- Current Pediatric Research (2016) Volume 20, Issue 1
Endothelial Progenitor Cells Cut-off and Relations to Cardiovascular Risk Factors in Obese Children and Adolescents.
- *Corresponding Author:
- Alaa Youssef Ahmed
Department of Paediatrics Ain Shams University Egypt
E-mail: dralaayoussef@gmail.com
| Date of Acceptance | May 30, 2016 |
Abstract
Background: Endothelial Progenitor Cells [EPCs] are involved in the regeneration of the endothelial lining following blood vessel injury. The reduction in the number of EPCs was postulated to be associated with the initiation and progression of cardiovascular disease.
Objectives: This study aimed at exploration of the number of EPCs in obese nondiabetic children and adolescents and their relation to fasting lipid levels, fasting glucose, fasting insulin, Homeostasis Model Assessment for Insulin Resistance [HOMAIR], Carotid Intima Media Thickness [CIMT], echocardiography as well as parameters of cardiac dysfunction on tissue Doppler imaging.
Methods: 56 children and adolescents 5-15 years were selected randomly from patients seeking medical advice for obesity management at the Obesity Clinic of the Paediatrics Hospital, Ain Shams University together with 36 age and sex matched controls. All underwent anthropometric evaluation, measurement of fasting lipids, glucose, insulin, HOMA-IR, CIMT, echocardiography and tissue Doppler imaging. EPCs, by flow cytometry, are the cells expressing positivity for CD34 and CD144.
Results: EPCs were significantly lower in patients compared to controls [p=0.00]. A ROC curve revealed a sensitivity and specificity each of 100% for the value of positivity ≤ 85 EPCs per million white blood cells. EPCs showed negative correlation with the left ventricular end diastolic diameter which is a known complication of obesity.
Conclusion: Impaired endothelial regeneration and the risk of atherosclerosis is incurred by obesity regardless of the state of dyslipidaemia and insulin resistance.
Keywords
Endothelial progenitors, Obesity, Cardiovascular risk, Children, Adolescents
Introduction
In the era of regenerative medicine and cell-based therapies, endothelial progenitor cells [EPC] attracted many researches as they represent one subtype of bone marrow derived cells that were shown to be able to replace old damaged endothelial cells by forming a patch that heals the endothelium after its injury [1]. The release of EPCs from the bone marrow occurs in response to ischaemic insults. Their number is reduced in adults with coronary ischaemia and in children with type 1 diabetes, therefore it has been speculated that this subtype of cells has an important role in diseases with adverse cardiovascular outcomes [2,3].
There is no universally agreed upon set of markers to identify EPCs, however, CD34, which is one marker of the haematopoietic cell lineage, appears to be a constant marker for the circulating EPCs [4].
High calorie diet induced endothelial changes and initiated hypothalamic angiopathy in mice thus contributing to the control of satiety at a central level through endothelial derived mechanisms [5]. Childhood obesity gives rise to a bunch of metabolic complications; namely dyslipidaemia, insulin resistance and hypertension with deleterious effects on the blood vessels and predisposition to early atherosclerosis whose mechanism is still largely unraveled [6,7]. Insulin resistance in adults has been associated with reduced sensitivity at the level of the PI3/AKT pathway in the skeletal muscles of adults and has been associated with reduced levels of EPCs [8,9]. The reduction in the number of the EPCs appears to be mediated by suppression of their recruiting signals in adults with metabolic syndrome [10].
More than 2 decades of follow up revealed that obesity is one major risk factor contributing to the development of cardiovascular disease and recommended control of obesity as a primary preventive measure to reduce cardiovascular mortality [11].
This study aimed at exploration of the EPC number in obese non-diabetic children and adolescents as a new proposed cardiovascular risk marker and their relations to the other well-known cardiovascular risk factors.
Methods
Study Population
This was a case control study including 56 children and adolescents with simple obesity randomly recruited from those seeking consultation at the Obesity Clinic, Children’s Hospital, Ain Shams University, Cairo, Egypt in the period from March 2013 to September 2013. Obesity was defined as having a body mass index more than 2 standard deviations for age and sex as recommended by the WHO [12]. Their ages ranged from 5-15 years with a mean of 9.13 ± 2.8 years. They were not receiving any medications or having any other systemic illness. The participants were 27 [48.2%] females and 29 [51.8%] males with 36 non-obese controls matched for their age, sex and Tanner stage chosen from children and adolescents attending the outpatient clinic with their sick relatives. None of the controls were hypertensive, receiving any treatment or having any systemic disease. All parents signed an informed consent before enrollment into the study. The study protocol was approved by the local ethics committee of Ain Shams University.
Clinical Evaluation
All patients and controls had weight measured in kilograms with a digital scale with minimal clothing. Height was measured to the nearest 0.1 cm by a Harpenden stadiometer [Holtain Ltd, Wales, UK] with calculation of the height SDS [13]. The Body Mass Index [BMI] was calculated as weight [kg]/height [m]2. BMI SDS was calculated from the age- and sex-specific reference values [14,15]. Measurement of the waist circumference was done midway between the lowest rib and the top of the iliac crest with a non-stretchable tape. Waist circumference SDS was calculated and compared to norms [16,17]. Hip circumference was measured in a horizontal plane at the maximum extension of the buttocks. Hip circumference SDS was calculated and compared to norms [16,18]. Waist/hip ratio SDS was calculated and compared to norms [16,17]. Tanner pubertal staging was done for girls ≥ 9 years and for boys 10 ≥ years [19,20]. Blood Pressure [BP] was measured and compared to norms according to the recommendations of the National High Blood Pressure Education Program [21].
Laboratory Investigations
For patients and controls, Fasting Blood Sugar [FBS] and fasting lipid profile [total cholesterol, high density lipoprotein-cholesterol [HDL-C], Low-Density Lipoprotein-Cholesterol [LDL-C] and triglycerides] were measured using Synchron CX7 [Beckman, Switzerland], fasting insulin was determined by the use of a double antibody immunochemiluminometric assay performed on an Access automated immunoassay system [Beckman Instruments]. Calculation of the glucose insulin ratio and calculation of homeostasis model assessment of insulin resistance [HOMA-IR] according to the formula: fasting insulin [microU/L] × fasting glucose [nmol/L]/22.5 [22].
Flow cytometry immunophenotyping for EPCs: Wholeblood EDTA-anticoagulated peripheral blood samples from patients and controls were collected. Optimal leukocyte count for staining was adjusted between 0.5 and 1.0 × 106 cells. Cells were labeled by incubating 100 μl of blood samples for 30 min at room temperature in the dark with the following monoclonal antibodies: 20 μl FITC anti-human CD 34, 5 μl PE anti-human CD144 [VE-cadherin] [R&D systems, UK]. For the purpose of erythrocyte lysis, cells were incubated for 5 min with 2 ml of 0.83% ammonium chloride then tubes were vortexed and washed with PBS [pH 7.2 +/- 0.2]. Cells were suspended in 0.5 ml PBS. Isotype matched control tubes [PE+FITC labeled IgG1] [R&D systems, UK] were used. Flow cytometry analysis was performed version III software, Coulter electronics on Epics XL FCM [Coulter electronics, USA]. At least 10,000 events were analyzed per case or control, analysis of EPCs was based on the surface expression of the following markers: CD34 and CD144 on the cells localised in the lymphocyte and monocyte gates. Results are presented as their absolute count per million White Blood Cells [WBCs].
Carotid Intima Media Thickness [CIMT]
CIMT was measured on the longitudinal views of the far wall of the bilateral distal common carotid arteries [1-3 cm proximal to the carotid bifurcation] at the diastolic phase and expressed as the mean of six measurements [3 on each side] using 7.5 MHz linear transducer [23].
Echocardiography and Tissue Doppler Imaging [TDI]
Cardiac morphology: Using M-mode echocardiography [Vivid 7, GE, Vingmed Horten, Norway], the interventricular septum thickness [SWT], left ventricular posterior wall dimension at end diastole [PWT], LV endsystolic [LVESD], end-diastolic [LVEDD] diameters, cardiac mass index, pulmonary pressure and systolic function; ejection fraction EF% and fractional shortening FS%. TDI was used for assessment of left and right ventricle diastolic and systolic functions: peak early [Em] [cm/s], late diastolic [Am] [cm/s] and systolic [Sm] [cm/s] velocities were measured using the sample volume that was placed at the lateral annulus of the mitral leaflets in the apical four- chamber view. Myocardial diastolic and systolic function of right ventricle [ET, AT and ST] were measured by the method described for the left ventricle regarding the sample volume of Doppler that was symmetrically set at the tricuspid valve instead of the mitral valve [24]. The ratios of peak myocardial velocities of the valves [Em/Am and ET/AT] were calculated.
Statistical Methods
Statistical analysis was performed using SPSS 17.0 statistical package. All results were expressed as mean & SD values for parametric data and median, IQR for non-parametric quantitative data. Student’s t-test was used for means, Mann-Whitney Test for non-parametric quantitative data and Chi-square test for comparing categorical variables. Pearson correlation and Spearman's rho were used for correlations in parametric and nonparametric data respectively. The one way Analysis of Variance [ANOVA] was used to detect any significant differences between three groups. A p value of <0.05 was considered significant. A ROC-curve [Receiver Operating Characteristic Curve analysis] was constructed to set the cutoff between cases and controls regarding the CD34, CD144 positive cells.
Results
All anthropometric measurements as well as the measured laboratory parameters differed significantly between patients and controls. All the studied patients had a BMI above +2SD with a mean of 3.5 ± 0.95. The waist circumference SDS was 5.27 ± 2.34 and waist height ratio was more than 0.5 [0.67 ± 0.09]. The studied patients have atherogenic dysplidaemia, higher fasting blood sugar, higher insulin level and higher HOMA-IR (Table 1).
| Patients (N=56) |
Controls (N=36) |
p | Patients (N=56) |
Controls (N=36) |
p | ||
|---|---|---|---|---|---|---|---|
| Height (SDS) | 0.76 ± 1.22 | -0.34 ± 0.75 | <0.001* | CIMT (mm) | 0.43 ± 0.02 | 0.37 ± 0.03 | <0.001 |
| BMI (kg/m2) | 30.17 ± 5.80 | 16.37 ± 1.84 | <0.001* | RV MI (gm/m2) | 27.08 ± 1.14 | 27.41 ± 0.71 | 0.084 |
| BMI SDS | 3.5 ± 0.95 | -0.21 ± 0.86 | <0.001* | LV MI (gm/m2) | 64.91 ± 3.53 | 68.17 ± 2.85 | <0.001 |
| Waist circumference (cm) | 92.03 ± 15.62 | 58.69 ± 7.15 | <0.001* | LAD (cm) | 2.43 ± 0.38 | 2.28 ± 0.17 | 0.026* |
| Waist circumference SDS | 5.27 ± 2.34 | -0.48 ± 0.69 | <0.001* | SWT (cm) | 0.65 ± 0.09 | 0.64 ± 0.06 | 0.752 |
| Hip circumference (cm) | 94.97 ± 14.35 | 68.47 ± 9.17 | <0.001* | PWT (cm) | 0.67 ± 0.08 | 0.65 ± 0.03 | 0.276 |
| Hip circumference SDS | 4.56-4.9 | -0.7 ± 0.79 | <0.001* | LVEDD (cm) | 3.53 ± 0.61 | 3.69 ± 0.43 | 0.168 |
| Waist/Hip ratio | 0.96 ± 0.06 | 0.85 ± 0.04 | <0.001* | LVESD (cm) | 2.5 (0.37) | 2.6 (0.52) | 0.285 |
| Waist/Hip ratio SDS | 2.32 ± 1.73 | 0.19 ± 0.91 | <0.001* | EF% | 69.96 ± 3.73 | 62.03 ± 6.02 | <0.001 |
| Waist/Height ratio | 0.67 ± 0.09 | 0.44 ± 0.02 | <0.001* | FS% | 30.71 ± 3.58 | 31.69 ± 5.11 | 0.320 |
| Cholesterol (mmol/L) | 5.24 ± 0.95 | 3.66 ± 0.36 | <0.001* | RVESP (mmHg) | 26.95 ± 6.5 | 13.14 ± 4.08 | <0.001 |
| Triglycerides (mmol/L) | 1.45 ± 0.49 | 0.81 ± 0.11 | <0.001* | Sm (cm/s) | 3.60 ± 0.80 | 4.18 ± 1.48 | 0.037* |
| LDL-c(mg/dl) | 3.26 ± 0.89 | 2.25 ± 0.22 | <0.001* | Em (cm/sec.) | 9.72 ± 1.00 | 11.48 ± 1.09 | <0.001 |
| HDL-c(mg/dl) | 1.15 ± 0.33 | 1.6 ± 0.14 | <0.001* | Am (cm/s) | 8.06 ± 3.73 | 4.36 ± 0.47 | <0.001 |
| TG/HDL ratio | 3.29 ± 1.81 | 1.16 ± 0.16 | <0.001* | Em/Am | 1.47 ± 0.52 | 2.68 ± 0.37 | <0.001 |
| CMIT | 2.25 ± Â 1.35 | 0.52 ± 0.079 | <0.001* | ST (cm/s) | 5.73 ± 0.43 | 5.96 ± 0.42 | 0.012* |
| Fasting blood sugar (mmol/L) | 4.5 ± 0.51 | 4.49 ± 0.32 | <0.001* | ET (cm/s) | 13.64 ± 0.97 | 13.85 ± 1.02 | 0.323 |
| Fasting insulin (μIU/mL) | 8.6 (7.38) | 4.33 ± 4.09 | <0.001* | AT (cm/s) | 6.83 ± 0.45 | 6.85 ± 0.49 | 0.880 |
| HOMA-IR | 2.15 (1.92) | 0.85 (0.8) | <0.001* | ET/AT | 2.01 ± 0.11 | 2.02 ± 0.10 | 0.413 |
| CD34/106WBCs | 105 (75.75) | 415 (295) | <0.001* | SBP (mmHg) | 109.11 ± 11.37 | 96.25 ± 8.65 | <0.001 |
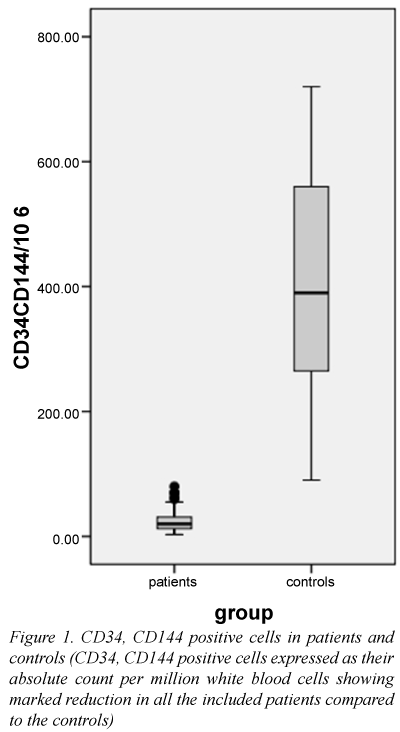
| CD34, 144/106 WBCs | 20 (19.25) | 390 (307.5) | <0.001* | DBP (mmHg) | 71.61 ± 10.45 | 57.78 ± 6.26 | <0.001 |
Table 1. Background clinical, laboratory data, CIMT, echocardiography and tissue Doppler imaging parameters of patients and controls
Both patients and controls differed significantly regarding the EPC count (Table 1 and Figure 1). A ROC curve analysis showed that an EPC count ≤ 85 EPCs per million WBCs has a sensitivity and specificity of 100% in differentiating between patients and controls.
The echocardiography data of the obese patients showed significant increase of the left atrial diameter, right ventricular end systolic pressure and ejection fraction compared to the controls; however the left ventricular mass index was significantly reduced. TDI showed significant reduction of systolic function at the mitral annulus [Sm], E wave at the mitral annulus [Em] in patients compared to controls. The A was at the mitral annulus significantly increased in patients; therefore, the Em/Am ratio was markedly reduced. The S wave at tricuspid annulus was markedly reduced in the studied group of patients (Table 1).
The patients had significantly higher systolic as well as diastolic blood pressures compared to the controls.
The CIMT was significantly higher in patients than in controls (Table 1); however, there was no correlation with the EPC count (Table 2). Similarly the EPC count didn’t show any significant correlation to metabolic risk factors associated with obesity; namely the anthropometric measurements, parameters of dyslipidaemia, fasting glucose, fasting insulin or calculated HOMA (Table 2). Systolic hypertension was present in 5 patients [8.9%] and systolic prehypertension in 10 [17.9%] while diastolic hypertension existed in 7 patients [12.5%] and diastolic prehypertension in 18 patients representing 32.1% of the sample.
| CD34; CD144/106WBCs | |||||
|---|---|---|---|---|---|
| Clinical and laboratory parameters | r | p | Cardiovascular parameters | r | p |
| Height (SDS) | -0.13 | 0.336 | CIMT(mm) | 0.1 | 0.445 |
| BMI (kg/m2) | -0.03 | 0.842 | RV MI (gm/m2) | 0.16 | 0.252 |
| BMI SDS | -0.18 | 0.188 | LV MI (gm/m2) | 0.04 | 0.784 |
| Waist circumference SDS | -0.08 | 0.577 | LAD (cm) | 0.49 | 0.717 |
| Hip circumference SDS | -0.23 | 0.089 | SWT (cm) | 0.02 | 0.874 |
| Waist/Hip ratio | 0.07 | 0.620 | PWT (cm) | -0.07 | 0.634 |
| Waist/Hip ratio SDS | 0.07 | 0.606 | LVEDD (cm) | -0.33 | 0.012* |
| Waist/Height ratio | -0.1 | 0.455 | LVESD (cm) | 0.07 | 0.605 |
| SBP | -0.1 | 0.457 | EF% | 0.12 | 0.367 |
| DBP | -0.09 | 0.500 | FS% | 0.23 | 0.089 |
| Cholesterol (mmol/L) † | -0.15 | 0.277 | RVESP (mmHg) | 0.03 | 0.822 |
| Triglycerides (mmol/L) † | 0.09 | 0.510 | Sm(cm/s) | 0.05 | 0.717 |
| LDL-c(mmol/L) † | -0.24 | 0.072 | Em(cm/s) | 0.02 | 0.892 |
| HDL-c(mmol/L) † | -0.01 | 0.959 | Am(cm/s) | 0.26 | 0.054 |
| TG/HDL ratio | 0.07 | 0.628 | Em/Am | -0.18 | 0.188 |
| CMI | 0.01 | 0.928 | ST (cm/s) | -0.04 | 0.767 |
| Fasting blood sugar (mmol/L) | 0.04 | 0.749 | ET(cm/s) | 0.1 | 0.464 |
| Fasting insulin(?IU/mL) | 0.13 | 0.356 | AT(cm/s) | 0.002 | 0.991 |
| HOMA† | 0.1 | 0.465 | ET/AT | 0.08 | 0.556 |
Table 2. Correlations of Endothelial Progenitor Cells (EPCs) expressing CD34; CD144 positivity/106 white blood cells with clinical; laboratory data; CIMT; Echocardiography and tissue Doppler imaging parameters of patients and controls
Despite the aforementioned differences in echocardiography between patients and controls, only the left ventricular end diastolic diameter [LVEDD] bore a significant correlation with the EPC count [r=-0.33, p=0.012, 95% CI=-0.545- -0.074] and neither of the measured TDI parameters nor the well-known cardiovascular risk factors [25] showed significant associations with the EPC count (Table 2). EPC count didn’t show significant differences between the groups of patients when the patients were divided categorically either by their lipid profile parameters into high, borderline and low or by their state of insulin resistance when taking into consideration the HOMA cutoff of ≥ 2.6 in pre-pubertal children and ≥ 3.2 in adolescents to diagnose insulin resistance (Table 3) [25,26].
| Lipid category | Cholesterol | TG | LDL | HDL | HOMA category | HOMA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Mean ± SD | p ¶ | Number | Mean ± SD | p ¶ | Number | Mean ± SD | p ¶ | Number | Mean ± SD | p¶ | Number | Mean ± SD | p¶ ¶ | |||
| EPCs | Acceptable | 13 | 27.62 ± 22.16 | 0.254 | 8 | 23.25 ± 18 | 0.926 | 18 | 21.3 ± 5 | 0.086 | 19 | 24.26 ± 18.01 | 0.896 | No IR | 39 | 23.33 ± 17.53 | 0.296 |
| Borderline | 15 | 29.8 ± 22.62 | 18 | 26.11 ± 18.47 | 13 | 22.47 ± 5 | 14 | 23.5 ± 18 | IR | 17 | 28.7 ± 17.42 | ||||||
| High | 28 | 21.14 ± 10.49 | 30 | 24.73 ± 17.38 | 25 | 8.2 ± 3 | 23 | 26.43 ± 17.49 | |||||||||
Table 3. Comparisons of endothelial progenitor cells (EPCs) expressing CD34; CD144 positivity/106 white blood cells with the different categorical classifications of patients according to the commonly identified cardiovascular risk factors
Discussion
This study was an exploratory study that aimed at assessing the EPCs that express CD34, CD 144 surface markers in obese children and adolescents and their relationships to CIMT as a marker of atherosclerosis, echocardiography changes as well as the different measurements of systolic and diastolic dysfunction on TDI [23].
EPCs were defined in this study as the cells that coexpressed CD34 and CD144 [27,28]. EPCs were determined using different units in various studies, but when we expressed their count as an absolute count per million WBCs, we reached a cutoff for a positive test of ≤ 85 EPCs per million WBCs in all patients included in our obese cohort [3,29]. This implies defective endothelial repair mechanisms after endothelial denudation in obesity which is one of the earliest steps in the pathogenesis of atherosclerosis [3]. In a similar study, obese males without cardiovascular events had reduced number of EPCs that were labeled by CD34, CD45 positivity [30].
A recent study defined EPCs using another combination of markers; either CD34 or CD133 positive cells plus positivity of either KDR or CD146 and showed that obese adolescents had elevated EPC counts so they speculated that there is an endothelial “activation” to compensate for and to repair the damage [31]. The discrepancy between the reduced EPC count in our study and the elevated count found by some authors could be explained by their assessment of both subtypes of endothelial progenitors; the CD34-/CD133+ as well as the CD34+/CD133+. The CD34-/ CD133+ cells were shown to be upregulated in reponse to impaired perfusion unlike the CD34 positive cells [31,32]. Thus, analysing both types of cells may push the results into the gap of endothelial progenitor cell heterogeneity.
To further resolve this conflict, we searched the mechanistic insights gained from animal studies and, in line with our data, animal experiments showed that mice given obesogenic diet for only 20 weeks had marked reduction of their EPC count due to defective nitric oxide production which is an important trigger for EPC release [27]. Others have demonstrated the scarcity of EPCs in the early stages of adipogenesis concluding that obesity is associated with early suppression of EPC number which triggers the associated metabolic derangements [33]. Only few studies addressed this cell population in paediatrics, and a search for definite markers for the EPCs has been done by some authors [31,32,34,35]. Determination of limited clear markers for this cell population is warranted to enhance validation of further research as well as increase the application of the use of EPC count in clinical practice at a reasonable cost.
This reduction in EPC count was not related to the state of dyslipidaemia or hyperinsulinaemia as it was demonstrated in all the randomly included patients which signifies the detrimental effects of obesity on vascular health whether it was associated with atherogenic dyslipidaemia and/or insulin resistance or not.
Statins increased the number of EPCs in obese males by upregulation of endothelial nitric oxide, a finding which favours the inclusion of the cut-off found in this study as a marker to be evaluated before and after statin therapy in obese children and adolescents [30,36].
EPCs didn’t show a significant correlation with the CIMT which may indicate that the reduction in their number may be an earlier step than the structural changes seen in the carotid intima in the process of atherosclerosis and may call for the assessment of the EPC count instead of measuring the CIMT particularly in children. Studying children and adolescents with obesity may give us some insight into the earlier stages of the evolution of obesity related atherosclerosis and it has been shown in this study that the earliest pathologic change, which is the reduced EPC number, is present even in the absence of other associated risk factors such as dyslipidaemia and hyperinsulinaemia. In the current study patients with and without insulin resistance had similar levels of EPCs and this may suggest that at the earlier end of the spectrum, obesity in children needs to have further mechanistic insights as to why all obese children had sharply demarcated decline in their endothelial regenerative capacity that is not related to insulin resistance as demonstrated in adults. Another explanation for this may reside in the differential assessment of the different clusters of differentiation on cells in different studies. This study demonstrated that the assessment of the CD34, CD144 positive cells was advantageous to be clearly differentiating patients from controls.
We explored the relation of EPCs to some other newly described cardiovascular risk factors namely the waist height ratio, triglyceride/HDL ratio and cardiometabolic index which equals the waist/height ratio X the TG/HDL ratio but none of them had significant correlations with the measured EPCs [37-39].
We checked the relationship of the EPCs to the structural cardiac changes that may be taking place in the obese subjects as well as to the systolic and diastolic functions on TDI to avoid the confounding effect of the increased plasma volume caused by obesity on the cardiac loading conditions [40]. When compared to the controls, patients showed diastolic dysfunction in the form of decreased E wave at the mitral annulus [Em], increased A wave at the mitral annulus [Am] and decreased Em/Am ratio which are the earliest manifestations of obesity cardiomyopathy [41]. Those changes could be attributed to adipokines secreted by excess adipose tissue which promote chronic inflammation, insulin resistance leading to an increase in cardiac mass, fibrosis, mitochondrial dysfunction which attenuate cardiac diastolic function and result in cardiovascular remodeling [42]. Patients had decreased systolic function at the mitral annulus [Sm], when compared to the control group, although the ejection fraction was in the normal range denoting early systolic dysfunction in patients [43]. This subtle dysfunction may result from insulin resistance which could lead to attenuated inotropic activity, fibrosis, deposition of fat globules, decreased glucose uptake and diminished mitochondrial respiration hindering the normal function of the myocytes [44]. Thus, the same inflammatory events that are implicated in the reduction of the EPC number associating obesity could lead to cardiac dysfunction [27]. Patients in this study showed evidence of structural cardiac changes in the form of increased left ventricular mass index, and left atrial diameter which agreed with previous studies [45]. Increased left atrial diameter and volume can be secondary to the increased filling pressure and could be viewed as an indicator of diastolic dysfunction [43].
EPCs showed a significant negative correlation with LVEDD. The increase in LVEDD is one of the known complications of longstanding obesity due to the chronic volume overload [46]. Patients included in this study were not significantly different from controls regarding the LVEDD indicating an earlier stage of obesity associated cardiomyopathy which is supported by the TDI data showing early diastolic dysfunction and subclinical systolic dysfunction. Therefore, we suggest that the reduction of EPCs was associating the earliest stages of obesity cardiomyopathy and therefore, the reduced EPC number could be used as a surrogate marker for detection of patients at risk of this type of cardiomyopathy. The timing and occurrence of such abnormalities need to be addressed by further longitudinal follow up of patients who remain non-compliant to the dietary advice and reluctant to lose weight in which case the EPC number is expected to remain suppressed.
To the best of our knowledge, this study was the first to clarify a sharply demarcated cutoff in obese children and adolescents regarding the EPCs using only CD34 and CD144 co-expression. We recommend using the absolute cell count per million WBCs in measuring the EPCs as it showed better sensitivity and specificity than using their percent of the mononuclear cells. This study also directly assessed the cardiac remodeling and its relation to the diminished endothelial repertoire in obesity as well as the tissue Doppler changes.
The clear-cut reduction in CD34, CD144 positive cells in obese children and adolescents may negotiate or even negate the recently described healthy obese phenotype which is defined by the absence of metabolic complications of obesity [47]. The study of EPC number may represent an open window through which we can inspect the cardiovascular health status of the individual as the reduction in their count is one of the important early steps of vascular dysfunction in obesity and may set a target for early therapeutic interventions to reduce the increased risk of cardiovascular death associated with obesity.
References
- Fadini GP, Agostini C, Boscaro E, et al. Mechanisms and significance of progenitor cell reduction in the metabolic syndrome. MetabSyndrRelatDisord 2009; 7:5-10.
- Patel RS, Li Q, Ghasemzadeh N, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res 2015; 116:289-297.
- GłowińskaOB, Moniuszko M, Hryniewicz A, et al. Relationship between circulating endothelial progenitor cells and endothelial dysfunction in children with type 1diabetes: a novel paradigm of early atherosclerosis in high-risk young patients. Eur J Endocrinol 2013; 168:153-161.
- Yoder MC. Defining human endothelial progenitor cells. J ThrombHaemost 2009; 7:49-52.
- Yi CX, Gericke M, Krüger M, et al. High calorie diet triggers hypothalamic angiopathy. MolMetab 2012; 1:95-100.
- Falaschetti E, Hingorani AD, Jones A, et al. Adiposity and cardiovascular risk factors in a large contemporary population of pre-pubertal children. Eur Heart J 2010; 31:3063-3072.
- Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005; 353:999-1007.
- Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 2000; 105:311-320.
- Dei CA, Spigoni V, Ardigò D, et al. Reduced circulating endothelial progenitor cell number in healthy young adult hyper-insulinemic men. NutrMetabCardiovasc Dis 2011; 21:512-517.
- Jialal I, Fadini GP, Pollock K, Devaraj S. Circulating levels of endothelial progenitor cell mobilizing factors in the metabolic syndrome. Am J Cardiol 2010;106:1606-1608.
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983; 67:968-977.
- http://www.who.int/growthref/who2007_bmi_for_age/en/
- Tanner JM, Whitehouse RH, Takaishi M. Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children, 1965. I. Arch Dis Child 1966; 41:454-471.
- Sempe M, Pedrong G, Roy-Ternot NP. Auxologie le methods et sequences. Theraplix Paris. 1979.
- Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK 1990. Arch Dis Child 1995; 73:25–29
- Schwandt P, Kelishadi R, Haas GM. First reference curves of waist circumference for German children in comparison to international values: the PEP Family Heart Study. World J Pediatr 2008; 4:259-266.
- Mederico M, Paoli M, Zerpa Y, et al. Reference values of waist circumference and waist/hip ratio in children and adolescents of Mérida, Venezuela: comparison with international references. EndocrinolNutr 2013; 60:235-242.
- Moreno LA, Fleta J, Mur L, et al. Waist circumference values in Spanish children--gender related differences. Eur J ClinNutr 1999; 53:429-433.
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44:291-303.
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45:13-23.
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004; 114 (2 Suppl 4th Report):555-576.
- Salgado AL, CarvalhoLd, Oliveira AC, et al. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. ArqGastroenterol 2010; 47:165-169.
- Dursun B, Dursun E, Suleymanlar G, et al. Carotid artery intima-media thickness correlates with oxidative stress in chronic haemodialysis patients with accelerated atherosclerosis. Nephrol Dial Transplant 2008; 23:1697-1703.
- Yu CM, Lin H, Ho PC, et al. Assessment of left and right ventricular systolic and diastolic synchronicity in normal subjects by tissue Doppler echocardiography and the effects of age and heart rate. Echocardiography 2003; 20:19-27.
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128 Suppl 5:S213-56.
- Yin J, Li M, Xu L, et al. Insulin resistance determined by Homeostasis Model Assessment (HOMA) and associations with metabolic syndrome among Chinese children and teenagers. DiabetolMetabSyndr 2013; 5:71.
- Tsai TH, Chai HT, Sun CK, et al. Obesity suppresses circulating level and function of endothelial progenitor cells and heart function. J Transl Med 2012; 10:137.
- Bai H, Liu Y, Xie Y, et al. Definitive hematopoietic multipotent progenitor cells are transiently generated from hemogenic endothelial cells in human pluripotent stem cells. J Cell Physiol 2016;231:1065-1076.
- Müller-Ehmsen J, Braun D, Schneider T, et al. Decreased number of circulating progenitor cells in obesity: beneficial effects of weight reduction. Eur Heart J 2008; 29:1560-1568.
- Westerweel PE, Visseren FL, Hajer GR, et al. Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy. Eur Heart J 2008; 29:2808-2817.
- Pires A, Martins P, Paiva A, et al. Circulating endothelial progenitor cells in obese children and adolescents. J Pediatr (Rio J) 2015;91:560-566.
- Jung C, Fischer N, Fritzenwanger M, et al. Endothelial progenitor cells in adolescents: impact of overweight, age, smoking, sport and cytokines in younger age. Clin Res Cardiol 2009; 98:179-188.
- Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J 2004; 18:983-985.
- Fadini GP, Baesso I, Albiero M, et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis 2008; 197:496-503.
- Mund JA, Case J. The ontogeny of endothelial progenitor cells through flow cytometry. CurrOpinHematol 2011; 18:166-170.
- Laufs U, La Fata V, Plutzky J, et al. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998; 97:1129-1135.
- Cai L, Liu A, Zhang Y, et al. Waist-to-Height Ratio and Cardiovascular Risk Factors among Chinese Adults in Beijing. PLoS One 2013; 8: e69298.
- Quijada Z, Paoli M, Zerpa Y, et al. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr Diabetes 2008; 9:464-471.
- Wakabayashi I, Daimon T: The "cardiometabolic index" as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. ClinChimActa 2015; 438:274-278.
- Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am CollCardiol 2004; 43:1399-1404.
- Di BV, Santini F, Di CA, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am SocEchocardiogr 2006; 19:1063-1071.
- Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 2009; 119:1272e1283.
- Dahiya R, Shultz SP, Dahiya A, et al. Relation of reduced preclinical left ventricular diastolic function and cardiac remodeling in overweight youth to insulin resistance and inflammation. Am J Cardiol 2015; 115:1222-1228.
- Hill DJ, Milner RD: Insulin as a growth factor. Pediatr Res 1985; 19:879e886.
- Chinali M, de Simone G, Roman MJ, et al. Impact of obesity on cardiac geometry and function in a population of adolescents: the Strong Heart Study. J Am CollCardiol 2006;47:2267e2273.
- Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 2001; 321:225-236.
- Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 2013; 36:2294-2300.