Research Article - Journal of Gastroenterology and Digestive Diseases (2025) Volume 10, Issue 3
Endoscopic evaluation of gastric mucosa to the H. pylori infection status in the recent advances.
Shinwari Abdullah Jan*, Hashimi Sayed Zekria
Department of Medicine, Nangarhar University, Nangarhar, Afghanistan
*Corresponding Author:
- Shinwari Abdullah Jan
- Department of Medicine,
- Nangarhar University,
- Nangarhar, Afghanistan
E-mail: abdullahjan07794@gmail.com
Received: 28-Jan-2024, Manuscript No. JGDD-24-126161; Editor assigned: 31-Jan-2024, JGDD-24-126161 (PQ); Reviewed: 14-Feb-2024, QC No. JGDD-24-126161; Revised: 11-
Feb-2025, Manuscript No. JGDD-24-126161 (R); Published: 18-Feb-2025, DOI: 10.35841/jgdd-10.1.241
Citation: Jan SA, Zekria HS. Endoscopic evaluation of gastric mucosa to the H. pylori infection status in the recent advances. J Gastroenterol Dig Dis. 2025;10(1):241
Abstract
Background: H. pylori infection has been recognized as a type 1 carcinogen of the gastric malignancy, and early diagnosis and treatment are very important for its eradication. Recent findings have also shown that atrophy and intestinal metaplasia remain after successful eradication, which moderately increases the risk of gastric cancer compared to those who have never infected, therefore, evaluation of the gastric mucosa during gastroscopy is useful.
Aims: The purpose of this review is to identify and summarize the reliable literature and proposed features of H. pylori status and gastritis in studies conducted on newly developed endoscopic models that influence clinical practice. Therefore, conventional white light endoscopic, image- enhanced endoscopic models and studies related to the Kyoto classification were searched and reviewed.
Results: The conventional white light endoscopic Kyoto classification modified model is a good model and method for evaluation of H. pylori current, past, and no infection status due to worldwide use, low-cost, and time-efficient with new findings. Advanced image enhanced endoscopic models such as NBI, LCI and BLI combine use with magnifying endoscopy provide a relatively cleared endoscopic features for H. pylori infection status and early gastric cancer.
Conclusion: According to H. pylori infection status, endoscopic prediction of gastric mucosal surface architecture analysis is possible, which influences clinical management. Endoscopic models might lead us to easier diagnose and treatment of H. pylori infection and be useful not only in the eradication of H. pylori infection but also in the diagnosis of early gastric cancer.
Keywords
Endoscopic appearance, H. pylori infection status, Kyoto classification, Endoscopic models, Conventional white light endoscopy, Image enhanced endoscopy.
Introduction
Newly developed models of endoscopic examination can detect the atrophy and intestinal metaplasia of the gastric mucosa that may persist after eradication of H. pylori infection and increase the risk of gastric cancer relative to those never infected. Accordingly, during endoscopy, evaluation of the gastric mucosa for H. pylori, along with other diagnostic tests such as UBT, SAT and RUT, is important not only for the diagnosis of H. pylori but also for pre-cancerous gastric lesions. The prevalence of H. pylori infection is around 50% worldwide and is the leading cause of chronic gastritis and is classified as class I carcinogen by World Health Organization and International Agency for Research of cancer. Gastric cancer is a fatal disease that only one in five people can survive for more than five years [1]. Most of gastric adenocarcinomas particularly of intestinal type is associated with phenotypic changes that result from chronic inflammation induced or initiated by H. pylori infection [2].
In addition to earlier WLI endoscopy, several new advanced endoscopic imaging modalities have been developed for the non-differentiated diagnosis of gastric malignancies. In current clinical practice using Magnifying Endoscopy (ME) and Image Enhanced Endoscopy (IEE) are necessary and useful to detect and describe the lesion with histologic features accurately [3].
In WLI endoscopy, images of the superficial gastric mucosa are presented only in gross form [4]. But in Image Enhanced Endoscopy (IEE), Magnifying Endoscopy (ME), Narrow Band Imaging (NBI) and Blue Laser Imaging (BLI) visualize also superficial vessels and structure of mucosal membrane along with diagnosis of H. pylori status and neoplastic lesions better than conventional white light endoscopy [5,6].
The objective of this review is to summarize the gastric mucosal imaging data presented in the CWLI and IEE endoscopic models to the status of H. pylori infection and demonstrate relevance in clinical practice. Therefore, CWLI, IEE models and related studies of the Kyoto classification were searched and reviewed.
Materials and Methods
Diagnostic performance of H. pylori infection in various endoscopic techniques and clinical applicability
Each endoscopic model is implemented to identify H. pylori infection according to the specific endoscopic features, nevertheless some endoscopic patterns are used to exclude H. pylori infection.
Conventional white light image endoscopy in clinical practice
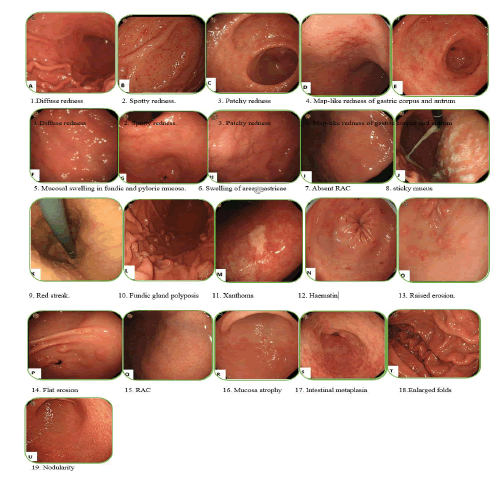
In 2013, Japan gastroenterological endoscopy society developed a series of nineteen CWLI endoscopic findings of chronic gastritis in the title “Kyoto classification’’ for the evaluation of gastric cancer risk and H. pylori infection status (Figure 1). Sticky mucus, diffuse redness, spotty redness, mucosal swelling, enlarged/tortuous fold, nodularity, xanthoma and hyperplastic polyp demonstrate current H. pylori infection. Normal mucosal appearance of the antrum, angle and body, presence of Regular Arrangement of Collecting venules (RAC), Fundic Gland Polyp (FGP), red streak and Hematin demonstrate absence or negative H. pylori infection. Regression of spotty redness indicates eradicated form of H. pylori infection. Mucosal atrophy, intestinal metaplasia, enlarged folds and nodularity have been suggested for precancerous status of gastric mucosa. Gastrointestinal mucosal swelling and redness have been demonstrated endoscopically for H. pylori-induced inflammation [7]. Diffuse redness like mucosal swelling is a key finding in the current H. pylori infection, which is clearly related to the degree of neutrophils and mononuclear cells infiltration caused by H. pylori infection [8,9].
Figure 1. Conventional endoscopic finding of Kyoto classification for chronic gastritis [10].
A Regular Arrangement of Collecting venules (RAC) is a starfish-like appearance seen on close endoscopic examination of the gastric body with WLI endoscopy, especially in young adults, which is characteristic feature of H. pylori negative or uninfected form, first time reported by Yagi, et al. In 2005 and correct or ideal location is in the lower part of lesser curvature, Hidaka, et al. demonstrated 100% and 90% sensitivity and specificity respectively [11]. Nevertheless specific view can be found for negative status of. H pylori infection in gastric antrum with standard endoscopy, therefore Magnifying endoscopy is a good model [12]. H. pylori-infected and inflamed mucosa is characterized by continuous mucosal breakdown and vascular remodeling due to severe inflammation, suggesting this mucosal changing appearance are essential for successfully eradicated infection [13,14]. The density of irregular small vessels decreases and RAC may reappear, which is caused by persistent inflammation in the atrophic mucosa, however these findings are not common for previous or past infection.
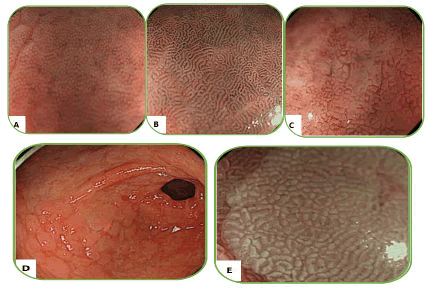
Atrophy caused by H. pylori infection, of course, refers to current infection, that gradually disappears after the eradication but, usually persists even though Its boundaries are unclear, therefore, individually atrophy is not a unique feature of current and eradicated infection [15,16]. In some previous studies, raised erosion and multiple white and smooth elevated lesions are suggested for previous infection. In 2010, Sheng Lei Yan et al. evaluated H. pylori infection in the gastric mucosa by standard endoscopy and divided it into four types as shown in Table 1. They found that standard endoscopy could detect H. pylori infection in the gastric mucosa without atrophy [17].
| Type | Appearances definition | ||||
| Type 1 | Cleft-like appearance | 1 and 2 types patterns are statistically significant in predicting H. pylori negative status. | |||
| Type 2 | Regular arrangement of red dots | ||||
| Type 3 | Mosaic mucosal pattern | 3 and 4 types patterns are statistically significant in predicting a H. pylori positive status. | |||
| Sensitivity | Specificity | PPV | NPV | ||
| 100% | 86% | 94% | 100% | ||
| Type 4 | Mosaic pattern with a focal area of hyperemia | ||||
Table 1. Four type gastric body mucosa in WLI endoscopic model.
Takuma, et al. illustrated over all gastric mucosal abnormal lesion’s sensitivity, specificity, PPV, NPV and accuracy of 93.3%, 89.1%, 92.3%, 90.6% and 91.6%, respectively.Carefully Observation of the gastric mucosa with standard endoscopy can represent H. pylori status [18].
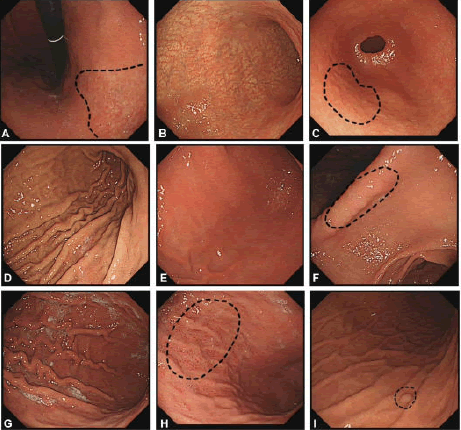
Recently, the Kyoto classification has been revised and a Kyoto scale score has been developed that consists of five endoscopic findings and eight points. Atrophy, intestinal metaplasia, enlarged folds, and nodularity evaluate gastric cancer risk, and red color and RAC appearance indicate H. pylori infection status Figure 2. A score of zero indicates the absence of H. pylori infection, 2 or higher score suggest H. pylori infection, and 4 or higher predicts the risk of developing gastric cancer (Table 2) [19]. The prevalence of H. pylori infection of Kyoto scale scores in these three 0, 1 and ≥ 2 point groups was 1.5%, 45% and 82%, respectively.
| Kyoto gastritis classification | Score | Descriptions |
| Atrophy | Kimura-Takemoto classification of endoscopic atrophy | |
| None, C1 | 0 | C1=Atrophy is limited to the antrum. |
| C2=Atrophy is limited to the minor area of the lesser curvature of the body. | ||
| C2 and C3 | 1 | C3=Atrophy exists in the major area of the lesser curvature of the body but does not extend beyond the cardia. |
| O1=Atrophy extends to the fundus over the cardia. Atrophic border of the body lies between the lesser curvature and anterior wall. | ||
| O2=Atrophic border of the body lies on the anterior wall. | ||
| O1-O3 | 2 | O3=Atrophy is widespread with the border between the anterior wall and greater curvature. |
| Intestinal metaplasia | ||
| None | 0 | |
| Confined to the antrum | 1 | |
| Extending to the gastric body | 2 | |
| Hypertrophy of the gastric fold | ||
| Absence (<5 mm) | 0 | |
| Presence (≥ 5 mm) | 1 | |
| Nodularity | ||
| Absence | 0 | |
| Presence | 1 | |
| Diffuse redness | ||
| None | 0 | |
| Mild (RAC can be seen in gastric body) | 1 | |
| Severe (RAC disappeared in gastric body) | 2 | |
| Total Kyoto scale | 0-8 | |
Table 2. Modified Kyoto scale.
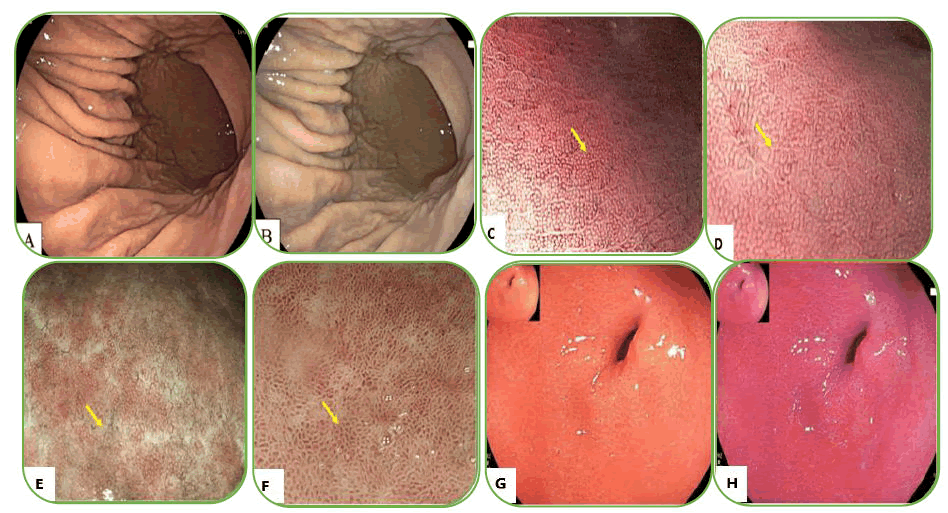
Figure 2. Endoscopic manifestations of the gastric mucosa. A) C-2 type atrophy; B) O-2 type atrophy; C) Nodularity; D) Hypertrophy of the gastric fold; E) Mild diffuse redness with blurry Regular Arrangement of Collecting venules (RAC); F) Severe diffuse redness with disappearing RAC; G) Sticky mucus; H) Spotty redness of the gastric fundus and body; I) Fundic gland polyp.
It was also confirmed by Yoshii, et al. [20]. Consistent with previous studies, RAC negative or non-infectious status (OR=32) and diffuse redness (OR=26.8) are prominent endoscopic features for current H. pylori infection and 82.9% is the overall diagnostic accuracy rate of H. pylori infection on the Kyoto scale. Two newly defined features, Unclear Atrophic Boundaries (UAB) and RAC reappearance, have been added to the Kyoto classification, and provide evidence for the clinical accuracy of H. pylori gastritis and strengthen the Kyoto classification. They assessed H. pylori infection in three states: Current, past, and uninfected. Three findings; unclear atrophic boundary, map-like redness and reappearance of RAC suggest past or eradicated infection. If one of these three findings is positive, it confirms past infection. Nodularity, diffuse redness and mucosal swelling predict current infection and have a high ROC/AUC (0.726). The presence of a RAC pattern is highly specific for the uninfected state. Furthermore Diffuse and map like redness suggested for the past or eradicated H. pylori infection. H. pylori infection status association to the gastric mucosal standard endoscopic features based on the recent studies are summarized in Table 3.
|
|
|
Odds ratio |
Sensitivity |
Specificity |
|
|
(24)
|
Mucosal oedema |
18.1 55 |
63.70% |
91.10% |
Predictor of H. pylori positive infection |
|
(23)
|
Nodularity |
11.7 |
Combined feature |
Combined feature |
Predictor of H. pylori positive infection |
|
MAP-like redness |
7.78
|
Combined feature |
Combined feature |
Predictor of H. pylori infection |
|
|
Hematin |
|
Combined feature |
Combined feature |
Predictor of H. pylori no infection |
|
|
(25) |
Diffuse redness Mucosal swelling |
|
|
|
Predictor of H. pylori positive infection |
|
Map-like redness |
|
|
|
Predictor of H. pylori infection Eradication |
|
|
RAC |
|
78.40% |
64.3 |
Predictor of H. pylori no infection |
Table 3. Summary of gastric mucosal appearance at standard endoscopy according to different status of H. pylori infection in recent studies.
Gastric distal part RAC appearance; diffuse redness and mucosal swelling; map-like redness are strongly suggest current and eradicated H. pylori infection respectively. Careful observation of mucosal atrophy and swelling in mildly atrophic patients may conform the diagnosis and treatment can potentially prevent risk of long-term carcinogenesis. Kaplan Meier analysis illustrated a significantly higher rate of cancer detection in current or cleared H. pylori infection than in those with never infected. In the conclusion, the new endoscopic technology with accurate and real-time practice of WLI endoscopy can implement for detection of H. pylori infection and pre-cancerous lesion.
Results
Image enhanced endoscopy models in clinical practice
Magnifying Endoscopy (ME), Narrow-Band Imaging (NBI), Linked Color Imaging (LCI) and Blue Laser Imaging (BLI) are known as IEE models that enhance the observation of gastric mucosa and mucosal surface vascular structure and have better role in the detection of H. pylori infection and pre-cancerous lesions of gastric mucosa than WLI endoscopic model. Advanced endoscopic imaging especially magnifying mode improve the observation and examination of gastric mucus membrane. Mucosal swelling in H. pylori associated gastritis caused by neutrophils and mononuclear cells infiltration. Vascular destruction and increasing irregular vessels causing gastric pits enlargement or elongation and inflammation disappear collecting venules.
Magnifying Endoscopy (ME)
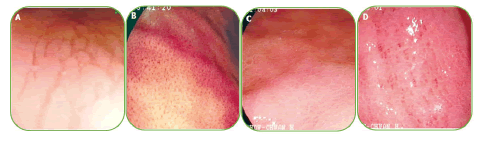
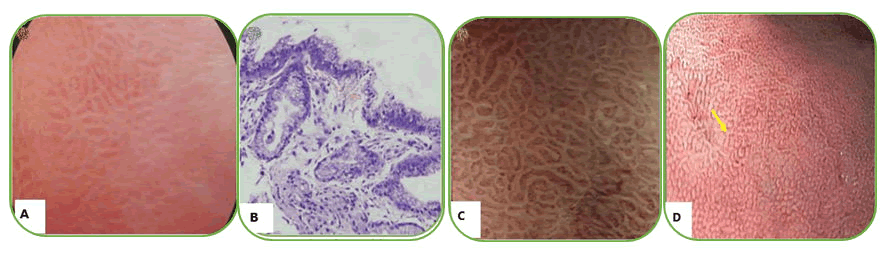
In this kind of endoscopic method, the image area is enlarged and as a result, the structures of the gastric mucous membrane can be observed in detail. The resolution of most magnifying endoscopes is less than 10 µm. ME during real-time endoscopy enables the observation of gastric mucosa at a magnification of 80-120 times. Membrane micro surface structure and microvascular architecture or network surrounding the gastric pit can be seen in detail. With the zoomed or magnifying endoscopic technique, the normal view of the fundus mucous glands with pit patterns and vascular details can be observed. In this part of the stomach glands, there are fixed, round or oval (mouth-like) crypt openings that look pin-like dark spots in the central part of these glands. These crypts are surrounded by Subepithelial Capillary Networks (SECNs) and give a honeycomb-like appearance. ME strength the relation between H. pylori associated gastritis and histological findings and a good interobserver agreement has been also reported. H. pylori infection becoming consistent or unchangeable chronic inflammation of gastric mucosa and infiltrated cells, degenerated epithelium and disruption of microvascular system disappear obvious features of collecting venules. Yagi, et al. develop a Yagi’s classification for the assessment of stomach mucus membrane by ME. Accordingly, Z0 demonstrate normal mucosal collecting venules and true capillaries that surrounded the pit as a network with 93.8% sensitivity and 96.2 specificity. Other three types Z1, Z2 and Z3 indicates H. pylori infected mucosa. In this manner Nakagawa’s classification divide the gastric mucosa in three pattens through the RAC morphology; R=Regular, I=Irregular and O=Obscured patterns. R pattern demonstrate non infected status of H. pylori with 63.9% and 100% sensitivity, specificity respectively. Kawamura, et al. focused on the crypts opening white mucosal membrane and divided it on white pit and dens white pit. White and dense white pit predict H. pylori infection that have 81.7%, 78.5% sensitivity and specificity respectively (Table 4). Anagnostopoulos, et al. gastric mucosa divided in four type by magnifying endoscopy in western population and identified that high resolution magnifying endoscopy is better and high model for diagnosis of H. pylori related gastritis than standard endoscopy (Figures 3 and 4).
| Type | Appearances | Sensitivity | Specificity |
| Type 1 | Regular arrangement of collecting venules and regular, round pits | ||
| Type 2 | Regular, round pits, but loss of collecting venules | For predicting H. pylori infection in type 2 and 3 | |
| Type 3 | Loss of normal collecting venules, with enlarged white pits surrounded by erythema | 100% | 92.70% |
| Type 4 | Loss of normal round pits, with irregular arrangement of collecting venules | 90% | 96% |
| Corresponded to atrophic gastritis |
Table 4. Gastric mucosal type in Western population.
Figure 3. (A) Type 1 mucosal pattern showing cleft-like appearance; (B) Type 2 mucosal pattern showing regular arrangement of red dots; (C) Type 3 mucosal pattern showing mosaic appearance; (D) Type 4 mucosal pattern showing mosaic appearance with focal area of hyperemia.
Figure 4. (B) Helicobacter pylori (H. pylori)-associated gastritis appears loss of the normal SECN and collecting venules, with enlarged white pits surrounded by erythema; C: Corresponding histologic features; (D) H. pylori-positive gastric mucosa is characterized by enlarged or elongated, varies in sized and shaped of pits with unclear SECNs in NBI; (C) Helicobacter pylori (H. pylori)-associated gastritis appears loss of the normal SECN and collecting venules, with enlarged white pits surrounded by erythema.
Magnifying with Narrow Band Imaging Endoscopy (ME-NBI)
Observation of gastric pit and vascular patterns in the gastric body with ME-NBI is useful in chronic H. pylori-associated gastritis. These findings include:
• Absent or disorganized collection of venules.
• Indistinct or irregular gastric pits.
• Disappearance of normal SECN (Figure 5).
A meta-analysis performed on six studies of ME-NBI H. pylori infection demonstrated 96% sensitivity and 91% specificity. The sensitivity, specificity, PPV and NPV of ME-NBI were found to be 100%, 92.7%, 83.8% and 100%, respectively, by Qi, et al. ME-NBI also illustrated high sensitivity (79%) and specificity (81%) in other studies.
Figure 5. ME-NBI findings of stomach mucosa according to location and Heliciobacter pylori infection status. (A) Fundal mucosa without H. pylori infection; (B) Antral mucosa without H. pylori infection; (C) Fundal mucosa with H. pylori infection. ME-NBI finding in intestinal metaplasia featuring white opaque substance. (A) White light endoscopy; (B) ME NBI. ME-NBI, magnifying endoscopy with narrow band imaging.
Magnifying Endoscopy with Blue Light Imaging (ME BLI)
Due to dark field patterns BLI technology is limited. However, BLI present clear mucosa and vascular structures appearance with high contrast laser light system. As ME-NBI ME-BLI is effective model for differentiation of H. pylori gastritis and have high sensitivity 98% and specificity 92%.
Linked Color Imagin (LCI)
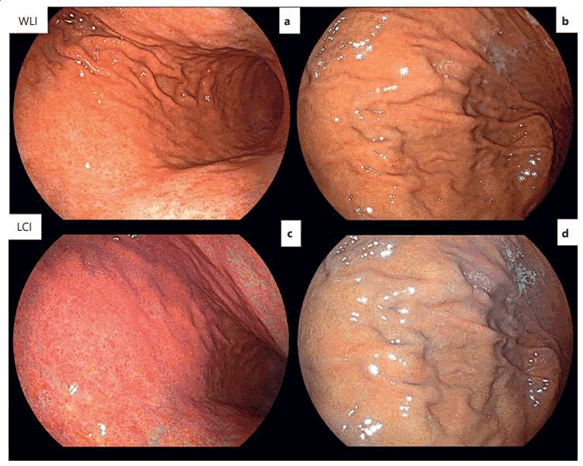
The color contrast of LCI is twice that of WLI and also in recent studies it has been reported that the ability of LCI in differentiating abnormal lesions is three times more than that of WLI. A study in Japan identified positive H. pylori gastric mucosa more accurately than WLI and had a sensitivity and specificity of 93.8% and 78.3%, respectively (Figure 6).
Figure 6. Endoscopic diagnosis of H. pylori-gastritis with WLI and LCI. A) Diffuse redness in WLI (active gastritis); B) Without diffuse redness in WLI (inactive gastritis); C) Crimson coloring in LCI (active gastritis); D) Apricot coloring in LCI (inactive gastritis). WLI, white light imaging; LCI, linked color imaging.
A randomized prospective comparative study using WLI, LCI, NBI and BLI techniques simultaneously for the diagnosis of H. pylori status between 220 and 2021 was conducted at Thamsat University Hospital, Thailand. Endoscopic appearance related to H. pylori presented as follows: diffuse redness, enlarged gastric folds and sticky mucus indicate current infection and the presence of RAC predict negative or uninfected status, that RAC having a high Negative Predictive Value (NPV). They also identified that IEE will improve diagnostic performance of H. pylori infection than WLI.
Spotty hemorrhage and diffuse redness in the fundus strongly suggest the presence of a positive H. pylori infection. IEE, especially the ME-BLI model, can improve the clear visualization of the mucosal and microvascular structures, which is important for evaluation of H. pylori infection status and precancerous lesions (Figure 7). Development of endoscopic techniques can reflect histologic patterns of endoscopic images during endoscopy for the real time diagnosis H. pylori infection.
Figure 7. (A) H. pylori-negative infection gastric mucosa in WLI; (B) Light orange/white apricot gastric mucosa in LCI; (C) H. pylori-negative gastric mucosa is characterized by homogeneous, round pits with regular honeycomb-like SECNs in NBI; (D) H. pylori-positive gastric mucosa is characterized by enlarged or elongated, varies in sized and shaped of pits with unclear SECNs in NBI; (E) H. Pylori-negative gastric mucosa is characterized by homogeneous, round pits with regular honeycomb-like SECNs in BLI; (F) H. pylori-positive gastric mucosa is characterized by enlarged or elongated, varied in sized and shaped of pits with unclear SECNs in BLI; (G) Diffuse redness in gastric antrum in WLI; (H) Diffuse redness in gastric antrum (deep red color) in LCI.
Artificial Intelligence (AI)
A large prospective randomized controlled study in Japan compared AI and expert endoscopists for the accuracy of diagnosis of H. pylori infection. A total of 32,208 endoscopic images from eight sections of the stomach were classified for H. pylori positive and negative status. In the result they identified that AI has higher sensitivity and specificity than endoscopists (81.9%, 83.4% vs. 79% 83.2%). Another experimental study in Japan compared BLI-bright LCI and WLI with AI, and the AUC of AI-BLI-bright and AI-LCI were 0.96 and 0.95, respectively, and the AUC of WLI was 0.66. AI is considered a strong focus method on the global health development system, therefore, studies of AI endoscopic models are increasing and trying to be applied together with IEE models to improve the endoscopic diagnosis of H. pylori infection status. The advantages of AI are providing a second opinion, finding some important features during endoscopy, reducing time consumption, and requiring less training and experience. In the future, AI may become the best diagnostic method for H. pylori infection (Table 5).
| Endoscopic method | Sensitivity | Specificity | PPV | NPV | Total Dx accuracy for HP | |
| WLI | 90.00% | 70.00% | 66.70% | 91.30% | 78.00% | |
| LCI | 95.00% | 76.70% | 73.10% | 95.80% | 84.00% | |
| BLI | 95.00% | 80.00% | 76.00% | 96.00% | 86.00% | |
| NBI | 85.00% | 80.00% | 73.90% | 88.90% | 82.00% | |
| WLI | 32.40% | 93.30% | 85.20% | 53.60% | 70.80% | |
| LCI | 57.40% | 91.30% | 88.70% | 64.30% | 78.8% | |
| LCI | 83.80% | 99.50% | ||||
| Corpus LCI | 85.41% | 79.71% | 59.42% | 94.02% | ||
| Current infection | Past infection | |||||
| WLI | 84.40% | 74.60% | 79.50% | 36.80% | ||
| LCI | 84.40% | 88.90% | 86.60% | 78.90% | ||
| LCI | 78.38% | 70.97% | 82.50% | 59.46% | 87.84% | |
| MI | 81.98% | 81.25% | 83.87% | 64.10% | 91.67% | |
| M-LCI | 78.38% | 80.65% | 76.25% | 57.78% | 92.42% | |
| NBI | 97% | 81% | 87% | 95% | ||
| BLI | 98% | 92% | 93% | 98% | ||
| M-BLE | 98% | 92% | ||||
| M-NBI | 100% | 92.7% | 83.80% | 100% | ||
| M-NBI | 96% | 91% | ||||
|
AI |
A reliable tool for endoscopic diagnosis of H. pylori infection. |
Lacking external validation performance and being conducted only in Asia should be overcome |
||||
|
Note: BLI: Blue Light Imaging; CI: Confidence Interval; LCI: Linked Color Imaging; NBI: Narrow-Band Imaging; M-BLE: Magnifying with Blue Light Imaging; M-LCI: Magnifying with Linked Color Imaging; M-NBI: Magnifying with Narrow-Band Imaging; NPV: Negative Predictive Value; PPV: Positive Predictive Value; WLI: White Light Imaging. |
||||||
Table 5. Sensitivity, specificity, PPV, NPV and total diagnostic accuracy of Helicobacter infection in different endoscopic models.
Endoscopic features of H. pylori infection status in various models
The gastric mucosal features of H. pylori positive, negative, and eradicated forms in different endoscopic models are summarized in Tables 6-8 and Figure 8, respectively.
| Endoscopic models | H. pylori-positive gastric mucosa | ||
| Endoscopic features | Sensitivity | Specificity | |
| WLI | Diffuse redness | 57.50% | 95.80% |
| Antral nodularity | 100% | 100% | |
| Spotty hemorrhage at fundus | 61.00% | 95.80% | |
| Enlarged gastric folds | 60.10% | 92.20% | |
| Sticky tenacious mucus | 53.30% | 95.10% | |
| Xanthoma | 11.20% | 98.00% | |
| LCI | Diffuse redness (deep red color) | 93.30% | 78.30% |
| Antral nodularity | 25% | 100% | |
| Spotty hemorrhage at fundus | 50% | 100% | |
| Enlarged gastric folds | 15% | 100% | |
| Sticky tenacious mucus | 5% | 100% | |
| Xanthoma | 5% | 100% | |
| NBI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 97.00% | 81.00% | |
| BLI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 98.00% | 92.00% | |
| Note: BLI: Blue Laser Imaging; H. pylori: Helicobacter pylori; LCI: Linked Color Imaging; NBI: Narrow-Band Imaging; WLI: White Light Imaging. | |||
Table 6. Summary of H. pylori-positive gastric mucosal endoscopic features in various models.
| Endoscopic models | H. pylori-eradicated gastric mucosa | ||
| Endoscopic features | Sensitivity | Specificity | |
| WLI | Diffuse redness | 57.50% | 95.80% |
| Antral nodularity | 100% | 100% | |
| Spotty hemorrhage at fundus | 61.00% | 95.80% | |
| Enlarged gastric folds | 60.10% | 92.20% | |
| Sticky tenacious mucus | 53.30% | 95.10% | |
| Xanthoma | 11.20% | 98.00% | |
| LCI | Diffuse redness | 93.30% | 78.30% |
| Antral nodularity | 25% | 100% | |
| Spotty hemorrhage at fundus | 50% | 100% | |
| Enlarged gastric folds | 15% | 100% | |
| Sticky tenacious mucus | 5% | 100% | |
| Xanthoma | 5% | 100% | |
| NBI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 97.00% | 81.00% | |
| BLI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 98.00% | 92.00% | |
Table 7. Summary of H. pylori-eradicated gastric mucosa endoscopic features in various models.
| Endoscopic models | H. pylori-negative gastric mucosa | ||
| Endoscopic features | Sensitivity | Specificity | |
| WLI | RAC | 92.40% | 94.50% |
| Fundic gland polyp | 14.60% | 95.50% | |
| Hematin spots | 12.80% | 93.80% | |
| Red streaks | 100% | 2.80% | |
| Raised erosion | 2.80% | 99.10% | |
| LCI | Light orange/white apricot mucosa | 96.70% | 50% |
| RAC | 76.70% | 90% | |
| Fundic gland polyp | 13.30% | 100% | |
| Hematin spots | 16.70% | 100% | |
| Red streaks | 16.70% | 100% | |
| Raised erosion | 10% | 100% | |
| NBI | Round homogenous sized pits and presence of RAC | 80% | 85% |
| BLI | Round homogenous sized pits and presence of RAC | 80% | 95% |
| Note: BLI: Blue Laser Imaging; H. pylori: Helicobacter pylori; LCI: Linked Color Imaging; N/A: Not Available; NBI: Narrow-Band Imaging; RAC: Regular Arrangement of Collecting venules; WLI: White Light Imaging. | |||
Table 8. Summary of H. pylori-negative gastric mucosa endoscopic features in various models.
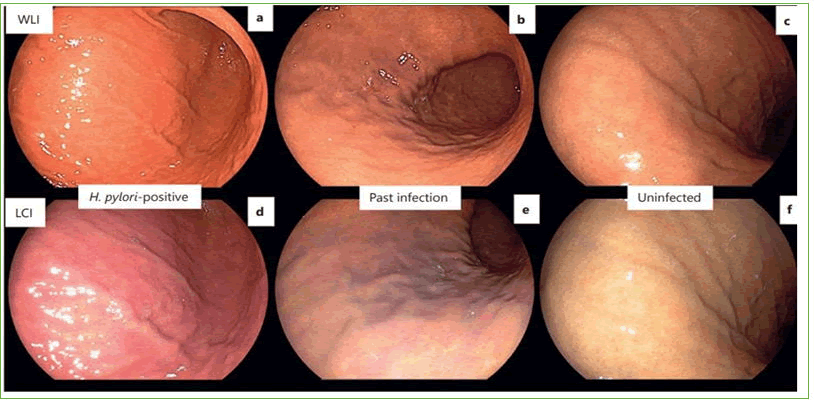
Figure 8. Endoscopic images with WLI and LCI according to H.pylori infection status. a,d) Active gastritis in a patient who had positive results in the H. pylori tests. Diffuse redness in WLI and crimson coloring in LCI are observed; b,e) Inactive gastritis in a patient who had negative results for the H. pylori tests but histologically confirmed atrophy and metaplasia. Orange mucosa in WLI and apricot coloring in LCI are observed. c,f) Inactive gastritis in a patient who had negative results in the H. pylori tests and was not infected. Orange mucosa without diffuse redness in WLI and apricot coloring in LCI are observed. WLI: White Light Imaging; LCI: Linked Color Imaging.
Discussion
Gastric mucosal changes to H. pylori status, in the various endoscopic models are area of current studies and present different characterized features for the current, eradicated and negative H. pylori infection in various endoscopic models.
Many previous studies of conventional white light endoscopy have consistently defined sticky mucus, atrophy, spotty redness, hyperplastic polyp, xanthoma, enlarged/tortuous folds, diffuse redness, and mucosal swelling for current H. pylori infection, and from of these nine characteristics, a single feature alone is not sufficient to diagnose current infection, instead the presence of two or more features can be closer to confirming the diagnosis. However, monitoring all of the above considerations and features is useful in assessing H. pylori status. CWLI Recent research focused on endoscopic evaluation of H. pylori infection all three status and concluded that eradicated H. pylori infection increased the risk of gastric cancer compared to those who had never had H. pylori infection. Also, recent studies have characterized on diffuse redness, mucosal edema and nodularity for current infection, presence of map-like redness, Unclear Atrophy Boundary (UAB) and reappearance of RAC for eradicated infection and presence of RAC appearance, hematin and red streak for uninfected H. pylori infection. The modified model scoring system of the Kyoto classification provides important and useful information for evaluating the status of H. pylori infection with gastric mucosal atrophy, intestinal metaplasia, diffuse redness, enlarged folds, nodularity and RAC features. In addition to WLI endoscopy, various other models such as Image-Enhanced Endoscopy (IEE) and Artificial Intelligence (AI) have been developed to generate and interpret clear images of the gastric mucosa to accurately observe the microstructure of the gastric mucosa, correlation with histological changes and clinical conditions and ultimately not only to diagnose H. pylori infection but also prepare sufficient information for diagnosis of chronic gastritis and precancerous lesions. ME and IEE models are superior to WLI in providing accurate and in-depth imaging of the mucosa and provide better diagnostic imaging for H. pylori status. Only MI and IEE endoscopic models have significantly higher sensitivity, specificity and accuracy than WLI to detect cases of H. pylori gastric mucosa infection. Currently, ME is practicing in combination with IEE especially NBI, CLI and LBI, which have high sensitivity and specificity not only for WLI but also for IEE.
Conclusion
Recent studies demonstrate that different endoscopic imaging of the gastric mucosa improves the evaluation and detection of H. pylori status and pre-cancerous lesion with the development of new equipment and models and can present valuable information for clinical and histopathological correlations.
Conventional white-light imaging endoscopy with a modified scoring system of the Kyoto classification is a good model for the diagnosis of H. pylori status in the gastric mucosa due to its availability, widespread use, less time-consuming, low cost, and no need for more experience. Newly developed IEE models alone or in combination with magnifying endoscopy are more useful models for detecting mucosal changes and obtaining accurate targeted biopsies than the WLI endoscopy. However, using IEE modules requires adequate training and skills along with sufficient experience, time, and specialized advanced equipment to perform the procedures. Therefore, its widespread use is limited and more studies have not been conducted. The use of AI is limited and there are no further researches, but it will be a good diagnostic model for the evaluation of the H. pylori infection status in the future because of the detection some important features during endoscopy, performed in a short time and require less training.
References
- Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420-9.
[Crossref] [Google Scholar] [PubMed]
- Colquhoun A, Arnold M, Ferlay J, et al. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64(12):1881-8.
[Crossref] [Google Scholar] [PubMed]
- Lee W. Application of current image-enhanced endoscopy in gastric diseases. Clin Endosc. 2021;54(4):477-87.
[Crossref] [Google Scholar] [PubMed]
- Kato T, Yagi N, Kamada T, et al. Diagnosis of Helicobacter pylori infection in gastric mucosa by endoscopic features: A multicenter prospective study. Dig Endosc. 2013;25(5):508-18.
[Crossref] [Google Scholar] [PubMed]
- Chatrangsun B, Vilaichone RK. Endoscopic diagnosis for H. pylori infection: white light imaging (WLI) vs. image-enhanced endoscopy (IEE). Asian Pac J Cancer Prev. 2021;22(9):3031.
[Crossref] [Google Scholar] [PubMed]
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64(9):1353-67. [Crossref] [Google Scholar] [PubMed]
- Dohi O, Yagi N, Onozawa Y, et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;4(07):E800-E5.
[Crossref] [Google Scholar] [PubMed]
- Nomura S, Terao S, Adachi K, et al. Endoscopic diagnosis of gastric mucosal activity and inflammation. Dig Endosc. 2013;25(2):136-46.
[Crossref] [Google Scholar] [PubMed]
- Uchiyama K, Ida K, Okuda J, et al. Correlations of hemoglobin index (IHb) of gastric mucosa with Helicobacter pylori (H. pylori) infection and inflammation of gastric mucosa. Scand J Gastroenterol. 2004;39(11):1054-60.
[Crossref] [Google Scholar] [PubMed]
- Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26(5):466.
[Crossref] [Google Scholar] [PubMed]
- Yagi K, Aruga Y, Nakamura A, et al. Regular arrangement of collecting venules (RAC): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophago-gastric adenocarcinoma. J Gastroenterol. 2005;40(5):443.
[Crossref] [Google Scholar] [PubMed]
- Hidaka N, Nakayama Y, Horiuchi A, et al. Endoscopic identification of Helicobacter pylori gastritis in children. Dig Endosc. 2010;22(2):90-4.
[Crossref] [Google Scholar] [PubMed]
- Hojo M, Miwa H, Ohkusa T, et al. Alteration of histological gastritis after cure of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16(11):1923-32.
[Crossref] [Google Scholar] [PubMed]
- Okubo M, Tahara T, Shibata T, et al. Changes in gastric mucosal patterns seen by magnifying NBI during H. pylori eradication. J Gastroenterol. 2011;46:175-82.
[Crossref] [Google Scholar] [PubMed]
- Haruma K, Kawaguchi H, Komoto K, et al. Helicobacter pylori infection is the major risk factor for atrophic gastritis. Gastroenterology. 1995;108(4):A110.
- Rokkas T, Pistiolas D, Sechopoulos P, et al. The long?term impact of Helicobacter pylori eradication on gastric histology: a systematic review and meta?analysis. Helicobacter. 2007;12:32-8.
[Crossref] [Google Scholar] [PubMed]
- Yan SL, Wu ST, Chen CH, et al. Mucosal patterns of Helicobacter pylori-related gastritis without atrophy in the gastric corpus using standard endoscopy. World J Gastroenterol. 2010;16(4):496.
[Crossref] [Google Scholar] [PubMed]
- Kim DB, Chung WC. Accuracy of endoscopic diagnosis of mild atrophic gastritis with Helicobacter pylori infection. Clin Endosc. 2018;51(4):310-2.
[Crossref] [Google Scholar] [PubMed]
- Wang K, Zhao J, Jin H, et al. Establishment of a modified Kyoto classification scoring model and its significance in the diagnosis of Helicobacter pylori current infection. Gastrointest Endosc. 2023;97(4):684-93.
[Crossref] [Google Scholar] [PubMed]
- Yoshii S, Mabe K, Watano K, et al. Validity of endoscopic features for the diagnosis of Helicobacter pylori infection status based on the Kyoto classification of gastritis. Dig Endosc. 2020;32(1):74-83.
[Crossref] [Google Scholar] [PubMed]