- Biomedical Research (2011) Volume 22, Issue 3
Elevation of serum methylglyoxal may be used as a screening marker in oral premalignant lesions
Utpal Kumar Biswas*, Santanu Banerjee, Amitabha Das, Arun Kumar
Department of Biochemistry, College of Medicine and JNM Hospital, The West Bengal University of Health Sciences,Kalyani, Nadia, India
- *Corresponding Author:
- Utpal Kumar Biswas
Department of Biochemistry
College of Medicine and JNM Hospital
The West Bengal University of Health Sciences
Kalyani, Nadia 741235,India
Accepted November 29 201
Abstract
Methylglyoxal (MG) is formed in several metabolic pathways as products, which are highly toxic, and detoxified by glyoxalase enzyme system to lactate. It is a tricky molecule as it has dual role, on one hand its synthesis is increased in oxidative stress and on the other hand it is considered as inhibitor of cell cycle particularly in the rapidly proliferating cells. Considering these aspects of methylglyoxal, the current study was designed to establish the potential role of MG in patients exhibiting oral premalignant lesions and in patients with established malignant disease. The aim of the present study was to determine the serum methylglyoxal concentration in pa-tients with oral premalignant lesions (OPL) as well as in established malignant dis-ease and compare it with the healthy controls. Further to evaluate the oxidative stress and antioxidant status in these OPL patients in comparison with the healthy individuals. Methylglyoxal (MG) was determined in thirty two cases of oral precancerous le-sions and thirty two cases malignant cancer patients and compared their findings with thirty-three age/sex-matched controls. Antioxidants and MDA were analyzed to observe the oxidative stress in OPL patients and healthy subjects. The OPL patients were further grouped in two cate-gories based on the extent of oxidative stress and antioxidant status in those patients in order to delineate whether oxidative stress is the sole cause of elevated MG concentration or is there any other factor responsible for elevation in them. The premalignant and malignant cases were con-firmed by histopathological examinations. The MG levels were further compared with the pa-tients with established biochemical cancer markers. Methylglyoxal was determined by method of Racker. Superoxide dismutase, Catalase and Glutathione peroxidase was determined by stan-dard methods. The values were expressed as means ± standard deviation (SD) and data from pa-tients and control was compared using student’s‘t’-test. Serum methylglyoxal levels was found to be significantly elevated (p<0.001) in oral premalignant lesions (OPL) and significantly de-creased levels (p<0.001) in established malignant disease patients compared to healthy controls. Further analysis among oral precancerous lesion patients was based on the oxidative stress and antioxidant status which were categorized into two groups. Serum malondialdehyde levels were significantly higher and antioxidants were significantly lower in (14/32) 43.75% (Group II) OPL patients where as no significant changes were observed in the remaining patients (18/32) 56.25% (Group I) when compared to controls. Levels of methylglyoxal and specific cancer markers in es-tablished cases of malignant disease are shown in Table 1II. Measurement of serum MG levels in suspected OPL- patients will help us to screen the subjects with or without risk of malignant transformation as decreased MG levels increases the risk of malignant change. These high risk subjects could be referred to specialized centers for further evaluation. This could be used as a screening marker which is simple and cost effective.
Keywords
Methylglyoxal, Superoxide dismutase, Catalase, Glutathione Peroxidase, Malondialdehyde, Oral Precancerous lesions, Malignant disease, India
Introduction
Methylglyoxal (MG), also called pyruvaldehyde is formed as a product of several metabolic pathways; however the impor tant source is glycolysis [1]. It is highly toxic compound which is detoxified by glyoxylase enzyme system to non-toxic form as lactate [2]. Elevated MG levels are observed in diabetes as a precursor of advanced glycation end product (AGE) [3]. Earlier studies have acclaimed methylglyoxal not only as toxic compound [4], but also as potent anti can-cer agent [5]. In 1963, Szent- Gyorgi identified methyl gly-oxal and named as ‘Retine’, which inhibited the rapidly mul-tiplying cells in cancer without hampering the normal cells. It was in 1967, when this molecule was first synthesized in his laboratory as carbonyl compounds which were sensitive to cancer cells than the normal ones, thus exploiting its ad-vantage in controlling the cancer.
Further scientists established that the crux to control the can-cer cell was by depleting their energy source which enables them to divide at faster rate, which was only possible when ATP synthesis was inhibited or depleted in those cancer cells [6]. Till late, evidence of methylglyoxal as cancer cell inhibi-tor were tried in animal models, but it was 2000, when clini-cal trials were conducted in humans by Indian cancer re-searchers from Kolkata (7). Their clinical trials showed that methylglyoxal selectively killed the cancer cells without affecting the normal cells, although much more is estab-lished as methylglyoxal levels being elevated in oxidative stress, aging process and in diabetes as AGEs (4, 8, 9, 10, 11).
Methylglyoxal is frequently generated under both physiological and pathological conditions. In animal cells the principal route for methylglyoxal catabolism is the glyoxalase pathway, which consists of two enzymes, glyoxalase I (EC 4.4.1.5, lactoylglutathione lyase) and gly-oxalase II (EC 3.1.2.6, hydroxyacylglutathione hydrolase). This pathway converts methylglyoxal to d-lactate via S-d-lactoylglutathione (11); reduced glutathione is a required cofactor. Despite advances in understanding the systemic effects of biogenic methylglyoxal, its prominence in meta-bolic network is still unearthed.
It is already established that oral premalignant lesions are associated with pathological changes in the linings of the mouth and upper aero digestive tract which are not cancer-ous, but have potency to become cancerous. If these cancer-ous cells are diagnosed in right time, then the future risk of cancer could be prevented. In India the detection of new le-sions of cancer is shown to occur in 0.8/ 1,000,000 persons annually (12), compared to an oral cancer incidence rate of 5/1,000,000 in the same population. The huge economy of the country is utilized in early detection and management of cancer patients. Since no biochemical test is ascertained till date for delineating the malignant potency of cancer cell, however antioxidant therapies are employed as evidence based medicine to patients who has more propensity to have future risk of cancer.
Considering the view points and established facts from all the observational and scientific studies on cancer, the current study was designed to evaluate the potential role of methyl-glyoxal in oral premalignant lesions as well as malignant disease. The study was conducted to observe the serum con-centration of methyl glyoxal in patients and its correlation with other parameters of oxidative stress. The finding of the current study was compared with the serum concentration of methylglyoxal with established cancer markers in patients with malignant diseases confirmed by histopathological ex-amination.
Materials and Methods
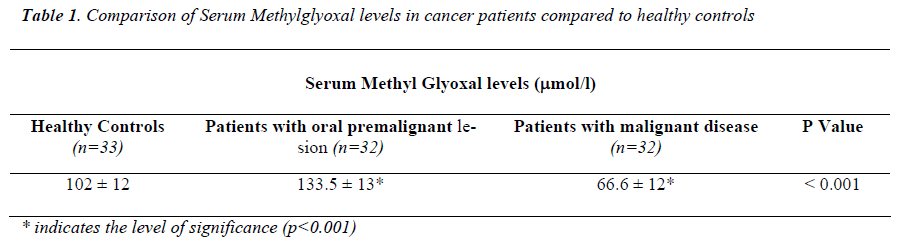
Ninety-seven subjects were enrolled for the present study with ages ranging from 20-40 years which included thirty-two patients with oral premalignant lesions, thirty-two pa-tients of confirmed malignant disease and the rest thirty-three subjects were healthy controls. The premalignant and malignant cases were confirmed by histopathological exami-nation. The details about the groups are furnished in Table 1.
Inclusion Criteria
Patients suffering from oral premalignant lesions and estab-lished malignant disease were included in the study.
Exclusion Criteria
Patients with diabetes mellitus, hypertension, dyslipidemia, smokers were excluded. Also patients on lipid lowering drugs and antioxidant vitamin supplements were also ex-cluded.
Serum methylglyoxal, catalase, superoxide dismutase, glu-tathione and malondialdehyde were determined in all the participants of the study using standard methods.
Serum Methylglyoxal assay
Methylglyoxals are estimated according to the modified method of Racker (13). Twenty-five μl of serum samples was added to 350 μl of DNPH [0.1% DNPH in 2N HCl].Then to each tube 2.125 ml of distilled water was added. Then it was incubated for 15 minutes at 37°C. After the incubation 1.5 ml 10% NaOH was added and absorbance was read at 576 nm using Microlab 200 semi-auto analyser. The results are calculated from the standard curve.
Catalase assay
The catalase assay was carried out by the method of Aebi (1984) (14). One milliliter of serum was taken with 1.9 ml of phosphate buffer in different test tubes (125 mM, pH 7.4). The reaction was initiated by the addition of 1 ml of hydro-gen peroxide (30 mM). Blank without serum was prepared with 2.9 ml of phosphate buffer and 1 ml of hydrogen perox-ide. The decrease in optical density due to decomposition of hydrogen peroxide was measured at the end of 1 min against the blank at 240 nm. Units of catalase were expressed as the amount of enzyme that decomposed 1 μM H2O2 per minute at 25°C. The specific activity was expressed in terms of units per milligram of protein.
SOD assay
The assay of SOD was based on the reduction of NBT to water insoluble blue formazan, as described by Fedovich (1976) (15). Serum (0.5 ml) was taken and 1 ml of 125mM sodium carbonate, 0.4 ml of 24μM NBT, and 0.2ml of 0.1mM EDTA were added. The reaction was initiated by adding 0.4ml of 1 mM hydroxylamine hydrochloride. Zero time absorbance was taken at 560 nm followed by recording the absorbance after 5 min at 25°C. The control was simulta- neously run without liver homogenate. Units of SOD activity were expressed as the amount of enzyme required inhibiting the reduction of NBT by 50.0%. The specific activity was expressed in terms of units per milligram of protein.
Glutathione peroxidase assay (GSH-PX)
GSH-PX was estimated by the method of Paglia and Valen-tine (1967) (16). The principal of the assay was based on ca-talyses the oxidation of glutathione (GSH) by cumene hy-droperoxide. In the presence of glutathione reductase (GR) and NADPH the oxidized glutathione (GSSG) is immedi-ately converted to the reduced form with a concomitant oxi-dation of NADPH to NADP+. The decrease in absorbance at 340 nm is measured.
Lipid peroxidation assay
Thiobarbituric acid (TBA) reacts with malondialdehyde (MDA) to form a diadduct, a pink chromogen, which can be detecte spectrophotometrically at 532 nm (Buege and Aust, 1978) (17). Liver homogenate (0.5ml) and 1ml of 0.15M KCl were taken. Peroxidation was initiated by adding 250 μL of 0.2 mM ferric chloride. The reaction was run at 37 oC for 30 min. The reaction was stopped by adding 2ml of an ice-cold mixture of 0.25 N HCl containing 15% trichloroaceticacid, 0.30% TBA and 0.05% BHT and was heated at 80oC for 60min. The samples were cooled and re-sults were expressed as MDA an equivalent, which was cal-culated by using an extinction coefficient of 1.56 X 105 M-1 cm-1. One unit of lipid peroxidation activity was defined as the amount of TBA that converts to thiobarbituric acid reac-tive substances (TBARS). The specific activity was ex-pressed in terms of units per milligram of protein.
Determination of specific cancer markers
The prostrate specific antigens-Total (TPSA) and Carci-noembryonic antigen (CA-125) levels in established cancer patients were determined by standard ELISA kit obtained from Ranbaxy Diagnostics.
The study was conducted for a period of six months from December 2009 till May 2010. The design of this study was pre-approved by the institutional ethical committee board of the Institution and informed consent was obtained from the participants.
Results
Serum methylglyoxal levels were significantly higher (p<0.001) in patients with oral premalignant lesions where as significantly lower (p<0.001) in patients with established malignant diseases compared to healthy controls (Table 1).
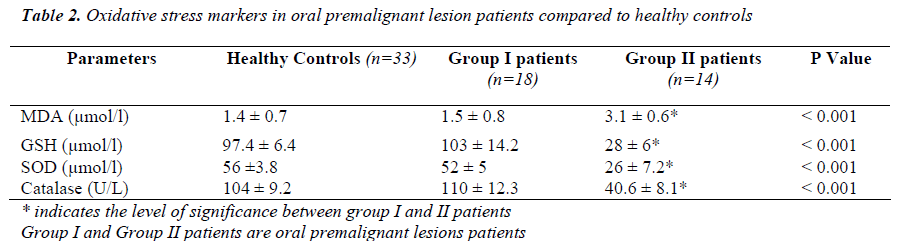
Serum malondialdehyde levels were significantly higher in 43.75% (group II) patients with oral precancerous lesions associated with significantly lower (p<0.001) levels of anti-oxidants in them compared to healthy controls where as no significant changes in oxidative stress parameters were ob-served in 56.25% (group I) patients (Table 1I).
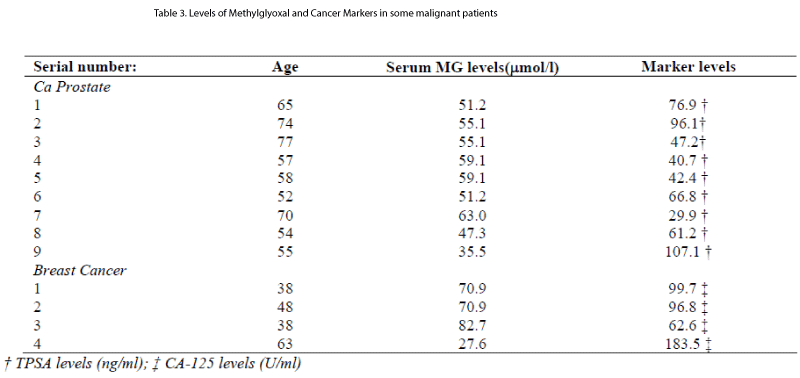
Levels of methylglyoxal and specific cancer markers in es-tablished cases of malignant disease are shown in Table 1II.
Discussion
The current study was conducted to determine the serum methylglyoxal (MG) levels in patients with oral premalig-nant lesions (OPL) and malignant diseases which were compared with healthy controls. Our study observed signifi-cantly higher levels of MG in the entire OPL patients when compared to controls. Report from the previous studies based on antioxidant and lipid peroxidation status in oral precancerous lesion (OPL) patients observed elevated perox-ides and depleted antioxidants with concomitant increase in serum methylglyoxal in all OPL patients (4, 8, 9, 10, 11). Our study did not conform with their findings as we observed only 43.75% of OPL patients had increased oxidative stress and depletion of antioxidants which is justified by signifi-cantly higher levels of malondialdehyde (MDA) and lower levels of superoxide dismutase, catalase and glutathione per-oxidase compared to healthy controls.
Study conducted by Subapriya et al on oral precancer pa-tients (18) observed enhanced lipid peroxidation and depletion of antioxidants levels in preoperative compared to post op-erative oral precancerous lesions patients. They observed decreased lipid peroxides and increased antioxidants levels twelve weeks after surgery. So it is evident that cancer is linked with derangements of oxidants and antioxidants. An-other study conducted by Khanna et al on twenty new cases of oral squamous cell carcinoma observed similar facts on oxidants/antioxidants misbalance. Both the above mentioned study does not comform with the current study as 56.25 % of OPL patients had increased serum MG levels without oxida-tive stress. Thus our study confirms the multifactorial nature and etiopathogenesis of OPL. There might be several unre-vealed factors other than oxidant/antioxidant imbalance which could elevate MG levels in OPL patients(19).
Earlier studies on cancer patients observed significantly higher levels of methylglyoxal (MG) in established cancer patients compared to controls (20) which is not in accordance with the current study since significantly decreased (p<0.001) MG levels was observed in malignant disease. As per published findings, methylglyoxal is toxic to cells which are involved in numerous pathologic processes in-vivo, causing carcinogenesis. Earlier studies conducted (Takahashi et al., 1989) on an experimental animal models proved me-thylglyoxal as a key molecule in carcinogenesis which has tumor promoting activity in adenocarcinomas of stomach in rat. Evidence based on their experiment observed sudden increase in glyoxalase I and II enzymes, a protective mecha-nism against methylglyoxal, when animal models were fed on diet containing 3′-methyl-4-dimethylaminoazobenzene to induce carcinogenesis. Apparently they observed drastic reduction of glutathione levels in animal models, which jus-tifies the fact when cells are exposed to carcinogens; it causes induction of methylglyoxal metabolizing enzyme to prevent the cells proceeding to cancerous state from precan-cerous state (21).
In another study the toxicity of methylglyoxal (MG) causing neuroblastoma was protected by phenolic acid which further proves the role of MG causing cancer (22). Contrary to the above study, researchers suggested the growth inhibitory, anticancer and tumoricidal properties of methyl glyoxal (23).
Study conducted elsewhere reported glyoxylase being as a pro-survival factor of the p53 family and plays a critical role in the normal development and in the pathogenesis of vari-ous diseases including cancer by controlling the levels of methylglyoxal in cells (24).
Based on the findings of the above studies it can be pre-sumed that alteration of MG level occurs at the time of oxi-dative stress which is involved in the pathogenesis of cancer but at times it acts as a protective mechanism as we observed in our study. The MG level alters at the stage of cell trans-formation from precancerous state to cancerous state indicat-ing the link in early detection of cancer in its premalignant stage.
Our study is an eye-opener since its points the alarming na-ture of cancerous cells much before they exhibits clinical signs and symptoms just by determining methylglyoxal lev-els by simple colorimetric measurement. This has merits of being adopted even in rural health set up due to its cost ef-fectiveness and affordability to lower socio economic strata of population. Though previous studies suggested increased levels of methylglyoxal is a phenomenon observed in oxida-tive stress, but our study presumes it as cell cycle checker in controlling the cell proliferation.
Its levels become optimized to safe guard the cellular prolif-eration but no sooner it fails the level of methylglyoxal drops back to normal. However, this is a preliminary report of our study. We are looking forward to perform an vitro study in future in cell culture model of oral cancer cell line (HSC2 or HSC3 etc ) and stimulate with MG in varying doses and per-form a simple flow cytometry analysis to see if cell cycle is perturbed. That would enable us with better understanding of real effect of MG on these cells. The drawback of our study is small sample size but our findings are justified by compar-ing the levels of MG in some established cases of prostate and breast cancer diagnosed with the specific cancer markers (Table 1II).
Conclusion
Measurement of serum MG levels in suspected OPL- pa-tients will help us to screen the subjects with or without risk of malignant transformation as decreased MG levels in-creases the risk of malignant change. These high risk sub-jects could be referred to specialized centers for further evaluation. This could be used as a screening marker which is simple and cost effective.
Limitations of the study
The results obtained from the current study is a tip of the iceberg though the study has limitations of involving less number of participants due to financial constraints and dura-tion of the study.
Future directions of the study
In future a multicentre study should be carried out involving large sample size to support the findings of the current study.
What this study contributes
Measurement of methylglyoxal levels in cancer prone pa-tients at the right time will avoid them from future cancer risk. As the measurement of methyglyoxal involves simple colorimetric assay, so it is cost effective and can be done in small laboratories. Also it can be used for screening of large scale population.
Conflict interest
The authors do not have any conflict interest from this study.
References
- Frank W R Chaplen. Incidence and potential implications of the toxic metabolite methylglyoxal in cell culture: A review. Cytotechnology 1998; 26(3): 173-183.
- Paoli T, Faulkner J, O’Kennedy R, Moore Eli Keshavarz.A Study of D-Lactate and extracellular methylglyoxal production in lactate reutilizing CHO cultures. Biotech- nology and Bioengineering 2010; DOI 10. 1002/ bit.22757.
- Cantero Anne-Valerie, Portero-Oti’n M, , Ayala V, Auge N, Sanson M, Elbaz M, Thiers Jean-Claude et al. Methyl- glyoxal induces advanced glycation end product (AGEs) formation and dysfunction of PDGF receptor-ß: implica- tions for diabetic atherosclerosis. The FASEB Journal 2007; 21:3096-3106.
- Mukhopadhyay S, Gachhui R and Kar M. The role of Methyl Glyoxal in relation to patho-physiological compli- cations in diabetes mellitus. Biomedical Research 2006; 17(2): 111-116.
- Talukdar D, Ray S, Ray M and Das S. A brief critical overview of the biological effects of methylglyoxal and further evaluation of a methylglyoxal-based anticancer formulation in treating cancer patients. Drug Metabol Drug Interact 2008; 23(1-2):175-210.
- Daniel A Tennant, Raul V Dura’n & Gottlieb E. Targeting metabolic transformation for cancer therapy. Nature Re- views Cancer 2010; 10: 267-277.
- Ray M, Ghosh S, Kar M, Datta S & Ray S. Implication of the bioelectronic principle in cancer therapy: Treatment of cancer patients by methylglyoxal-based formulation. In- dian Journal of Physics 2001; 75B (2):73-77.
- Bakris GL, Bank AJ, Kass DA, et al. Advanced glycation end-product cross-link breakers. A novel approach to car- diovascular pathologies related to the aging process. Am J Hypertens 2004; 17(12 Pt 2):23S-30S.
- Bhatwadekar AD, Glenn JV, Figarola JL, Scott S, Gar- diner TA, Rahbar S et al. A new advanced glycation in- hibitor LR-90 prevents experimental diabetic retinopathy in rats. Br J Opthalmol 2008; 92:545-547.
- Cooper ME. Importance of advanced glycation end prod- ucts in diabetes-associated cardiovascular and renal dis- ease. Am J Hype 2004; (12 Pt 2):31S-38S.
- Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structuralchanges. Circ Res 2003; 92(7):785-792.
- Bouquot JE. The pathology and progression of oral pre- malignancy. Proceedings, Epithelial Dysplasia Sympo-sium, 5 International Congress on Oral Cancer, Royal College of Physicians, London, United Kingdom; Sep- tember, 1997.
- Racker, E., in S. P. Colowick and N. 0. Kaplan (Editors), Methods in Enzymology, Vol. III, Academic Press, New York,1963, p. 283.
- Aebi, H. Catalyse in vitro methods of analysis. Methods Enzymol 1984; 105: 121-125.
- Fedovich, B.C. Superoxide dismutase; improved assayand an assay applicable to acrylamide gel. Anal. Biochem 1976;10: 276-287.
- Paglia DE, Valentine WN. Studies on quantitative and qualitative characterization of erythrocyte glutathione per- oxidase. J Lab Clin Med 1967; 70: 158-169.
- Buege, J.A. and S. D. Aust. Microsomal lipid peroxida-tion. Enzymol 1978; 52: 302-310.
- Subapriya R. Kumaraguruparan R. Nagini S. Than- gavelu A. Oxidant-Antioxidant Status in Oral Precancerand Oral Cancer Patients. Toxicology Mechanisms and Methods 2003; 13 (1):77-81.
- Khanna R, Thapa PB, Khanna HD, Khanna S, Khanna AK and Shukla HS. Lipid peroxidation and antioxidant en- zyme status in oral carcinoma patients. Kath Univ Med J 2005; 3 (4): 334-339.
- Germanová A, Germanová A, Tesarová P, Jáchymová M, Zvára K, Zima T, and Kalousová M. Glyoxalase I Glu111Ala Polymorphism in Patients with Breast Cancer. Cancer Investigation 2009; 27(6):655-660.
- Takahashi M, Okamiya H, Furukawa F,Toyoda K, Sato H, Imaida K and Hayashi Y. Effects of glyoxal and methyl- glyoxal administration on gastric carcinogenesis in Wistar rats after initiation with N-methyl-N' -nitro-N- nitrosoguanidine. Carcinogenesis 1989; 10(10):1925-1927.
- Huang SM, Chuang HC, Wu CH and Yen GC. Cytopro- tective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol Nutr Food Res 2008; 52(8):940-949.
- Malcolm J. Kavarana, Elena G. Kovaleva, Donald J. Creighton, Megan B. Wollman, and Julie L. Eiseman. Mechanism-Based Competitive Inhibitors of Glyoxalase I: Intracellular Delivery, in Vitro Antitumor Activities, and Stabilities in Human Serum and Mouse Serum. J Med Chem 1999; 42 (2): 221-228.
- Yang Xu and Chen Xibin. Glyoxalase II a Detoxifying Enzyme of Glycolysis Byproduct Methylglyoxal and a Target of p63 and p73, Is a Pro-survival factor of the p53 family. J Biol Chem 2006; 281:26702-26713.