- Biomedical Research (2010) Volume 21, Issue 4
Depression of cellular immunity correlated to stress: questions on welfare and protection in italian trotters after running performance
L. Passantino1, A.Passantino2,A. Cianciotta1, A. Ostillio3, F. Jirillo1, C. Russo1, R. Patruno1, Passantino G. F1*
1Department of Animal Health and Welfare, Faculty of Veterinary Medicine, University of Bari (Italy). Str. Prov.le per Casamassima Km 3, 70010, Valenzano (BA), Italy.
2Department of Veterinary Public Health, Faculty of Veterinary Medicine - University of Messina. Polo Universitario Annunziata, 98168 Messina, Italy.
3Research Doctorate in “EC countries’ norms concerning animal welfare and protection” University of Messina
- *Corresponding Author:
- G.F. Passantino
Dip. Sanità e Benessere Animale
Facoltà di Medicina Veterinaria
Str. Prov.le per Casamassima Km 3
70010, Valenzano (BA)
Italy.
Phone: 0039 080 4679904
Fax: 0039 080 4679813
E-mail: anatomia.normale@veterinaria.uniba.it
Accepted date: March 09 2010
Abstract
In all Equestrian sports, the welfare of the horse must be paramount and must never be subordinated to competitive or commercial influences. It is necessary to prohibit any train-ing methods which are abusive or cause fear or for which the horse has not been properly trained. The purpose of this study was to examine the effects of prolonged and intensive physical training on the immune response in trotter horses in order to recommend at the legislator the adoption of guidelines for the welfare of these animals. In fact, during their athletic life and some of them undergo lethal lung infections, therefore it is likely that modi-fications of physiologic cellular parameters could account for the increased susceptibility to microbial disease. Noteworthy, chronic stress has been shown to be immunosuppressive, whereas acute stress seems to lead to immunoenhancing effects. In particular, we have stud-ied some immune parameters as blood cells distribution, hemoglobin, hematocrit, erythro-cyte sedimentation rate, phagocytosis activity, macrophage Migration Inhibiting Factor (MIF) and finally the levels of (1-3)-ß-D-Glucan, as indicator of clearance. Taken together, these findings indicate a condition of reduced immune response in seven trotters after race, to identify possible biomarkers of stress dependent on physical exercise.
Keywords
stress, immunity, trotter, welfare, legislation.
Introduction
Trotters are exposed to a prolonged and continuous physi-cal stress; some of those horses are subjected to move from different race tracks and it often happens that after long transportation in trailers they became more sensitive to res-piratory infections [1]. Several authors [2-8] have showed that strenuous exercise, training and competition may be a factor which predisposes horses to the development of pleuropneumonia.
The lung’s immune system is protected by the alveolar macrophages activities and that high concentrations of cor-tisol in blood suppress the function of these leukocytes [9].
In animals as in humans it’s important to evaluate the im-mune system, considering the scheme of distribution and some blood cells and serum parameters.
In fact, studies on animal welfare evidenced similarities among species concerning the adaptive response of im-mune system to different stressing conditions [10-12]. It is known today that different types of stressors, including physical activity, can cause neuro-endocrine and hormonal changes that may mediate alterations in immune cell func-tion and thus influence susceptibility to disease [13-14]. In fact, stress, such as excessive muscular exercise could lead to a number of responses influencing hypothalamic-pituitary-adrenocortical axis and spymphatetic system func-tions. The activity of this systems is demonstrated by rapid increases in circulating levels of ACTH, adrenaline, noradrenaline and cortisol allowing these hormones to be useful in the evaluation of exercise-induced stress. The ex-ercise induced responses of catecholamines in the horse are much greater than in human [15].
The nervous system reacts to stressors modulating the se-cretion of neuropeptides [16]. The innervation of specific regions of primary and secondary lymphoid organs establishes the links necessary for neural modulation of immu-nity. The expression of beta-adrenoceptors on a variety of immune cells, including lymphocytes and macrophages, provides the molecular bases for these cells to be targets for catecholamine signalling. It is apparent that a multifactorial interaction between the cells of the immune system and the neuroendocrine hormones exists [17-18]. Conversely, there is now evidence of production of neuroendocrine hormones by the cells of immune systems, suggesting that the signal from the immune system can activate the central nervous system and control the function of the neuroendocrine sys-tem [19]. The first evidence that the cells of the immune system could produce a peptide was the finding that human lymphocytes could produce ACTH in connection to virus infections [20]. Since then, numerous peptides have been shown to be produced by different immune cells [21]. Moderate stress leads to highly stereotyped changes within leukocyte subsets in peripheral blood in which both neutro-phil and lymphocyte concentrations increased markedly [22]. Conversely, strenuous exercise induces lymphocyte reduction. Athletes in endurance are reported to experience increased susceptibility to respiratory tract infections after periods of heavy training. Therefore, strenuous exercise re-sults associated to depression of immunity but the intrinsic molecular mechanisms are not well known. Recently, Hsu et al. [23] observed that stress can induce mitochondrial membrane alterations in leukocytes. Reactive oxygen spe-cies produced during vigorous exercise may permeate into cell nuclei and induce oxidative DNA damage. These phe-nomena may be one of the mechanisms behind the immune dysfunctions after exhaustive exercise [24]. Moderate exer-cise has potent stimulatory effects on phagocytosis, antitu-mour activity, reactive oxygen and nitrogen metabolism and chemiotaxis.
Therefore in Equine Sport Medicine there is an increasing interest in the study of metabolic and neuroendocrine re-sponses to exercise in order to assess physical adaptation to stress elicited by exhaustive training and/or frequent com-petions and to improve animal welfare. Animal welfare is a relevant theme also within the European Community that has recently presented the “Animal Welfare Action Plan 2006-2010” with the aim to emphasize that well-being of animals represents a scientific discipline that needs to be adequately supported by research.
At present, there isn’t corresponding legislation in Italy that provides a mechanism for establishing detailed standards for the welfare of horses and, in particular, of the trotters. In fact, in Italy we have only the following specific rules, produced by associations and federations:
− Vet Regulation F.I.S.E. (Italian Equestrian Federa-tion) (updated to March, 27th, 2006);
− U.N.I.R.E. Regulation (National Union of Improved Horse Species) of harness racing updated to January, 30th, 2004;
− UNIRE Regulation of gallop races updated to Feb-ruary, 1st, 2004;
− Bets Rules, of D.P.R. (Decree) April, 8th 1998, n. 169;
− Code of equestrian behaviour F.E.I. (International Equestrian Federation) destined to all the people who work in the equestrian field. It is a code of good conduct and it acts as guidelines.
Current Italian legislation relating to horse includes:
-Law on Transport of horse (Regulation EC no. 1/2005);
-Law on Veterinary Medicinal Products (Legislative De-cree no. 193/2006 following EC Directive 2004/28);
-Law on Identification of equidae (Ministerial Decree 5 May 2006, Commission Decision 2000/68/EC, Regula-tion EC no. 504/2008);
-Law on Maltreatment of animals (Law 189/2004).
Economic interests that turns around the trotters and their sport are massive and this leads to neglect the health and welfare of these animals.
Considering that the welfare of the horse remains para-mount, it is necessary to prohibit any training methods which are abusive or cause fear or for which the horse has not been properly trained.
Although the control of welfare at the physical level was improved by the FEI [32], the problem is that if the inter-pretation of some types of abuse (such as beating, exhaus-tion, prolonged and intensive physical training, incompe-tent poling) is straightforward, other practices need more objective evidence in order to be considered as unethical.
For example, inappropriate physical training is probably a frequent and underestimated source of decreased welfare, but it will be difficult to con-vince riders because personal pride is involved. There is a need for scientific information in this context.
On the basis of these consideration, the current investiga-tions were undertaken to study the effects of prolonged and intensive physical training on the immune system, consid-ering also some cellular, cytochemical and serum parame-ters as white and red blood count (WBC and RBC), hemo-globin (HB), hematocrit (HTC), erythrocyte sedimentation rate (ESR), leucocyte enzyme pattern, phagocytosis activity and levels (1-3)-ß-D-Glucan, the last as indicator of clear-ance.In fact ß-D-Glucans posses their own receptor on macrophages as demonstrated by [25] Brown et al. (2002). Noteworthy the immune response was evaluated by MIF activity, as a reduced cell-mediated immune response, after race in trotter horses in order to recommend at the legislator the adoption of guidelines for the welfare of these animals.
Materials and Methods
Trotters
This study was carried out on no. 7 trotters, [five females (age range 2-4 years) and two males (age range 5-8 years)] weighing approximately 300-350 kilos, kept in stall hous-ing in the province of Bari (Italy). All animals, clinically healthy, were investigated for the physiological stress be-fore and after race.
Blood samples were taken with and without anticoagulant from the jugular vein - before and after race - for haemato-logical and biochemical analysis - using 18 gauge needles (Venoject Terumo, Haasrode, Belgium); sera stored at – 30°C until use. Heparinized blood was used for cellular studies.
Cellular parameters and cytochemical methods
The following cellular parameters, WBC, RBC,HB,HTC and ESR, were performed by means of a hemocytometer (K 1000 SYSMEX-TOA, DASIT S.p.A., Milan, Italy) using Sysmex-Dasit reagents.
Lymphocyte, neutrophils and monocyte counts were micro-scopically read on peripheral blood films stained with May-Grünwald-Giemsa (MGG).
Besides, same smears were stained with cytoenzymatic methods: α-naphyl acetate esterase (ANAE) (Sigma Diag-nostics, St. Louis, MO, USA) and Peroxidase (Perox) (Sigma Diagnostics).
Determination of (1-3)-β-D-Glucan in serum
The G-test was a generous gift from Associates of Cape Cod/Seikagaku (Cape Cod MA). Equine sera were assayed for (1-3)–β-D-Glucan according to the manufacturer’s in-structions.
Peripheral blood mononuclear cell (PBMC) isolation
PBMC were isolated from heparinized blood diluted 1:4 with Hanks’ Balanced Salt Solution (HBSS) by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation [26]. Cell suspensions recovered at the inter-face were resuspended in RPMI 1640 (Flow Laboratories, Milan, Italy) supplemented with penicillin 100 IU/ml, streptomycin 100 μg/ml, glutamine 2mM and 5% heat-inactivated foetal calf serum (FCS) (complete medium).
Monocyte (Mo) isolation
Aliquots of PBMC, resuspended in complete medium at a concentration of 10x106 cells/ml, were introduced into FCS-pretreated plastic Petri dishes and incubated for 2 hrs at 37°C to allow Mo adhesion [27]. After gentle agitation, the nonadherent cells (purified lymphocytes) were dis-carded; the purity of the resulting enriched plastic adherent Mo population was about 99%, as evaluated by non-specific esterase staining and immunofluorescence, using fluorescein isothiocyanate (FITC)-conjugated anti-CD14 monoclonal antibody (Becton Dickinson, Milan, Italy).
MØ phagocytosis
At 1 ml of medium containing MØ (15 x 106 cells) 400 μl of horse serum and a known volume of Candida albicans (Ca) (cell/micete ratio 1:2) were added.
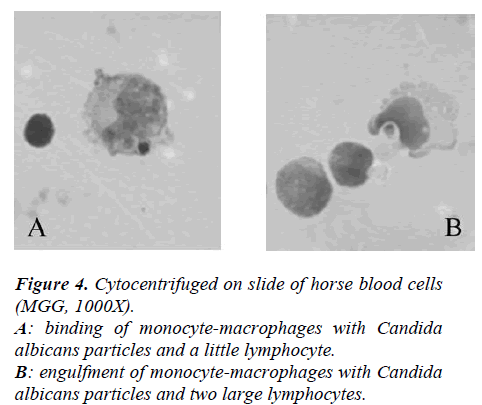
The suspensions were incubated for 30 minutes at 14 °C, cytocentrifuged on slides at 1200g, and, finally, cells were fixed with methyl alcohol and stained with MGG. Percent of phagocytosis was microscopically evaluated and at least 300 elements for slides, that have engulfed 1 or 2 or 3 Ca, were counted.
PBMC stimulation with Phytohemagglutinin (PHA)
In parallel experiments, aliquots of PBMC (2x106/ml) were stimulated with PHA (1μg/ml), and incubated for 1 h in 5% CO2 -5% humidified air atmosphere at 37°C. At the end of this incubation period, the mononuclear cells were centri-fuged at 400X g for 10 min and the PHA-containing super-natant was discarded. Mononuclear cells were resuspended at the concentration of 5 x 106cell/ml in complete medium and transferred to plastic tissue culture flasks for a 48 hrs incubation. Control supernatants were prepared by incubat-ing mononuclear cells without the mitogen. At the end of incubation period, samples were centrifuged at 400X g for 10 min and, then, supernatants were collected.
Preparation of agarose
The solution of agarose contained 1% agarose, 1X me-dium199, 10% human AB+ serum, 200U of penicillin/ml, 200μg of streptomycin/ml, and the HEPES and bicarbonate buffer (pH 7.20 to 7.40). The final pH of the agarose need to be maintained between 7.20 and 7.40, as measured MIF activity is consistently reduced at a low plate pH (< 7.10) and the ability of monocytes (MØ) to migrate is inhibited above pH 7.40. Fractions (5.5 ml) of the complete agarose were placed in Petri dishes and allowed to solidify for 5 to 10 min. Six-nine wells were cut into the agarose with a 2.5mm gel punch [27].
Incubation of MØ with supernatants
An aliquot of cells (15 x 106 ) were incubated with acti-vated and control supernatants. To cells were added 15μl of AB + serum and 85 μl of the test supernatant. The sample were then placed in the incubator for 30 min at 37° C with gentle shaking every 5 min. After the incubation, 10 μl samples (containing 1.5 x 106 cells) were pipetted into the wells cut in agarose plates. All samples were tested in trip-licate. The Petri dishes were incubated for 18 h at 37°C in 5% CO2-95% humidified air atmosphere.
Measurement of MIF activity
The areas of migration were measured microscopically at low magnification by using a microscope with a grid at-tachment.
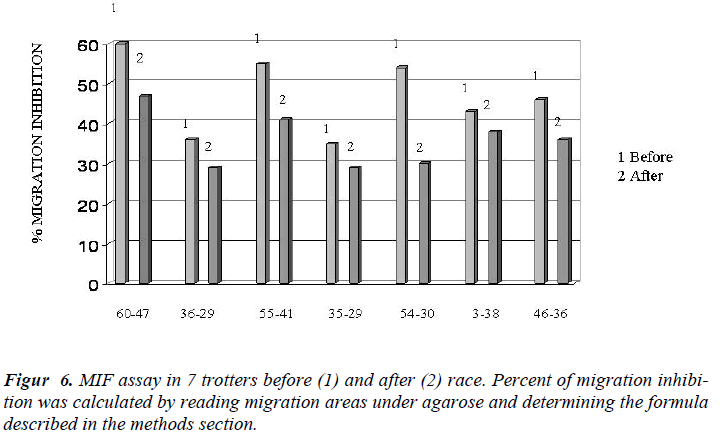
The percentage of migration inhibition was determined by the formula [28].
Percent inhibition= 1- area of migration in experimental wells x 100 area of migration in control wells
Statistical studies
Statistical differences were determined by the Student’s t test.
Results
Cellular and serum parameters
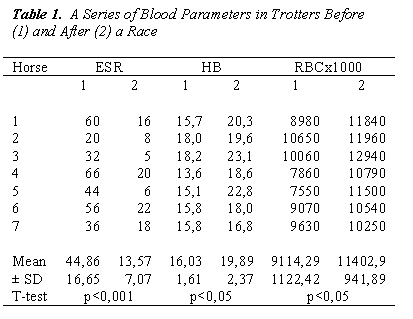
Increased values of RBC, HCT and HB may depend on the high hemoconcentration resulting from the fluid loss through sweating (Table 1).
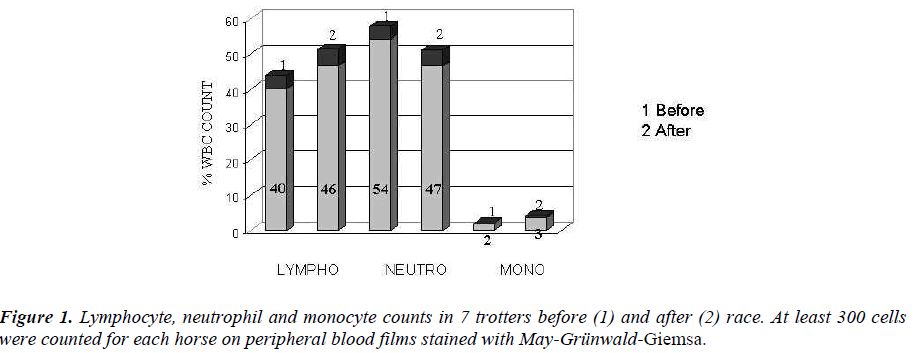
In trotters an increased counts of lymphocytes and monocytes were showed, while neutrophils were de-creased (Figure 1).
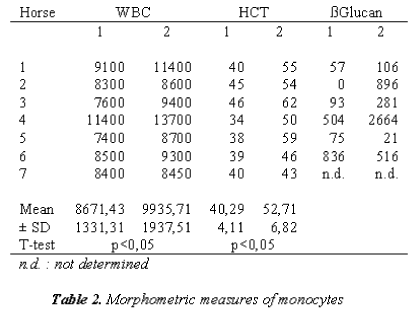
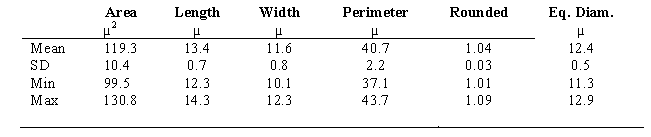
In Table 2, MØ were evaluated morphometrically; they appear smaller than what is reported in literature [31].
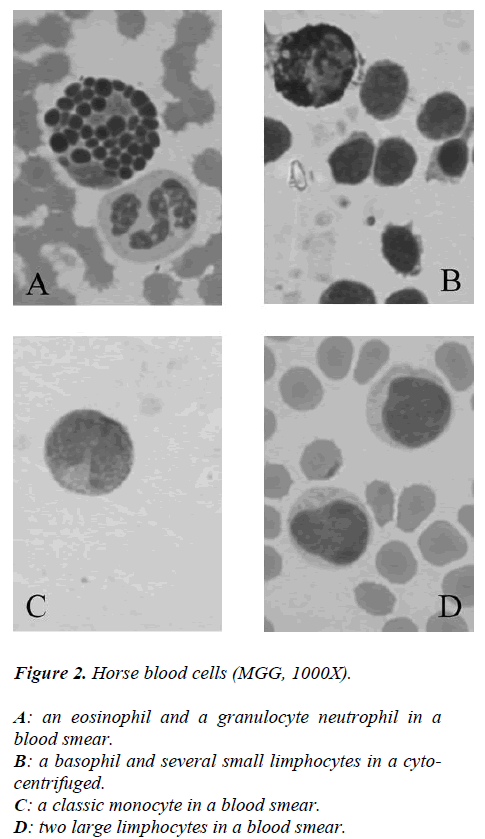
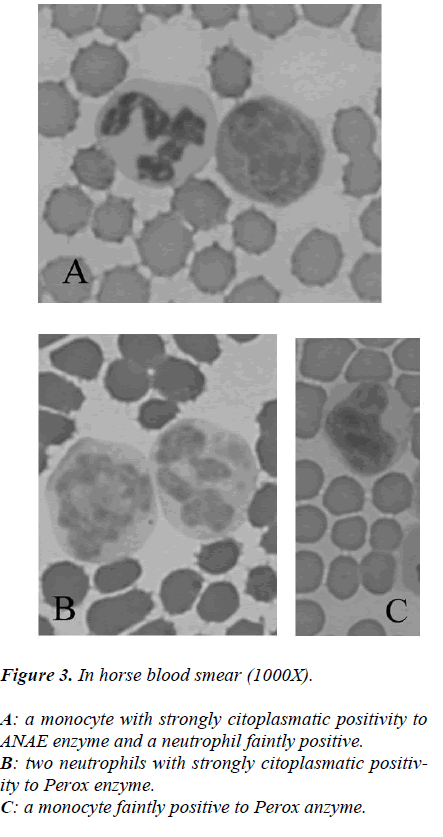
While from the point of cytochemical view, the pattern enzyme did not show any particular interest because a classic citoplasmatic positivity to Perox and ANAE enzymes were showed in monocytes and neutrophils to reveal its lisosomial activity (Figures 2-3).
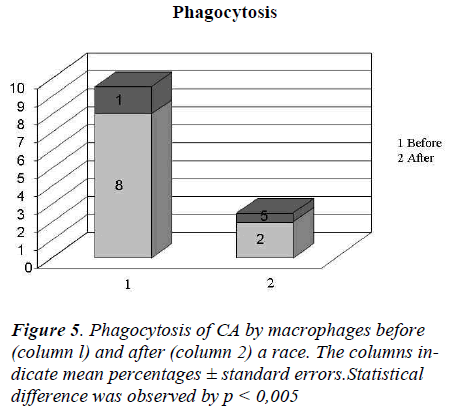
In Figure 4 it showed the activity of phagocytosis by MØ, against a common micete as Ca, after the race was strongly declined (Figure 5) to confirm this result is the test of MIF (Table 3 and Figure 6).
In Table 1 was also showed the increase of ß-D-glucan after the race can be seen both as a temporary reduction in the capacity of this molecule.
Discussion
The improvement of animals’ welfare gives animals a better quality of life, which means also better health status by avoiding chronic stress reactions that lower the organism’s coping ability. Such stress reactions may induce the development of pathologies due to e.g. the impairment of the immune system.
Our results have shown how extreme and intensive physical training induces a chronic prolonged stress and, consequently, discomfort to horses.
Our findings regards about lymphocyte and monocyte increases are in contrast with data of others [29], who demonstrated, following acute stress, a rapid and re-versible decrease of T cells, B cells, natural killer cells and monocytes in the blood. Such a transient decrease in WBC numbers has been considered as a sort of re-distribution or redeployment of leukocytes from the blood to other compartments of the body where an en-hanced immune function is required [30]. In our case, we hypothesize that the short duration of the race (2 min. about) does not allow us to take the exact moment of leukocyte migration to other sites. It is likely that collection of our blood samples, immediately after race, coincides with the time of lymphocyte and mono-cyte extravasation before their redeployment from the blood to other districts.
The quantitative analysis of the elements figured blood, red cells and white blood cells, has always shown an increase in the number of cells after the race, statisti-cally significant, which led to a inspessitio sanguinis shown by the values of ESR. The increase of red blood cells is determined by a greater demand for oxygen. The increase in white blood cells is typical of a state of stress.
In particular, this increase occurred borne by lympho-cytes and monocytes, confirming the hypothesis that stress to which they were subjected horses were chronic in nature and not sharp, or acute stress condi-tion.
Noteworthy the increase of ß-D-glucan after the race could be as a result of an immune-response inhibited, both as a non-receptor function of macrophages. So we can confirm our hypothesis that the immune inhibiting of MØ observed was a result of repeated stress, is closely related to chronic stress.
We believe that the training represent therefore a po-tential welfare risk that must not be ignored.
It is necessary to underline that the welfare of trotters depends on its ability to sustain fitness and avoid suf-fering. In fact, improved keeping conditions as well as performance-adapted training methods will help to avoid defects.
Any person responsible for the welfare of horse should acquire maximum possible expertise, because the well-being and usefulness of horse depend on the skill and attitude of the individuals who manage them. Assis-tance or advice on management of horse can be ob-tained from veterinarians.
Veterinarians have many opportunities to improve the welfare of horses, but owners will not always be happy with their veterinarian’s comments on these issues. However, veterinarians have the duty to insure the horses health and welfare. Nowadays, many horse owners lack the basic knowledge of horse care and they are not aware of the welfare needs of their horses, nor are they capable of safeguarding their horses under all foreseeable conditions.
We would propose that the following key concepts should be considered:
1. Decisions regarding care and management in equine husbandry and associated activities based on sound scientific principles;
2. Oversight and regulation of the care and man agement should be clearly defined.
3. An industry’s “buying in” to the process will likely be most productive. To move beyond the issue of whether or not horses should participate at all, industries must demonstrate the ability to safeguard the well-being of the horse.
In conclusion, given the current lack of laws, the Au-thors recommend, in accordance with the FEI Code of Conduct, at the Italian legislator the adoption of a law to supervise and ensure the horse’s wellness during the horse training, practice and the planning to compete in a horse race.
References
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol 2004; 25: 187-192.
- Ainsworth DM, Erb HN et al. Effects of pulmonary abscesses on racing performance of horses treated at referral veterinary medical teaching hospitals: 45 cases (1985-1997). J Am Vet Med Assoc 2000; 216: 1282-1287
- Sweeney CR, Divers TJ, Benson CE. Anaerobic bac-teria in 21 horses with pleuropneumonia. J Am Vet Med Assoc 1985; 187: 721-724.
- Carlson GP, O’Brien MA. Anaerobic bacterial pneumonia with septicaemia in two racehorses. J Am Vet Med Assoc 1990; 196: 941-943.
- Rackyleft DJ. Studies on bacterial infections of the equine lower respiratory tract. Phd thesis, University of Sydney 1990.
- Collins MB, Hodgson DR, Hutchins DR. Pleural ef-fusion associated with acute and chronic pleuropneu-monia and pleuritis secondary to thoracic wounds in horses: 43 cases (1982-1992). J Am Vet Med Assoc 1994; 205: 1753-1758.
- Austin SM, Foreman JH, Hungerford LL. Case-control study of risk factors for development of pleu-ropneumonia in horses. J Am Vet Med Assoc 1995; 207: 325-328.
- Raidal SL, Love DN, Bailey GD, Rose RJ. The ef-fect of high intensity exercise on the functional ca-pacity of equine pulmonary alveolar macrophages and BAL-derived lymphocytes. Res Vet Sci 2000; 68: 249-253.
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribu-tion. Role of adrenal steroid hormones. J Immunol 1996; 157: 1638-1644.
- Hwang HJ, Kwak YS, Yoon GA, et al. Combined effects of swim training and ginseng supplementa-tion on exercise performance time, ROS, lympho-cyte proliferation, and DNA damage following ex-haustive exercise stress. Int J Vitam Nutr Res 2007; 77: 289-96.
- Cappelli K, Felicetti M, Capomaccio S, et al. Exer-cise induced stress in horses: selection of the most stable reference genes for quantitative RT-PCR normalization. BMC Mol Biol 2008; 9: 49.
- Williams CA, Gordon ME, Betros CL, McKeever KH. Apoptosis and antioxidant status are influenced by age and exercise training in horses. J Anim Sci 2008; 86: 576-583.
- Ortega E, Giraldo E, Hinchado MD, et al. Neuro-immunomodulation during exercise: role of cate-cholamines as “Stress Mediator” and/or “Danger Signal” for the innate immune response. Neuroimm-unomodulation 2007;.14: 206-212.
- Yoshizaki F, Nakayama H, Iwahara C, et al. Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochimt Biophys Acta 2008; 1780: 383-392.
- Snow DH, Harris RC, MacDonald IA, et al. Effects of high-intensity exercise on plasma catecholamines in the thoroughbred horse. Equine Vet J 1992; 24: 462-467.
- Meeusen R, Watson P, Hasegawa H, et al. Brain neurotransmitters in fatigue and overtraining. Appl Physiol Nutr Metab 2007; 32: 857-864.
- Elenkov IJ, Chrousos GP. Stress hormones, proin-flammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci 2002; 966: 290-303.
- Covelli V, Pellegrino NM, Jirillo E. A point of view: The need to identify an antigen in psyconeuroimmu-nological disorders. Curr Pharm Des 2003; 9: 1951-1955.
- Hada T, Onaka T, Takahashi T, et al. Effects of nov-elty stress on neuroendocrine activities and running performance in thoroughbred horses. J Neuroendo-crinol 2003; 15: 638-648.
- Dawson TR, Horohov DW, Meijer WG, Muscatello G. Current understanding of the equine immune re-sponse to Rhodococcus equi. An immunological re-view of R. equipneumonia. Vet Immunol Immuno-pathol. 2010; 135: 1-11.
- Madden KS. Catecholamines, sympathetic innerva-tion, and immunity. Brain Behav Immun 2003; 1: 5-10.
- Ronsen O, Pedersen BK, Øritsland TR, et al. Leuko-cyte counts and lymphocyte responsiveness associ-ated with repeated bouts of strenuous endurance ex-ercise. J Appl Physiol 2001; 91: 425-434.
- Hsu TG, Hsu KM, Kong CW, et al. Leukocyte mito-chondria alterations after aerobic exercise in trained human subjects. Med Sci Sports Exerc 2002; 34: 438-442.
- Tsai K, Hsu TG, Hsu KM, et al. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med 2001; 31: 1465-1472.
- Brown GD, Taylor PR, Reid DM, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002; 196: 407-412.
- Bruyninckx WJ, Blancquaert AM. Isolation of horse mononuclear cells, especially of monocytes, on Iso-paque-Ficoll neutral density gradient. Vet Immunol Immunopathol. 1983; 4: 493-504.
- Kumagai K, Itoh K, Hinuma S, Tada M. Pretreat-ment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods 1979; 29: 17-25.
- Satoskar AR, Bozza M, Rodriguez Sosa M, et al. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania majorinfection. Infect Immun 2001; 69: 906-911.
- Pedersen BK, Woods JA, Nieman DC. Exercises-induced immune changes-an influence on metabo-lism ? Trends Immunol 2001; 22: 473-475.
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dyna-mics and hormonal mechanisms. J Immunol 1995; 154: 5511-5527.
- McKane SA, Slocombe RF. Alveolar fibrosis and changes in equine lung morphometry in response to intrapulmonary blood. Equine Vet J Suppl 2002; 34: 451-458.
- Atock MA, Williams RR. Welfare of competition horse: In: The Thinking Horse. Equine Research Centre Seminar Series, Guelph. 1995; pp 51-65.