Research Article - Journal of Molecular Oncology Research (2017) Volume 1, Issue 1
EBV LMP1 regulates cancer growth through IRF-7 in nasopharyngeal carcinoma.
Jianguo Wu1*, Dongsheng Xu1,2, Qian Cheng3, Luwen Zhang4, Dan Cheng5 and Jing Zhao5
1State Key Laboratory of Virology, College of Life Sciences, Wuhan University, Wuhan 430072, P.R. China
2Department of Spine Surgery and Neural Rehabilitation Center, Tongji Hospital, Tongji University, School of Medicine, Shanghai 20065, P. R. China
3Department of Anesthesiology, Fudan University Shanghai Cancer Center, Shanghai Medical College, Fudan University, Shanghai 200032, P. R. China
4Nebraska Center for Virology, School of Biological Sciences, University of Nebraska, Lincoln, Nebraska 68588, P. R. China
5Department of Neurology, Shanghai Minhang District Central Hospital, Shanghai 201199, P. R. China
- *Corresponding Author:
- Jianguo Wu
State Key Laboratory of Virology, College of Life Sciences
Wuhan University, Wuhan 430072, PR China
E-mail: jwu@whu.edu.cn
Accepted date: 01 August, 2017
Citation: Xu D, Zhao J, Cheng Q, et al. EBV LMP1 regulates cancer growth through IRF-7 in nasopharyngeal carcinoma. J Mol Oncol Res. 2017;1(1):47-52.
DOI: 10.35841/molecular-oncology.1.1.47-52
Visit for more related articles at Journal of Molecular Oncology ResearchAbstract
The cellular Interferon (IFN) regulatory factor-7 (IRF-7) was originally identified in cells with Epstein-Barr virus (EBV) infection, which constituted of positive circuit with viral oncoprotein latent membrane protein 1 (LMP1), and both proteins are involved in neoplasia. EBV is known to be associated with the development of various cancers, including Hodgkin lymphoma, non-Hodgkin lymphoma (NHL), Burkitt lymphoma, gastric lymphoma, and nasopharyngeal carcinoma (NPC). Although the exact role of IRF-7 in EBV-related oncogenesis is not clear, in this study, we demonstrated the role of IRF-7 in angiogenesis suppression via LMP1 down-regulation. Additionally, we employed knock down of IRF-7 and verified the inhibition of IRF-7 in NPC growth in vivo. These findings suggest that IRF-7 could be a potential therapeutic target in EBV-infected NPC tumors.
Keywords
Epstein-Barr virus, LMP1, IRF-7, C666-1, Nasopharyngeal Carcinoma
Introduction
Nasopharyngeal carcinoma (NPC) is characterized by its distinct geographic distributions and is a very common cancer in the Southern and Southeast regions of China [1]. The undifferentiated or poorly differentiated NPC type is consistently associated with the Epstein-Barr virus (EBV). There is compelling evidence to suggest that EBV is a causal agent of NPC, and that EBV is likely involved in the oncogenesis of NPC [2].
EBV is a member of the herpesviridae family, also known as human herpes virus 4 (HHV-4), and was discovered by Michael Epstein and Yvonne Barr in 1964 during their research on Burkitt lymphoma (BL) [3,4]. Of note, EBV can be found in the latent stage of both lymphoma and carcinomas, and EBV is known to be associated with Burkitt lymphoma, Hodgkin's lymphoma, immunosuppression-related lymphoma, T and NK cell lymphoma, and nasopharyngeal and stomach carcinomas [5,6]. In the initiation and progression of neoplasia, the viral protein LMP1 of EBV was best recognized to be oncogenic in both B lymphocyte and epithelial cells. LMP1 is highly associated with cellular interferon regulation factors (IRF family, IRFs) in EBV transformed cells and EBV-associated tumors. However, the relationship between LMP1 and IRFs in oncogenesis is unclear.
IRF-7 is one of nine members of the interferon regulatory factor (IRF) proteins family, which was originally identified as transcriptional regulators of the type I interferon system, and the IRF proteins family has multiple functions that includes reproduction, development, stress responses, immune defense, and carcinogenesis[7-9]. To note, IRF-7 was cloned in the context of EBV latency. There is a series of evidence suggesting that IRF-7 has oncogenic properties, and IRF-7 may be an oncoprotein that interacts with LMP1 in EBV [10,11]. Furthermore, studies have suggested that the interaction of IRF-7 and LMP1 may initiate oncogenesis in EBV transformed cancers [12-14].
LMP1 is one of five key EBV-encoded viral proteins that is essential for B cell immortalization, although its expression in human epithelial cells is not normally associated with growth transformation [15-17]. Research studies have revealed that LMP1 can induce profound effects in epithelial cell, such as epidermal hyperplasia, cell motility and invasion, and the possession of oncogenic properties[15,16-18]. LMP1 is constitutively activated in various signaling pathways in EBVinfected cells, including NF-kappaB and AP-1 pathways in NPC [19]. LMP1 plays a central role in both initiation and progression of NPC, in which LMP1 modulates the expression of key tumor suppressor genes p53, modulates the G1-S cellcycle checkpoint, imparts resistance to apoptosis induces an epithelial-mesenchyme-transition (EMT), and promotes angiogenesis [15]. High expression levels of LMP1 or RAGE have been observed with higher microvessel counts in NPC tumors, and through the mechanisms involved in the induction of cyclooxygenase 2 (COX-2), hypoxia inducible factor 1 alpha (HIF-1α), and MMP-9, IL-8 [15].
Although LMP1 promotes angiogenesis in various pathways, whether IRF-7, which is intimate to LMP1, plays a role in angiogenesis in EBV-infected tumor is still uncertain. We have shown that siIRF-7 suppresses NPC tumor growth in vivo, in which siIRF-7 down-regulates LMP1 blood vessel; therefore, siIRF-7 could be a potential therapeutic target for NPCassociated tumor.
Materials and Methods
Cell line culture
EBV positive C666-1 cells were cultured in RPMI-1640 (Hyclone), supplemented with 10% fetal bovine serum (FBS; Sijiqing, China), 50 U/mL penicillin G and 50 U/mL streptomycin (Gibco, Carlsbad, CA, USA) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The medium was changed every 2 days.
Cell proliferation and MTT assay
The 3-(4,5-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) growth assay was used to measure the cell proliferation rate using a Cell Titer 96 nonradioactive cell proliferation assay kit (Promega) according to the manufacturer’s recommendations. On day 3, a total 1 × 104 live cells were collected and placed into 85 μl RPMI medium plus 10% FBS in a 96-well plate. 15 μl of dye solution was added to each well. The cells were incubated in 37°C for 4 h. 100 μl of solubilization solution/stop mix was added and incubated for an additional hour. The absorbance at a wavelength of 490 nm was recorded using a 96-well plate reader. There was no dramatic changes in cell number within the time frame, and procedures were described in a previous study [8].
Construction of recombinant adenovirus
Ad-siLMP1 and Ad-siIRF-7, designed sequence of LMP1 and IRF-7 via GenBank, acquired LMP1 expression vector, synthesized interference sequence specific to LMP1 and IRF-7, inserted adenovirus expression vector and gained recombinant adenovirus Ad-siLMP1 and Ad-siIRF-7, inserted siLuc into control group, and determined by the company. Procedures of siLMP1, siP53, siLuc, and siIRF-7 plasmids can be found in a previous study [20].
Animal model and cell implantation
The NU/NU nude mice were ordered and delivered from Charles River.
The selected 6-8 week-old nude mice were injected with 1 × 106 C666-1 nasopharyngeal carcinoma cells, Ad-siluc-infected C666-1 cells, Ad-siIRF-7-infected C666-1 cells, and AdsiLMP1- infected C666-1 cells subcutaneously. The course of tumor formation was observed for five weeks continuously, and the tumor weight and volume were recorded.
Immunofluorescence method
Stripped tumor tissue samples from nude mice were embedded in optical cutting temperature to prepare for frozen sections, which were fixed by paraformaldehyde and sealed with 10% goat serum. DAPI to nuclear staining and antibody to LMP1 were proportionally diluted and dripped onto the tissue slides, and were coated and incubated overnight at 4°C. On the following day, the sections were rinsed with PBS three times. Secondary antibodies were proportionally diluted and dripped onto the tissue section, respectively, and incubated at 37°C for 1 h and rinsed with PBS three times. Subsequently, the slides were covered with cover slips and were observed under a fluorescence microscope at 400X magnification.
RNA extraction, reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. The RNA samples were treated with DNase I before RT reactions. 2 μg of total RNA was used for cDNA synthesis of random hexamer with reverse transcriptase (Invitrogen) (RT+) or without RT (RT-). The same amount of cDNAs was used for PCR amplification for LMP1 and Actin. For LMP1-forward gene, TCGTTATGAGTGACTGGACTGG and LMP1-reverse gene, CCTGTCCGTGCAAATTCCA. For actin gene: Actin1, 5′TTC TAC AAT GAG CTG CGT GT-3′and Actin 2, 5′GCC AGA CAG CAC TGT GTT GG-3. PCR was performed with Go Taq Flexi DNA Polymerase (Promega) following manufacturer's protocol. Different PCR cycles were used to ensure the products were in the linear ranges of the amplifications, and procedures were described in the previous study [21].
Gene array analysis
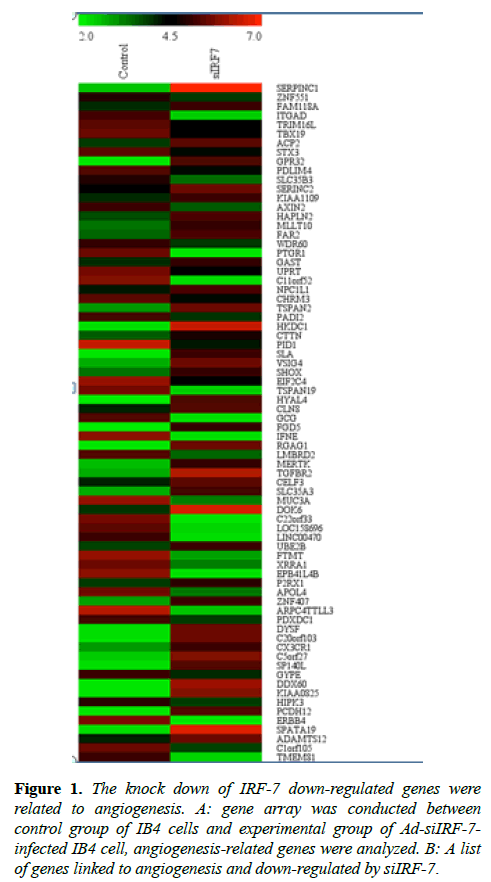
Total RNA was quantified by the NanoDrop ND-2000 (Thermo Scientific) and RNA integrity was assessed using Agilent Bioanalyzer 2100 (Agilent Technologies). The sample labeling, microarray hybridization, and washing were performed based on the manufacturer’s standard protocols. Briefly, total RNA was transcribed to double-stranded cDNA, and then synthesized into cRNA and labeled with Cyanine-3- CTP. The labeled cRNAs were hybridized onto the microarray (Figure 1). After washing, arrays were scanned by the Agilent Scanner G2505C (Agilent Technologies). Feature Extraction software (version10.7.1.1, Agilent Technologies) was used to analyze array images to obtain the raw data. Genespring was employed to carry out basic analysis with the raw data.
Figure 1: The knock down of IRF-7 down-regulated genes were related to angiogenesis. A: gene array was conducted between control group of IB4 cells and experimental group of Ad-siIRF-7- infected IB4 cell, angiogenesis-related genes were analyzed. B: A list of genes linked to angiogenesis and down-regulated by siIRF-7.
Results
Knock down of IRF-7 down-regulated genes related to angiogenesis
IRF-7 was cloned in the context of EBV latency, and IRF-7 has an intimate relation with EBV transformation [21]. It has been well demonstrated that IRF-7 exhibited oncogenic property by phosphorylation and nuclear translocation [22,23]. Previous study revealed that knock-down of IRF-7 expression reduced the proliferation rate and enhanced apoptosis in IB4 cell line [24]. To unravel the role of IRF-7 in relation to angiogenesis, which is an essential pathophysiological process in tumor growth and metastasis, we employed gene array technique in EBV positive IB4 lymphoma cell line. Our results revealed that IRF-7 correlated strongly with genes related to angiogenesis in EBV positive cells (Table 1). The related genes consisted of GRTP1SORCS3FOXJ1, as well as other angiogenesis-related genes.
| Gene Symbol ( angiogenesis) | siIRF-7 | Control | Difference value |
|---|---|---|---|
| GRTP1 | 2.2660594 | 7.951235 | -5.6851756 |
| SORCS3 | 2.2795262 | 6.299667 | -4.0201408 |
| FOXJ1 | 2.4101117 | 6.203555 | -3.7934433 |
| GK5 | 2.2695155 | 6.0348005 | -3.765285 |
| OLFML2A | 2.2992408 | 5.911006 | -3.6117652 |
| TSPEAR | 2.3100839 | 5.901888 | -3.5918041 |
| IFNE | 2.25637 | 5.826875 | -3.570505 |
| EPB41L4B | 2.242587 | 5.810715 | -3.568128 |
| ERBB4 | 2.1626709 | 5.6823845 | -3.5197136 |
| PTGR1 | 2.1692169 | 5.4852505 | -3.3160336 |
| FGFBP2 | 2.2433496 | 5.547415 | -3.3040654 |
| DNAH5 | 2.244691 | 5.5385365 | -3.2938455 |
| CA1 | 2.2585793 | 5.3886175 | -3.1300382 |
| LANCL1 | 4.069931 | 7.191886 | -3.121955 |
| GCG | 2.2340565 | 5.32301 | -3.0889535 |
| PLCL1 | 2.247598 | 5.3347855 | -3.0871875 |
| FTMT | 2.8479733 | 5.927552 | -3.0795787 |
Table 1. IB4 cells and experimental group of Ad-siIRF-7-infected IB4 cell, angiogenesis-related genes were analyzed.
Knock down of IRF-7 expression suppressed tumor growth of NPC C666-1 cells in Nu/Nu mice
IRF-7 itself has oncogenic potential and is associated with EBV transformation in vitro and in vivo in central nervous system lymphoma [25]. IRF-7 may interact with LMP1 physically, and then induces the expression and activation of IRF-7 [26]. A recent study has unveiled that Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibited NPC growth in vivo [27], and the findings implied that IRF-7 may negatively regulate tumor growth through LMP1.
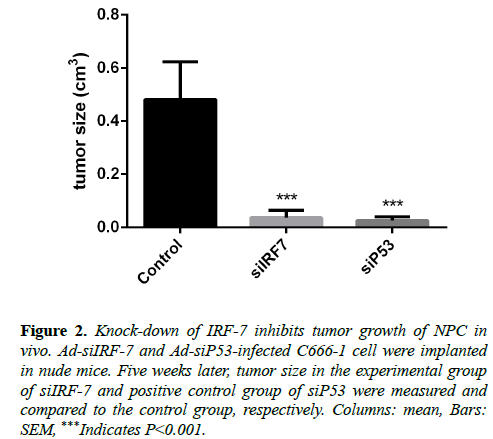
To determine whether IRF-7, which is involved in angiogenesis and interacts intimately with LMP1, could be a therapeutic target in nasopharyngeal carcinoma, we implanted Ad-siIRF-7-infected C666-1 cells into Nu/Nu mice model, and observed tumor size continuously for five weeks. Results showed that knock-down of IRF-7 expression reduced tumor size of NPC in vivo as compared to the control group (Figure 2).
Figure 2: Knock-down of IRF-7 inhibits tumor growth of NPC in vivo. Ad-siIRF-7 and Ad-siP53-infected C666-1 cell were implanted in nude mice. Five weeks later, tumor size in the experimental group of siIRF-7 and positive control group of siP53 were measured and compared to the control group, respectively. Columns: mean, Bars: SEM, ***Indicates P<0.001.
Knock-down of LMP1 suppressed tumor growth of NPC in vivo
There is an overwhelming evidence supporting that EBVencoded LMP1 plays critical pathological roles in NPC development; for instance, LMP1 modulates key tumor suppressor genes, modulates the G1-S cell-cycle checkpoint, induces the pro-inflammatory cytokines, imparts resistance to apoptosis, induces an epithelial-mesenchyme-transition, promotes cell motility, invasion and metastasis, and modulates cellular micro-RNAs [15].
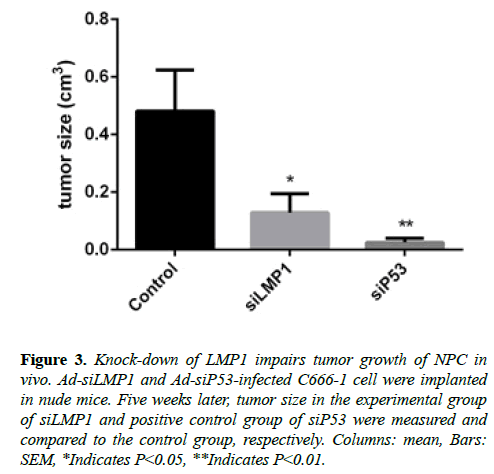
But whether down-regulation of LMP1 expression could inhibit NPC tumor growth in vivo is of interest. We constructed Ad-siLMP1-infected C666-1 cells, implanted to Nu/Nu mice, and compared the tumor size to the control group five weeks afterwards.
We discovered that the tumor size in the experimental group was smaller than that in the control group. This finding of in vivo experiment with RNA interference to LMP1 (Figure 3) is consistent with findings from the previous study that employed LMP1 antibody to NPC in vivo [28].
Figure 3: Knock-down of LMP1 impairs tumor growth of NPC in vivo. Ad-siLMP1 and Ad-siP53-infected C666-1 cell were implanted in nude mice. Five weeks later, tumor size in the experimental group of siLMP1 and positive control group of siP53 were measured and compared to the control group, respectively. Columns: mean, Bars: SEM, *Indicates P<0.05, **Indicates P<0.01.
Knock-down of IRF-7 down-regulated LMP1 expression in vitro and in vivo
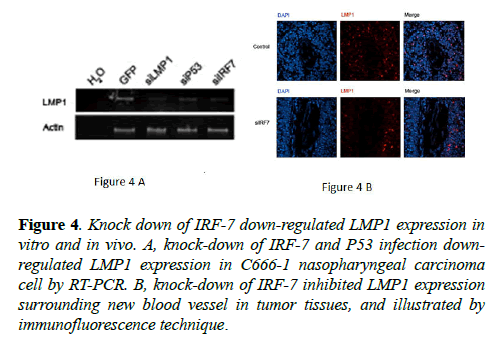
Gene array results have revealed IRF-7 to be strongly correlated to angiogenesis-relevant genes. LMP1 is involved in angiogenesis via multiple pathways that include RAGE, COX2, HIF-1α, and IL-8 [1,29,30]. To elucidate the role of LMP1 in siIRF-7-induced tumor growth suppression in vivo, we verified LMP1 expression by RT-PCR technique in C666-1 cell line and by immunofluorescence technique in tumor tissue.
The results showed that knock-down of IRF-7 (Figure 4) expression reduced LMP1 mRNA level in the cell line and LMP1 protein level around new blood vessel of tumor tissue, respectively. These results could be explained based on findings from the previous studies, in which LMP1 could influence tumor development from various aspects, including angiogenesis, invasion, metastasis, apoptosis, as well as proliferation [1-15]. Therefore, down-regulation of LMP1 may be one of the mechanisms in siIRF-7-induced tumor growth suppression of NPC in vivo.
Figure 4: Knock down of IRF-7 down-regulated LMP1 expression in vitro and in vivo. A, knock-down of IRF-7 and P53 infection downregulated LMP1 expression in C666-1 nasopharyngeal carcinoma cell by RT-PCR. B, knock-down of IRF-7 inhibited LMP1 expression surrounding new blood vessel in tumor tissues, and illustrated by immunofluorescence technique.
Discussion
The findings reported in this study demonstrate that knock down of IRF-7 reduces LMP1 expression level in new blood microvessel, and inhibits NPC tumor growth in vivo. To our knowledge, this is the first study to investigate the functions of IRF-7 on angiogenesis in EBV-associated tumors in vivo.
Although there are an abundant of studies focused to understand IRF-7 in areas of activation, regulation, modification, function, molecular structure, protein interaction and gene level, fewer studies have examined the pathological initiation and progression of cancers with EBV latency. For instance, angiogenesis and lymphangiogenesis play essential roles in tumor metastasis and recurrence. Co-localized with IRF-7 in EBV-related cancers, LMP1 has been confirmed to be involved in angiogenesis through the induction of VEGF production in epithelial cell by COX-2, FGF-2, MMP-9, and HIF-1α [1,29,30]. Studies have found that the angiogenic chemokine, IL-8, correlates with increased angiogenesis and poor prognosis in NPC tumor by p38-MAPK, NF-kB, and ATF2 [1,31,32].
Our results indicated that IRF-7 correlates with LMP1 in new blood microvessel in NPC tumor, which is consistent with findings from a previous study that LMP1 promote angiogenesis through various pathway. For the first time, we revealed that IRF-7 plays roles in angiogenesis in EBVinfected NPC tumor. We speculate that IRF-7 is essential in the angiogenesis of pathological process in stomach carcinoma, which is another reported EBV-related epithelial cell transformed cancer.
Through the VEGF-C/VEGF receptor-3 axis, and the reported correlation with lymph node metastasis in NPC, LMP1 has been confirmed to promote lymphangiogenesis [33]. In combination to our study, we speculate that, in addition to the inhibition of angiogenesis, knock down of IRF-7 expression could suppress lymphangiogenesis in NPC. Both of these phenomenon are critical determinants in metastasis and the recurrence of tumor.
We observed the effect of knock down of IRF-7 expression on NPC tumor growth in vivo. The results are consistent and are explainable based on the suppression effect of IRF-7 on angiogenesis. Therefore, we conclude that IRF-7 could be a potential therapeutic target in EBV-related tumor.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81471936 and 81271420).
References
- Yoshizaki T, Kondo S, Wakisaka N, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337(1):1-7.
- Ng MH, Chan KH, Ng SP,et al. Epstein-Barr virus serology in early detection and screening of nasopharyngeal carcinoma. Ai Zheng. 2006;25(2):250-6.
- Grywalska E, Markowicz J, Grabarczyk P, et al. Epstein-Barr virus-associated lymphoproliferative disorders. Postepy Hig Med Dosw (Online). 2013;67:481-90.
- Epstein MA, Achong BG, Barr YM. Vitus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964;1(7335):702-3.
- Morales-Sanchez A, Fuentes-Panana EM. Human Viruses and Cancer. Viruses. 2014;6(10):4047-79.
- Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res. 2004;10(3):803-21.
- Yanai H, Negishi H, Taniguchi T. The IRF family of transcription factors: Inception, impact and implications in oncogenesis. Oncoimmunology. 2012;1(8):1376-86.
- Xu D, Zhang Y, Zhao L, et al. Interferon regulatory factor 7 is involved in the growth of Epstein-Barr virus-transformed human B lymphocytes. Virus Res. 2015;195:112-8.
- Nehyba J, Hrdlickova R, Bose HR. Dynamic evolution of immune system regulators: the history of the interferon regulatory factor family. Mol Biol Evol. 2009;26(11):2539-50.
- Ning S, Pagano JS, Barber GH. IRF-7: activation, regulation, modification and function. Genes Immun. 2011;12(6):399-414.
- Kim IW, Park HS. Colocalization of interferon regulatory factor 7 (IRF-7) with latent membrane protein 1 (LMP1) of Epstein-Barr virus. J Korean Med Sci. 2006;21(3):379-84.
- Ning S, Huye LE, Pagano JS. Interferon regulatory factor 5 represses expression of the Epstein-Barr virus oncoprotein LMP1: braking of the IRF-7/LMP1 regulatory circuit. J Virol. 2005;79(18):11671-6.
- Ning S, Hahn AM, Huye LE, et al. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J Virol. 2003;77(17):9359-68.
- Bentz GL, Shackelford J, Pagano JS. Epstein-Barr virus latent membrane protein 1 regulates the function of interferon regulatory factor 7 by inducing its sumoylation. J Virol. 2012;86(22):12251-61.
- Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol. 2012;22(2):144-53.
- Tsao SW, Tramoutanis G, Dawson CW, et al. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):473-87.
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757-68.
- Li HP, Chang YS. Epstein-Barr virus latent membrane protein 1: structure and functions. J Biomed Sci. 2003;10(5):490-504.
- Liu HD, Zheng H, Duan Z, et al. LMP1-augmented kappa intron enhancer activity contributes to upregulation expression of Ig kappa light chain via NF-kappaB and AP-1 pathways in nasopharyngeal carcinoma cells. Mol Cancer. 2009;8:92.
- Xu D, Zhao L, Del Valle L, et al. Interferon regulatory factor 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J Virol. 2008;82(13):6251-8.
- Zhang L, Pagano JS. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17(10):5748-57.
- Au WC, Moore PA, LaFleur DW, et al. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273(44):29210-7.
- Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17(22):6660-9.
- Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333(11):693-8.
- Zhang L, Zhang J, Lambert Q, et al. Interferon regulatory factor 7 is associated with Epstein-Barr virus-transformed central nervous system lymphoma and has oncogenic properties. J Virol. 2004;78(23):12987-95.
- Song YJ, Izumi KM, Shinners NP, et al. IRF-7 activation by Epstein-Barr virus latent membrane protein 1 requires localization at activation sites and TRAF6, but not TRAF2 or TRAF3. Proc Natl Acad Sci U S A. 2008;105(47):18448-53.
- Chen R, Zhang D, Mao Y, et al. A human Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibits nasopharyngeal carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2012;11:594-603.
- Mao Y, Zhang DW, Wen J, et al. A Novel LMP1 Antibody Synergizes with Mitomycin C to Inhibit Nasopharyngeal Carcinoma Growth in vivo Through Inducing Apoptosis and Downregulating Vascular Endothelial Growth Factor. Int J Mol Sci. 2012;13(2):2208-18.
- Wakisaka N, Murono S, Yoshizaki T, Epstein-barr virus latent membrane protein 1 induces and causes release of fibroblast growth factor-2. Cancer Res. 2002;62(21):6337-44.
- Wakisaka, N, Kondo S, Yoshizaki T, et al. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol. 2004;24(12):5223-34.
- Eliopoulos AG, Gallagher NJ, Blake SM, et al. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274(23):16085-96.
- Horikawa T, Kaizaki Y, Kato H, et al. Expression of interleukin-8 receptor A predicts poor outcome in patients with nasopharyngeal carcinoma. Laryngoscope. 2005;115(1):62-7.
- Wakisaka N, Hirota K, Kondo S, Murono S, et al. Induction of lymphangiogenesis through vascular endothelial growth factor-C/vascular endothelial growth factor receptor 3 axis and its correlation with lymph node metastasis in nasopharyngeal carcinoma. Oral Oncol. 2012;48(8):703-8.