Research Article - Biomedical Research (2018) Volume 29, Issue 8
Effects on the expression of pro-inflammatory cytokines in the liver and spleen after oral administration of Porphyromonas gingivalis in mice
Jingyi Ren1, Ye Ding1, Hongqiang Yu1, Yanmin Zhou1* and Weixian Yu2*
1Department of Implantology, School of Stomatology, Jilin University, Changchun, PR China
2Key Laboratory of Mechanism of Tooth Development and Jaw Bone Remodeling and Regeneration in Jilin Province, Changchun, PR China
- *Corresponding Authors:
- Yanmin Zhou
Department of Implantology
School of Stomatology
Jilin University, Changchun, PR China
Weixian Yu
Key Laboratory of Mechanism of Tooth Development and Jaw Bone Remodeling and Regeneration
Jilin Province, Changchun, PR China
Accepted date: February 12, 2018
DOI: 10.4066/biomedicalresearch.29-17-3173
Visit for more related articles at Biomedical ResearchAbstract
Periodontitis initiated by periodontopathic bacteria is associated with several systemic diseases. Porphyromonas gingivalis (P. gingivalis) is one of the major pathogens causing periodontal diseases, and is thought to also play a critical role in possible mechanisms linking periodontitis with other systemic disorders. We explored whether the production of pro-inflammatory cytokines interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α) in the liver and spleen changed as a result of oral administration of P. gingivalis in mice. mRNA expression of pro-inflammatory cytokines was analysed by Real-time Quantitative polymerase chain reaction (RT-qPCR) and cytokine protein levels were measured by Enzyme-Linked Immunosorbent Assay (ELISA) and immunohistochemistry. In addition, histological changes of liver and spleen tissues were monitored using haematoxylin and eosin staining. The results showed that liver and spleen tissue of P. gingivalis treated mice had higher mRNA and protein levels of pro-inflammatory cytokines compared to the control group. The production of proinflammatory cytokines in the liver and spleen was therefore suggested to increase as a result of oral administration of P. gingivalis in mice, and may provide further understanding of the mechanisms linking periodontitis and systemic disorders.
Keywords
Periodontitis, Porphyromonas gingivalis, Pro-inflammatory cytokines, Liver, Spleen
Introduction
Periodontitis, a chronic inflammatory condition of the periodontium, is one of the most common oral diseases worldwide. Many reports have revealed the correlation between periodontitis and systemic diseases including cerebrovascular and cardiovascular disease, rheumatoid arthritis, diabetes mellitus, chronic obstructive pulmonary disease and preterm low birth weight [1-6]. Periodontitis is caused by gram negative microorganisms and their production of including lipopolysaccharide (LPS), peptidoglycan, DNA which can damage the connective tissues and alveolar bone, finally leading to the loss of the tooth [7]. P. gingivalis, a gram negative anaerobe, is the putative predominant pathogenic bacteria associated with periodontitis [8]. Oral infection by P. gingivalis has indeed been demonstrated to induce periodontitis and activate an immune response [9].
Pattern recognition receptors such as Toll-like-receptors (TLRs) are key to innate immune system as they recognise microbial structural motifs known as pathogen-associated microbial patterns (PAMPS) [10]. The TLR signalling pathway is critical for the initiation of periodontitis pathology [11]. Of ten human TLRs, TLR2 and TLR4 have previously been related to periodontal disease [12]. Moreover, TLR2 and TLR4 may also contribute to the progression of metabolic disorders like insulin resistance and hepatic steatosis [13]. In addition to the local production of inflammatory mediators, periodontitis also causes a significant up-regulation in circulating inflammatory mediators such as TNF-α, IL-6 and IL-1β [14,15].Periodontitis is often associated with systemic disorders. The underlying mechanism possibly involves PAMPS entering the systemic circulation and activating host defence cells to induce the secretion of cytokines, chemokines and other kinds of immune factors in endothelial cells and hepatocytes [16]. In addition, various locally produced proinflammatory stimuli such as IL-1, TNF-α and IL-6 can also invade circulation and induce a systemic response [17,18].
The release of inflammatory mediators (TNF-α) in circulation has been related to various systemic diseases including insulin resistance, diabetes, atherosclerosis as well as non-alcoholic fatty liver disease (NAFLD) [19,20]. NAFLD is the most common liver disease worldwide and has recently been integrated with the metabolic syndrome to include steatosis, steatohepatitis, liver fibrosis, cirrhosis, and carcinoma [21-23]. Human trials reported that NAFLD patients were at greater risk of P. gingivalis infection compared to healthy patients. The study implied that endotoxins from P. gingivalis and inflammatory mediators released by the microorganisms can enter the blood circulation and may participate in the mechanism linking periodontitis and NAFLD [24]. To conclude, there is a lot of data supporting the hypothesis that periodontitis can trigger a systemic inflammatory response and exert an inflammatory response in distant tissues. However, whether periodontitis can induce alterations in the expression of pro-inflammatory cytokines in remote organs such as the liver and spleen has remained unproven until now. The study presented here explored the effect of oral administration of P. gingivalis on the production of pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in the liver and spleen in mice.
Materials and Methods
Animals and study design
This study followed guidelines from the Institutional Authority for Laboratory Animal Care of Jilin University and received the ethics committee approval of Hospital of Stomatology of Jilin University No.201703020000286. Thirty female C57BL/6 mice aged between four and six weeks old were purchased from the Animal Experiment Center of Jilin University. Animals were separated into two groups (n=15 per group): P. gingivalis group and the control group. Both groups received the same amount of feed and were kept under the same conditions. Mice were sacrificed by deep anesthesia after five weeks of oral administration and samples were taken from the liver and spleen.
Oral administration of P. gingivalis
P. gingivalis ATCC33277 was cultured in Columbia Blood Agar (BIO-KONT, Wenzhou, China). The density of P. gingivalis in an overnight culture was examined by spectrophotometry at 550 nm. 100 μl phosphate buffered saline with 2% carboxymethyl cellulose (Aladdin Industrial Corporation, Shanghai, China) containing 109 colony forming units of live P. gingivalis was given to the mice in the P. gingivalis group with a feeding needle. The suspension was given every other day for five weeks. Similarly, the control group was given the control solution of phosphate buffered saline with 2% carboxymethyl cellulose.
Quantitative Real-time PCR for gene expression of IL-1β, IL-6 and TNF-α in the liver and spleen
TRIzol reagent (Invitrogen Corp, Carlsbad, CA) was used to extract total RNA of liver and spleen samples according to manufacturer’s instructions. The isolated RNA was used as the template with the PrimeScript RT reagent Kit and gDNA Eraser (DRRO47A, TaKaRa, China) to obtain cDNA. RTqPCR was performed using a 25 μl reaction with SYBR Premix Ex Taq II (RR420Q, TaKaRa, China) containing cDNA from 25 ng of total RNA per sample (Mx3005P Real-Time QPCR System; Agilent Technologies, USA). The housekeeping gene mouse β-actin was used as a loading control. Gene expression was calculated using the 2-ΔΔCt method. The following primers were used for RT-qPCR:
IL-1β: (F5’-TCCAGGATGAGGACATGAGCAC-3’) and (R5’-GAACGTCACACACCAGCAGGTTA-3’);
IL-6: (F5’-CCACTTCACAAGTCGGAGGCTTA-3’) and (R5’-CCAGTTTGGTAGCATCCATCATTTC-3’);
TNF-α: (F5’-ACTCCAGGCGGTGCCTATGT-3’) and (R5’- GTGAGGGTCTGGGCCATAGAA-3’);
β-actin: (F5’-CATCCGTAAAGACCTCTAGCCAAC-3’) and (R5’-ATGGAGCCACCGATCCACA-3’).
ELISA assay
Protein levels of IL-6, IL-1β and TNF-α in the liver and spleen were measured using Mouse IL-6, IL-1β and TNF-α ELISA kits (Lengton Bioscience Co, Shang Hai, China) in accordance with respective manufacturer’s protocols. In brief, the monoclonal antibody specific for either mouse TNF-α, IL-6 or IL-1β was coated on the 96-well plates. Then, specimens and biotin-labelled antibodies against mouse TNF-α, IL-6, IL-1β and specimens were added. After adding the HRP-conjugated antibody into each well, the plates were incubated at 37°C for 1 h and rinsed five times. Chromogen solutions A and B were used as substrate and the reaction was terminated with Stop reagent after colour formation. The absorbance of each well was read at 450 nm.
Immunohistochemistry
Tissue samples taken from the liver and spleen were fixed overnight in 10% formalin. The sections from the liver and spleen were embedded in paraffin and cut to make 5 μm slides. After hydrating and treatment with 0.3% H2O2 in methanol, the sections were incubated with primary rabbit polyclonal TNF-α (1:100, Abcam, ab6671), IL-6 (1:100, Bioss, bs-0379R, China), IL-1β (1:100, Bioss, bs-0812R, China) antibodies in a humidified chamber for 1 h at 37°C. The sections were washed with phosphate buffered saline, followed by incubation with polymer helper and secondary antibodies (goat anti-rabbit IgG HRP) from the Polink-2 plus kit (ZSGB-BIO, PV-9001). Finally, colour was developed with 3, 3-diaminobenzidine (ZSGB-BIO, ZLI-9018) and counterstained with hematoxylin. Positive expression was evident as brown staining. Images were captured with an Olympus BX 51 (Japan) operated with the micro imaging software cellSens (Olympus, Japan). Five fields were randomly selected from each section and assessed at magnification X200. Quantification of positive expression in each field was quantified as the mean optical density (MOD; integral optical density/total area) with Image Pro Plus 6.0.
Histopathology
After fixing overnight in 10% formalin, sections of liver and spleen tissue were cut as described in immunohistochemistry and stained with haematoxylin and eosin for histological examination. Five randomly selected fields from each section were analysed under a light microscope (Olympus BX 51, Japan) at magnification X200. The histological changes of liver and spleen tissue were evaluated by a specialist blind to the grouping.
Statistical analysis
All data are present as mean ± standard deviation (SD). The significance of the differences between the P. gingivalis group and the control group was analysed using the Student's t-test in SPSS version 17.0.0. P<0.05 was considered as significant difference.
Results
Gene and protein expression of pro-inflammatory cytokines in liver tissue
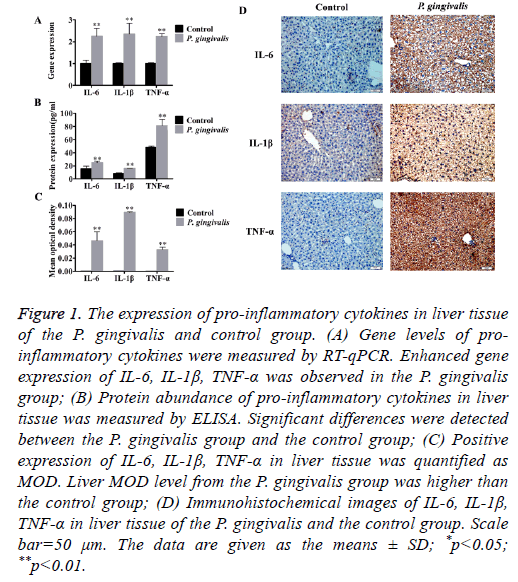
Elevated mRNA levels of the pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were found in the P. gingivalis group (Figure 1A and Table 1).
| Control | P. gingivalis | P-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| IL-6 | 1.00 ± 0.15 | 2.26 ± 0.36 | <0.01** |
| IL-1β | 1.00 ± 0.03 | 2.36 ± 0.49 | <0.01** |
| TNF-α | 1.00 ± 0.04 | 2.25 ± 0.12 | <0.01** |
Table 1. Analysis of TNF-α, IL-6, IL-1β mRNA expression levels in mice liver tissues by qRT-PCR.
Figure 1: The expression of pro-inflammatory cytokines in liver tissue of the P. gingivalis and control group. (A) Gene levels of proinflammatory cytokines were measured by RT-qPCR. Enhanced gene expression of IL-6, IL-1β, TNF-α was observed in the P. gingivalis group; (B) Protein abundance of pro-inflammatory cytokines in liver tissue was measured by ELISA. Significant differences were detected between the P. gingivalis group and the control group; (C) Positive expression of IL-6, IL-1β, TNF-α in liver tissue was quantified as MOD. Liver MOD level from the P. gingivalis group was higher than the control group; (D) Immunohistochemical images of IL-6, IL-1β, TNF-α in liver tissue of the P. gingivalis and the control group. Scale bar=50 μm. The data are given as the means ± SD; *p<0.05; **p<0.01.
In addition, oral administration of P. gingivalis increased protein levels of TNF-α, IL-6 as well as IL-1β (Figure 1B). Immunohistochemical staining of liver tissues also indicated up-regulation of protein expression compared with the control group. There was a significant difference in MOD between the P. gingivalis and the control group (p<0.01) (Figures 1C and 1D).
Gene and protein expression of the pro-inflammatory cytokines in spleen tissue
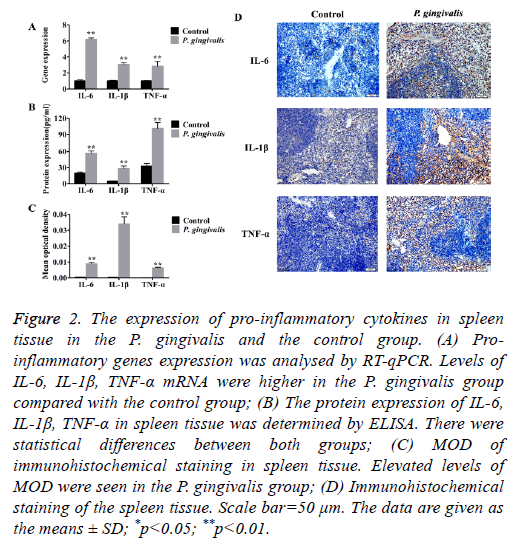
Production of pro-inflammatory genes TNF-α, IL-6 and IL-1β were significantly up-regulated in the P. gingivalis group as compared to the control group (p<0.01) (Figure 2A and Table 2).
| Control | P. gingivalis | P-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| IL-6 | 1.00 ± 0.13 | 6.20 ± 0.20 | <0.01** |
| IL-1β | 1.00 ± 0.06 | 3.07 ± 0.20 | <0.01** |
| TNF-α | 1.00 ± 0.04 | 2.82 ± 0.64 | <0.01** |
Table 2. Analysis of TNF-α, IL-6, IL-1β mRNA expression levels in mice spleen tissues by qRT-PCR.
Figure 2: The expression of pro-inflammatory cytokines in spleen tissue in the P. gingivalis and the control group. (A) Proinflammatory genes expression was analysed by RT-qPCR. Levels of IL-6, IL-1β, TNF-α mRNA were higher in the P. gingivalis group compared with the control group; (B) The protein expression of IL-6, IL-1β, TNF-α in spleen tissue was determined by ELISA. There were statistical differences between both groups; (C) MOD of immunohistochemical staining in spleen tissue. Elevated levels of MOD were seen in the P. gingivalis group; (D) Immunohistochemical staining of the spleen tissue. Scale bar=50 μm. The data are given as the means ± SD; *p<0.05; **p<0.01.
Correspondingly, protein levels of TNF-α, IL-6 and IL-1β were also higher (Figure 2B). Furthermore, the immunohistochemical staining of spleen tissue from the P. gingivalis group indicated elevated levels of MOD which implied positive expression of TNF-α, IL-6 and IL-1β proteins (Figures 2C and 2D).
Histological analysis of liver tissue
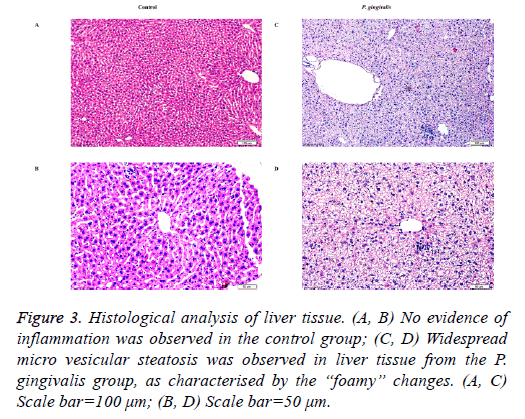
Scattered micro vesicular steatosis was observed in hepatocytes of the P. gingivalis group. A majority of hepatocytes had “foamy” changes where the nuclei were typically centrally located. Furthermore, a few inflammatory cells had infiltrated the tissue (Figure 3). In contrast, liver sections from the control group showed no obvious alterations in the hepatic cord structure or hepatic cells (Figures 3).
Figure 3: Histological analysis of liver tissue. (A, B) No evidence of inflammation was observed in the control group; (C, D) Widespread micro vesicular steatosis was observed in liver tissue from the P. gingivalis group, as characterised by the “foamy” changes. (A, C) Scale bar=100 μm; (B, D) Scale bar=50 μm.
Histological analysis of spleen tissue
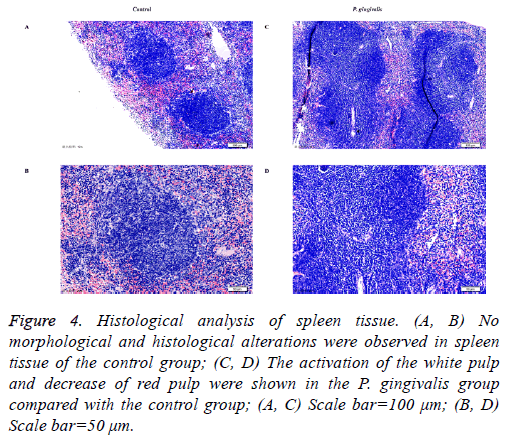
Several visible histological changes were found in the spleen tissue of P. gingivalis administered mice. We observed obvious activation of the white pulp and reduction of the red pulp compared with the control group. In addition, neutrophil infiltration was detected in the spleen tissue of the P. gingivalis group (Figure 4). In contrast, no histological changes of inflammation were detected in spleen tissue obtained from the control group (Figure 4).
Figure 4: Histological analysis of spleen tissue. (A, B) No morphological and histological alterations were observed in spleen tissue of the control group; (C, D) The activation of the white pulp and decrease of red pulp were shown in the P. gingivalis group compared with the control group; (A, C) Scale bar=100 μm; (B, D) Scale bar=50 μm.
Discussion
To our knowledge, this is one of the first animal model studies revealing the effects of periodontitis on the expression of proinflammatory cytokines in liver and spleen by oral administration of P. gingivalis. P. gingivalis can initiate periodontitis; LPS from P. gingivalis can enter gingival tissues and elicit an inflammatory response leading to an increase in pro-inflammatory cytokines [25]. The proposed pathways linking periodontitis and its systemic effects include the direct impact of bacterial products from the oral cavity and the dissemination of inflammatory mediators and immune complexes, including cytokines and chemotactic factors, generated by periodontitis lesions [26,27]. Recent studies reported that elevated levels of inflammatory cytokines such as IL-6 and IL-1β were secreted by the host in response to P. gingivalis stimulation [28,29]. Although the precise mechanisms remain unclearthe accumulation of inflammatory mediators in the circulation is thought to trigger the inflammatory response in remote tissues.
The liver is pivotal in protecting the host against microorganisms and microbial components as it can trigger an immunological response against endogenic and exogenic toxins present in the portal blood [30]. To date, an increasing number of studies have evidenced a correlation between periodontal diseases and the pathogenesis of liver diseases such as NAFLD, cirrhosis and hepatocellular carcinoma [31]. However, there is no direct evidence implicating the oral administration of P. gingivalis with the induction of proinflammatory cytokines expression in the liver. In this study, we observed that livers from mice in the P. gingivalis group exhibited increased levels of pro-inflammatory cytokines TNF- α, IL-6, IL-1β compared with the control group. Numerous studies demonstrated a close correlation between hepatic inflammation and chronic liver disease [32,33]. Cytokines produced by hepatic and inflammatory cells significantly contributed to the development of liver disease. TLRs are capable of recognising endotoxins such as LPS and induce the secretion of pro-inflammatory cytokines like IL-1, IL-6 and TNF-α from macrophages and adipocytes; thus promoting the liver disease [34]. A number of studies have shown that Kupffer cells play a pivotal role in the development of NAFLD involving TLR4 response to LPS and the consequent activation of Kupffer cell to release inflammatory cytokines [35-37]. In agreement, mice lacking TLR4 were less likely to develop NAFLD and were insulin resistance [38-40]. In the present study, we observed a significant up-regulation of TNF-α, IL-6, IL-1β in the liver of P. gingivalis administered mice. TNF-α is a key inflammatory mediator and can be secreted directly by hepatic cells and Kupffer cells [41]. Both human and animal studies have confirmed the role of TNF-α in the development of NAFLD and non-alcoholic steatohepatitis [42]. Moreover, a study using the rat periodontitis model found that after chronic administration of LPS from Escherichia coli and Streptomyces griseus proteases, rats developed periodontitis and the liver manifested steatosis, with inflammation and fibrosis following the production of TNF-α in the liver [43]. As a powerful mediator in the acute phase response of hepatocytes, IL-6 is generated by a number of cells including activated macrophages and lymphocytes [44]. The secretion of IL-6 is induced by IL-1 and TNF-α. In turn, IL-6 is able to regulate the production of IL-1, by activating the secretion of the IL-1 receptor antagonist and TNF-α directly [45]. IL-6 is therefore a key to local pathological processes as well as systemic inflammation. Mas et al. stated that IL-6-deficient mice showed a reduction in diet-induced non-alcoholic steatohepatitis in comparison with controls [46]. Further, IL-6 accumulated in the livers of patients with non-alcoholic steatohepatitis compared to control patients, suggesting a positive relationship between IL-6 expression in the liver and the degree of NAFLD [47]. In the case of IL-1β, an in vivo study has shown that IL-1β deficient mice had a marked reduction in the development of steatosis to steatohepatitis and liver fibrosis [48]. Thereby demonstrating the important role for IL-1β in the promotion of liver disease. Apart from the upregulation of pro-inflammatory cytokines, we also observed the presence of diffuse micro vesicular steatosis in the majority of hepatocytes in the P. gingivalis group. Micro vesicular steatosis has been implicated in the advanced histology of NAFLD. A clinical trial has demonstrated the association between micro vesicular steatosis and hepatocyte injury including the presence of steatosis, ballooning cell injury and fibrosis [49]. Based on our results, we propose that P. gingivalis participates in the development of liver disease by activating the production of pro-inflammatory cytokines including TNF-α, IL-1β and IL-6 in the liver. However, the precise mechanism of P. gingivalis mediated pathogenesis of liver disease is still unknown. As for the liver, the levels of TNF-α, IL-1β and IL-6 were significantly up-regulated in the spleen of mice following oral administration of P. gingivali. Also histological changes were observed in this tissue. The spleen is the largest peripheral lymphoid organ, composed of two distinct components: the white and red pulp. The white pulp is made up of lymphoid tissue, mostly lymphocytes (T cells and B cells) and macrophages, while the red pulp is composed of parenchyma and lots of vascular sinuses and sinusoids [50]. The white pulp is thus regarded as the main site of immunological function in the spleen. The spleen interacts with the circulatory, reticuloendothelial, and immune systems and is key to developing an immune response to antigens. As the first line of defence, the spleen is capable of recognising specific PAMPS such as LPS. Moreover, the spleen is considered a reservoir for inflammatory monocytes [51,52]. A study in vivo showed that LPS from Escherichia coli could activate dendritic cells of the spleen and elevate the expression of pro-inflammatory cytokines including IL-6, interleukin 12 p40, and TNF-α compared to the control group [53]. Another study reported that pro-inflammatory cytokines IL-6 and TNF-α and the antiinflammatory cytokine IL-10 were secreted locally in the spleen after LPS injection [54]. In addition, Semaeva et al. found that during LPS-induced inflammation, the spleen showed a significant efflux of lymphocytes and up-regulation of pro-inflammatory cytokines released into the systemic circulation through the isolation of spleen lymph [55]; thus confirming the central role of the spleen in a systemic immune reaction. There are some limitations to our study. Histological changes in the liver were for example examined by haematoxylin and eosin staining which may have underestimated the appearance of micro vesicular steatosis. Compared to haematoxylin and eosin stain, fat-specific oil red O stain would have been better for the purpose of our study. Future work is needed to determine whether the oral administration of P. gingivalis changes the expression of hepatic injury markers including aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase.
Acknowledgement
This study was supported by the National Natural Science Foundation of China (81570983).
References
- Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med 2000; 160: 2749-2755.
- Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc 2006; 137: 14-20.
- Ribeiro J, Leão A, Novaes AB. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol 2005; 32: 412-416.
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol 1998; 3: 51-61.
- Hayes C, Sparrow D, Cohen M, Vokonas PS, Garcia RI. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol 1998; 3: 257-261.
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996; 67: 1103-1113.
- Hans M, Hans VM. Toll-like receptors and their dual role in periodontitis: a review. J Oral Sci 2011; 53: 263-271.
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017; 22: 17038.
- Baker PJ, Howe L, Garneau J, Roopenian DC. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol Med Microbiol 2002; 34: 45-50.
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5: 987-995.
- Kouich T, Kazuhisa Y, Sachiko A, Kensuke M, Hidefumi K, Toshio U, Hiromasa Y. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect Immun 2000; 68: 3731-3735.
- Swaminathan V, Prakasam S, Puri V, Srinivasan M. Role of salivary epithelial toll-like receptors 2 and 4 in modulating innate immune responses in chronic periodontitis. J Periodontal Res 2013; 48: 757-765.
- Erridge C. Diet, commensals and the intestine as sources of pathogen-associated molecular patterns in atherosclerosis, type 2 diabetes and non-alcoholic fatty liver disease. Atherosclerosis 2011; 216: 1-6.
- Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madaliński K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol 2010; 30: 1046-1052.
- Ide M, Jagdev D, Coward PY, Crook M, Barclay GR, Wilson RF. The short-term effects of treatment of chronic periodontitis on circulating levels of endotoxin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6. J Periodontol 2004; 75: 420-428.
- Marcaccini AM, Meschiari CA, Sorgi CA, Saraiva MC, de Souza AM, Faccioli LH, Tanus-Santos JE, Novaes AB, Gerlach RF. Circulating interleukin-6 and high-sensitivity C-reactive protein decrease after periodontal therapy in otherwise healthy subjects. J Periodontol 2009; 80: 594-602.
- Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 1998; 279: 1477-1482.
- Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol 2011; 38: 60-84.
- Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature 2008; 454: 470-477.
- Ferrante AW Jr. Obesity‐induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med 2007; 262: 408-414.
- Satapathy SK, Sanyal AJ. Novel treatment modalities for nonalcoholic steatohepatitis. Trends Endocrinol Metab 2010; 21: 668-675.
- Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis 2010; 30: 391-401.
- Brunt EM. Non-alcoholic fatty liver disease: what's new under the microscope? Gut 2011; 60: 1152-1158.
- Yoneda M, Naka S, Nakano K, Wada K, Endo H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, Murata S, Tohnai I, Sumida Y, Shima T, Kuboniwa M, Umemura K, Kamisaki Y, Amano A, Okanoue T, Ooshima T, Nakajima A. Involvement of a periodontal pathogen, Porphyromonas gingivalis on the pathogenesis of non-alcoholic fatty liver disease. BMC Gastroenterol 2012; 12: 16.
- Page RC. The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontol 1998; 3: 108-120.
- Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic Diseases Caused by Oral Infection. Clin Microbiol Rev 2000; 13: 547-558.
- Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 1999; 100: 20-28.
- Hernichel-Gorbach E, Kornman KS, Holt SC, Nichols F, Meador H, Kung JT, Thomas CA. Host responses in patients with generalized refractory periodontitis. J Periodontol 1994; 65: 8-16.
- Hasegawa-Nakamura K, Tateishi F, Nakamura T, Nakajima Y, Kawamata K, Douchi T, Hatae M, Noguchi K. The possible mechanism of preterm birth associated with periodontopathic Porphyromonas gingivalis. J Periodontal Res 2011; 46: 497-504.
- Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010; 7: 691-701.
- Han P, Sun D, Yang J. Interaction between periodontitis and liver diseases. Biomed Rep 2016; 5: 267-276.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860-867.
- Li XH, McGrath KC, Nammi S, Heather AK, Roufogalis BD. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol 2012; 110: 238-244.
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology 2001; 34: 101-108.
- Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 2000; 31: 932-936.
- Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009; 51: 212-223.
- Honda Y, Yamagiwa S, Matsuda Y, Takamura M, Ichida T, Aoyagi Y. Altered expression of TLR homolog RP105 on monocytes hypersensitive to LPS in patients with primary biliary cirrhosis. J Hepatol 2007; 47: 404-411.
- Björkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 2004; 10: 416-421.
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116: 3015-3025.
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005; 11: 191-198.
- Montecucco F, Mach F. Does non-alcoholic fatty liver disease (NAFLD) increase cardiovascular risk? Endocr Metab Immune Disord Drug Targets 2008; 8: 301-307.
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-: Direct role in obesity-linked insulin resistance. Science 1993; 259: 87-91.
- Tomofuji T, Ekuni D, Yamanaka R, Kusano H, Azuma T, Sanbe T, Tamaki N, Yamamoto T, Watanabe T, Miyauchi M, Takata T. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J Periodontol 2007; 78: 1999-2006.
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000; 148: 209-214.
- Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol 1994; 96: 437-443.
- Mas E, Danjoux M, Garcia V, Carpentier S, Ségui B, Levade T. IL-6 deficiency attenuates murine diet-induced non-alcoholic steatohepatitis. PLoS One 2009; 4: e7929.
- Dogru T, Ercin CN, Erdem G, Sonmez A, Tapan S, Tasci I. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol 2008; 103: 3217-3218.
- Kamari Y, Shaish A, Vax E, Shemesh S, Kandel-Kfir M, Arbel Y, Olteanu S, Barshack I, Dotan S, Voronov E, Dinarello CA, Apte RN, Harats D. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 2011; 55: 1086-1094.
- Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Ünalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol 2011; 55: 654-659, 2011.
- Cesta MF. Normal Structure, function, and histology of the spleen. Toxicol Pathol 2006; 34: 455-465.
- Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612-616.
- Robbins CS, Chudnovskiy A, Rauch PJ, Figueiredo JL, Iwamoto Y, Gorbatov R, Etzrodt M, Weber GF, Ueno T, van Rooijen N, Mulligan-Kehoe MJ, Libby P, Nahrendorf M, Pittet MJ, Weissleder R, Swirski FK. Extramedullary hematopoiesis generates Ly-6C (high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012; 125: 364-374.
- Xu L, Kwak M, Zhang W, Lee PC, Jin JO. Time-dependent effect of E. coli LPS in spleen DC activation in vivo: Alteration of numbers, expression of co-stimulatory molecules, production of pro-inflammatory cytokines, and presentation of antigens. Mol Immunol 2017; 85: 205-213.
- Wiig H. Pathophysiology of tissue fluid accumulation in inflammation. J Physiol 2011; 589: 2945-2953.
- Semaeva E, Tenstad O, Skavland J, Enger M, Iversen PO, Gjertsen BT, Wiig H. Access to the spleen microenvironment through lymph shows local cytokine production, increased cell flux, and altered signaling of immune cells during lipopolysaccharide-induced acute inflammation. J Immunol 2010; 184: 4547-4556.