of engineered hWJMSC in a chronic model of epilepsy for the long term. We mimic human miR 30 for the design of stemloop and flanking sequences of an anti-ADK miR-shRNAs to intensify the productivity of RNAi-based gene therapy. To evaluate the therapeutic application of engineered hWJMSCs, we xenograft these cells into the CA3 region of the hippocampus of PTZ-induced epileptic rats. The chronic model of epilepsy in rats induced by PTZ can make tonic-clonic seizures and is a suitable model for refractory epilepsy.
Stable knockdown of the ADK gene by anti-ADK miR-shRNA lentiviral systems made possible long-term production of adenosine. We investigate the effect of bilateral xenograft transplantation on seizure frequency, duration, latency, and amplitude of EEG recording for two weeks in four groups of animals. All of the male rats before injection of cells were kindled by PTZ. Animals in the control group (n=10) did not receive any substance (culture medium or cells) but created to clear any damage of surgical events in the time of injection or implantation of electrodes for EEG recordings. However, animals in vehicle groups (n=10) received 5 μl of culture medium on each side. Transplantation groups received 1 × 105 hWJMSCs transduced by anti-ADK miR-sh7 (WJMSC-miRsh7 group) or scrambled control (WJMSC-miR-shSC group) viruses in each side. On days 7 and 14 after xenograft, EEG recording was employed to demonstrate the therapeutic. Potential of this cell-based gene therapy approach. Analysis of data showed that the amplitude and duration of seizure spikes diminished significantly in the WJMSC-miR-sh7 group compare to the control, vehicle, and WJMSC-miR-shSC group [18-20]. Out reads demonstrated a significant reduction of seizure spike frequency in the WJMSC-miR-sh7 group in comparison to other groups. Results also implicated significantly increasing the latency of seizure spike after induction with PTZ in the WJMSC-miR-sh7 group in comparison to control, vehicle, and WJMSC-miR-shSC group.
One week after cell transplantation, visible cells that expressed GFP were seen under the fluorescent microscope in the zone of injection. Also, 1 month and 2 months after xenograft, hWJMSC was detected in the marginal and further zone of injection. The monitoring of behavioral seizures was continued for 8 weeks after xenograft. Complete seizure protection was perceived in 100% (n=20) of rats after one week and more than 83% (n=18) of rats after two weeks post- transplantation. It proved transplanted cells stay alive, functional, and could release adenosine that has a paracrine therapeutic effect on neighbors. Complete seizure protection continued in 55% (n=18) and 50% (n=16) of rats for the third and fourth week in order. One animal showed complete seizure protection after 8 weeks [21-26]. This diminishing in seizure protection was according to the decreasing of detected engineered hWJMSC in the hippocampi of rats after 4 and 8 weeks posttransplantation. We did not use any immunosuppressive drugs for cell graft. It can explain the possibility of cell loss after 8 weeks. Here we explained engineered hWJMSCs as novel adenosine-releasing stem cells that sufficiently suppress seizure activity in an animal model of epilepsy in 50% of animals (n=16) at least for 4 weeks. The substitution of lentiviral vectors associated vectors or non-integrative lentiviral vectors could provide a safer gene transfer system for the prevention of insertional mutation and tumorigenesis in the next studies. Differentiation of hWJMSCs to neurogenic cells. Like GABAergic could improve the potential of these cells for structural integration into neural network and compensation of neuron loss and cell damage during the seizure. Using novel genome editing tools such as CRISPR/Cas9 could possible long-term survival of hWJMSC- derived brain implants. These approaches may provide a clinical application of these cells in the treatment of epilepsy and other neurological disorders in the future.
Conclusion
The results show that hWJMSC with adenosine releasing property significantly decreases seizure activity in EEG recording and behavioral seizure monitoring. Ameliorate of seizure implicates the antiepileptic effect of engineered cells. These properties suggest that manipulated hWJMSCs could be a suitable source in cell-based gene therapy of epilepsy.
Acknowledgement
We would like to thank Space Medicine BV (Rotterdam, Netherlands) and the Iranian Institute of Cell Gene Therapy (Tehran, Iran) for their support.
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
References
References
- Campos G, Fortuna A, Falcao A, et al. In vitro and in vivo experimental models employed in the discovery and development of antiepileptic drugs for pharmacoresistant epilepsy. Epilepsy Res. 2018;146:63-86.
- Detlev Boison KAS. Therapeutic epilepsy research: From pharmacological rationale to focal adenosine augmentation. Biochem Pharmacol 2009;78:1428-37.
- Boison D. Adenosine Augmentation Therapies (AATs) for epilepsy: Prospect of cell and gene therapies. Epilepsy Res. 2009;85:131-41.
- Fedele DE. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383-95.
- Li T, Ren G, Kaplan DL, et al. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84: 238-41.
- Boison D. Engineered adenosine-releasing cells for epilepsy therapy: Human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics 2009;6:278-83.
- Dulak J, Szade K, Szade A, et al. Adult stem cells: Hopes and hypes of regenerative medicine. Acta Biochim Pol. 2015;62:329-37.
- Upadhya D. Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration. Proc Natl Acad Sci. 2019;116:287-96
- Josse C. Systematic chromosomal aberrations found in murine bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:1167-73.
- Rebuzzini P, Zuccotti M, Redi CA, et al. Chromosomal abnormalities in embryonic and somatic stem cells. Cytogenet Genome Res. 2015;147:1-9.
- Frausin S. Wharton’s jelly derived mesenchymal stromal cells: Biological properties, induction of neuronal phenotype and current applications in neurodegeneration research. Acta Histochem. 2015;117:329-38.
- Watson N. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy. 2015;17:18-24.
- Liu S. Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. J Biosci Bioeng. 2014;117:229-35.
- Kamal MM, Kassem DH. Therapeutic potential of wharton’s jelly mesenchymal stem cells for diabetes: Achievements and challenges. Front Cell Dev Biol. 2020;8.
- Abdolhossein Zadeh B. Lentiviral-mediated BCL2 gene knockdown using comparative microRNA adaptive shRNAs. Cell Mol Biol. 2018;64:1-25.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72.
- Luttjohann A, Fabene PF, van Luijtelaar GA, et al. Revised racine’s scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579-86.
- Steinlein OK. Genetic mechanisms that underlie epilepsy. Nat Rev Neurosci. 2004;5: 400-08.
- Weinberg MS. McCown TJ. Current prospects and challenges for epilepsy gene therapy. Exp Neurol. 2013;244:27-35.
- Ingusci S, Cattaneo S, Verlengia G. A matter of genes: The hurdles of gene therapy for epilepsy. Epilepsy Curr. 2019;19:38-43.
- Dunwiddie TV. Endogenously released adenosine regulates excitability in the in vivo hippocampus. Epilepsia. 1980;21:541-48.
- Boison D. Adenosine dysfunction in epilepsy. Glia. 2012;60:1234-43.
- Joerger-Messerli MS. Mesenchymal stem cells from wharton’s jelly and amniotic fluid. Best Pract Res Clin Obstet Gynaecol. 2016;31:30-44.
- Huang PY. Xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly as a potential therapy for rat pilocarpine-induced epilepsy. Brain Behav Immun. 2016;54:45-58.
- Ren G. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: A novel perspective for seizure control. Exp Neurol. 2007;208:26-37.
- Dhir A. pentylenetetrazol (ptz) Kindling model of epilepsy. Curr Protoc Neurosci. 2012;58