- Biomedical Research (2016) Volume 27, Issue 2
Correlation between macrophage infiltration and prognosis of ovarian cancer-a preliminary study.
|
Zhu Yafei1*, Gao Jun2, Gao Guolan3 |
| Corresponding Author: Zhu Yafei, Department of Obstetrics and Gynecology, Gnannan Medical University, PR. China |
| Accepted: January 20, 2016 |
Abstract
Objective: There is controversy in the prognostic value of infiltrating macrophages density within the human ovarian tumor microenvironment. The aim of this study is to examine the prognostic significance of tumor-associated macrophages (TAMs) that have an M2 polarized function in ovarian cancer.
Methods: Formalin-fixed, paraffin- embedded blocks were obtained from 42 ovarian cancer and 30 benign ovarian tumor patients who underwent surgery. The number of infiltrating macrophages and M2-polarized macrophages within tumor tissue by both CD68 and CD163 were evaluated. The correlation of CD68/CD163-positive macrophages number with clinical pathology staging, lymphatic metastasis, prognosis value, and post-surgery survival were caculated. All have the permission of ethical approval for the use of human tissue samples.
Results: A high number of macrophages expressing CD68 or CD163 were found in ovarian cancer tissue, there was significant difference according to the FIGO stage (P<0.05). The number of CD68- positive macrophages has no correlation with prognosis, while density and ratio of CD163-positive macrophages correlate negatively with prognosis.
Conclusion: Cancer related inflammation characterized by tumor associated macrophages (TAMs) was found in human ovarian cancer tissues. The ratio of TAMs rather than density of CD68-positive macrophages is associated with poor prognosis of ovarian cancer.
Keywords |
||||||||||
| Inflammation, Ovarian cancer prognosis, Tumor associated macrophage. | ||||||||||
Introduction |
||||||||||
| The macrophage plays an important role in both inflammation and cancer, it is a bridge between inflammation and cancer, and most inflammatory cells in the solid tumor tissue are macrophages, which account for more than 50%. The infiltration of tumor associated macrophages (TAMs) representing smouldering inflammation, named cancer related inflammation, is considered as the seventh feature of cancer [1]. | ||||||||||
| Infiltration density of TAMs is closely related with tumor prognosis. Many clinical studies in solid tumors show that infiltration density of TAMs is independent factors of poor prognosis. But there are inconsistent reports about TAMs in the prognosis in human ovarian carcinoma tumor. Wan Ting et al. [2] considered that the 5-year survival rate of the patients with the invasion of high density of CD68-positive macrophages was significantly lower, high-density infiltration of CD68- positive macrophage is an independent factor of poor prognosis. But Shah et al. [3] found that the CD68-positive macrophage expression has nothing to do with the persistence rate And Tanaka et al. [4] found that Bikunin, secreted by CD68-positive macrophages, a protease inhibitor, could active the expression of factor uPA by down regulating urokinasetype plasminogen, and it was an independent prognostic factor of increasing disease free survival and overall survival. Similar situation was also seen in other tumors such as non-small cell lung cancer [5]. | ||||||||||
| It was previously considered that macrophages infiltrated in tumor were important anti-tumor cells which could kill tumor cells directly or by tumor-associated immune response, but now only M1 type (classic-activated) macrophages were considered as the anti-tumor cells. Recently it was found that macrophages can be reeducated by tumor microenvironment and be induced for differentiation and the polarization and then become M2 macrophages, which can attenuate acute inflammatory reactions, inhibit the immune response, and promote the proliferation and metastasis of tumor cells, vascular regeneration and the induce the local immune tolerance, which in the contrary can promote tumor development. Currently, it is believed that TAMs have a similar phenotype with M2 macrophages, highly expressing the scavenger receptor CD163, and promote tumor development [1]. | ||||||||||
| The present study was based on the tumor-associated inflammation theory, and we marked total macrophages with CD68 and TAMs with CD163, respectively, and tried to analyze the value of macrophages for ovarian cancer prognostic from the view of tumor-associated inflammation and differentiation of macrophages. | ||||||||||
Materials and Methods |
||||||||||
Patients |
||||||||||
| Sample estimation: Each group needs at least 30 cases, twotailed test method (α=0.05, 1-β=0.8) were used to compare two means to estimate the sample. Mean of CD68, 163 density, standard deviation from the pre-test. | ||||||||||
| Design: This was a retrospective study, we selected patients with ovarian cancer from cases admitted to the First Affiliated Hospital of Nanchang University from 2004 to 2006, the including criteria were as follows: epithelial ovarian cancer first treated; not received any chemotherapy, radiotherapy, hormone therapy or anti-inflammatory therapy preoperatively; treated by ovarian cancer cytoreductive surgery and the post-operative stage was stage II-III or stage IV; all subjects had complete clinical and pathological data and follow-up data, and completed the platinum-based combination chemotherapy on time after surgery (patients received radiotherapy after surgery were evacuated). Total of 42 cases of ovarian cancer cases were met the above criteria. Selected 30 cases as the control group with ovarian cancer tumor surgical resection, but the postoperative pathological diagnosis was benign ovarian lesions. All specimens were subjected for paraffin-embedding and immunohistochemical staining. | ||||||||||
| Clinical information | ||||||||||
| Verify and record age and diagnosis of all selected cases, and record the clinical stage, postoperative residual lesion size, histological type and differentiation, number of postoperative chemotherapy of ovarian cancer patients. All patients were followed-up by phone combined with the hospital outpatient reexamination. Record the death date if the patients were dead, and record the date and data of last follow-up if the patients were lost to follow-up. The pathology and clinical features of the 42 cases of ovarian cancer included in this study were as follows: most of the patients had bilateral ovarian cancer (66.7%, 28 cases), unilateral ovarian cancer accounted for 33.3% (14 cases); the common pathological type was serous (71.4%, 30 cases), and the mucinous cancer accounted for about only 21.4% (9 cases); patients with lymph node metastasis took up for 52.4% (22 cases). The clinicopathological of stage III to IV epithelial ovarian cancer accounted for 64.3% (27 cases), early stage I+II was 35.7% (15 cases). The high-differentiated and mid-differentiated cancer occupied for 16.7% (7 cases) and 83.3% (35 cases), respectively. There were 21 cases of over and less than 50 years, respectively. | ||||||||||
| Agents and detection methods | ||||||||||
| Sections of 4 μm thickness were cut from the formalin-fixed and paraffin embedded by specimens. Expressions of CD68 and CD163 inside tumor and on tumor edge were detected by two-step immunohistochemical staining. CD68 is molecular marker of macrophages. CD163 is marker for M2 macrophages. Immunohistochemistry and immunofluorescence: Cancer specimens were sliced into 4-μm sections. Antigen retrieval was performed using a microwave at >90°C for 15 min, and the samples were allowed to cool to room temperature. The non-specific binding sites were blocked with 5% bovine serum albumin (BSA) for 1 h. For immunohistochemical staining, the sections were sequentially incubated with a mouse anti-human CD68 monoclonal antibody (clone KP1, ZM-0060, Zhongshan,beijing) and a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody ((clone: LN3,ZM-0136, Zhongshan). The antibodybinding sites were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Zhongshan), and the cell nuclei were counterstained with hematoxylin. For immunofluorescent staining, the sections were incubated with a mouse anti-human CD68 polyclonal antibody (clone ZM0464, Zhongshan; 1:100) mouse anti-human CD163 monoclonal antibody (clone 10D6, Zhongshan);. The nuclei were counterstained with SYTO 40 (Life Technologies, Grand Island, NY, USA). Two pathologists with no knowledge of the patient population independently reviewed the immunostained sections. Ten representative 200× or ×400fields were selected from each section, and only nucleated cells were analyzed. The obtained TAM densities were calculated as cells/mm2.Tonsil was used for CD68 and CD163 positive control, and in negative control, antibody was replaced with PBS. | ||||||||||
| Results analysis | ||||||||||
| CD68 was positively expressed within the cytoplasm of macrophages on immunohistochemical staining slices; cells containing tan to brown-yellow granular particle in cytoplasm were positive cells. CD163 were positively expressed in the membrane and cytoplasm of macrophages, cells containing tan to brown-yellow granular particle in cytoplasm were positive cells. CD68+ cells in tumor tissue were tumor associated macrophages (TAMs) and CD163+ cells were M2 type macrophages. | ||||||||||
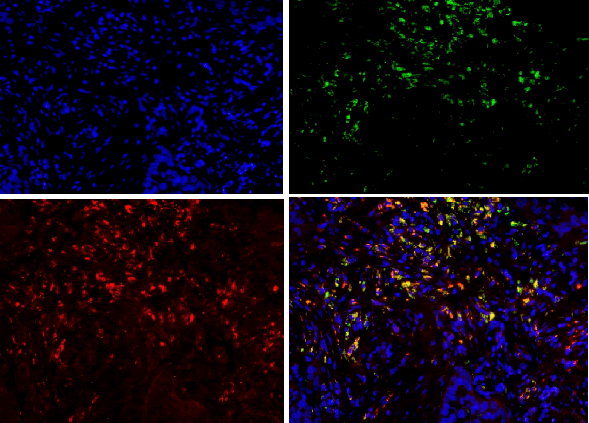
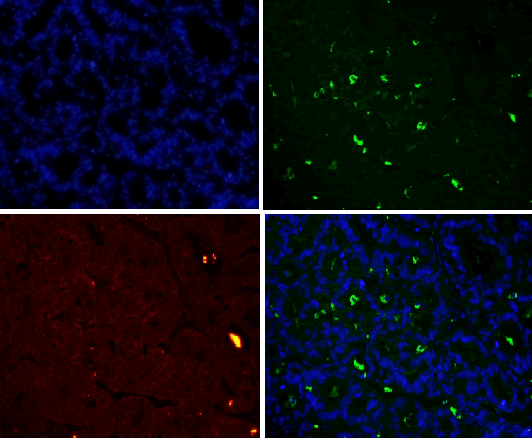
| For results analysis of immunofluorescence double staining, we use serum of the same species of the secondary antibody as negative control. Currently CD163 is considered to be a specific marker for M2 type macrophage, CD68 is marker for macrophage, so we labeled CD68 with FITC (green), CD163 with TRIC (red), and nuclear with DAPI staining (blue). If green and red are presented in the same position, the overlapping merge displays yellow, i.e. CD163 and CD68 are co-expressed in the same cell (Figure 1), suggesting that the macrophages are polarized as M2 phenotype. If there is only green without red, it indicates that CD163 is not expressed in CD68-positive macrophage (Figure 1), and it suggests that the macrophage is polarized into non-M2 phenotype. | ||||||||||
| For TAMs counting, the tumor tissue is divided into two regions (inside of the tumor, i.e. cancer nests and tumor edge, i.e. cancer interstitial). We observed TAM intensive region in cancer tissue and neighboring stromal, which is HotSpot, count the TAM in five visions under light microscope (400X) and take the average for TAM infiltration density. All specimens were analyzed by two experts from the Department of Pathology, and take the average of their separate counting. The benign lesions criteria are the same with the above. | ||||||||||
| Take the median of TAM infiltration density of all ovarian cancer cases as the cut-off point of high low-density, the ovarian cancer group was divided by TAM infiltration density to low-density group and high-density group. | ||||||||||
| Statistical analysis | ||||||||||
| Data were described as Mean ± S.D. All data were analyzed using the SPSS/Win13.0 software (SPSS, Inc., Chicago, Illinois). P value less than 0.05 was considered statistically significant. Comparison of the density of infiltration of macrophages between ovarian cancer and benign ovarian lesions was conducted by one-way ANOVA and then by independent t-test. The samples were divided into high/low density group according to macrophage density median, frequency distribution difference was analyzed by Fisher's chi-square test (χ2 test), if 1<theoretical frequency <5, the data were then analyzed by fisher exact test. Kaplan-Meier method and Cox regression model was used for survival curves analysis and multivariate analysis of prognostic factors respectively. | ||||||||||
Results |
||||||||||
| Expression of CD68 and CD163 in ovarian tumor tissue | ||||||||||
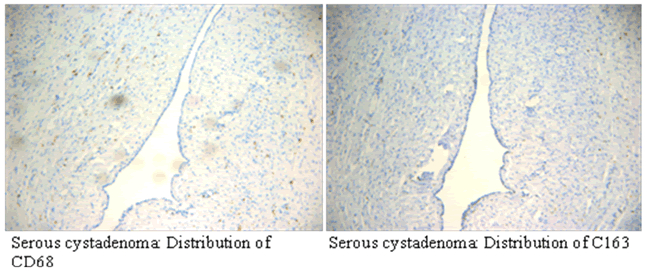
| Immunohistochemical results showed that CD68 and CD163 were mainly expressed in cytoplasm of macrophages in ovarian cancer tissue, macrophages were mainly distributed in the tumor mesenchyme. | ||||||||||
| CD68 and CD163 were both expressed in ovarian cancer, while in benign ovarian tumors, CD68 and CD163 were rarely expressed (Figure 2-1). Immunofluorescence double staining prompted that CD68 and CD163 were co-expressed in some cells, which meaned that part of macrophages polarizated to M2 phenotype (Figure 2-2). In earlystage, CD163 was not expressed in the CD68-positive macrophage, suggesting that part of the macrophages were non-M2 phenotype (Figure 2-3). | ||||||||||
| The average infiltration density of CD68 in ovarian cancer was 74.4 ± 7.2/HP, the average infiltration density of CD68 in benign ovarian tumors was 18.4 ± 4.2/HP, the CD68 expression in ovarian cancer tissues was significantly higher than that in benign ovarian tumor (P=0.000). | ||||||||||
| The average infiltration density of CD163 in ovarian cancer was 63.8 ± 8.2/HP, The average infiltration density of CD163 in benign ovarian tumors was 14.4 ± 3.6/HP, the CD163 expression in ovarian cancer tissues was significantly higher than that in benign ovarian tumor (P=0.000). | ||||||||||
| CD163 was expressed in Distribution region of CD68. CD68 and CD163 had significant positive correlation, (P<0.01) suggested that most macrophages in ovarian cancer tissue were M2 type. | ||||||||||
| The expression of CD68 and CD163 in lymphatic metastasis and FIGO stage (III + IV) group were higher in non-lymphatic metastasis and FIGO stage (I + II) (P<0.01). | ||||||||||
| The tissue sections of advanced stage human ovarian cancer M2 macrophages cell ratio (CD163/CD68), 79.2 ± 18.2%, while the proportion of early tumor (I + II) was only 48.2 ± 12.1%. The difference was statistically significant (P<0.05). | ||||||||||
| The relationship of the expression of CD68 and CD163 in ovarian cancer tissues with clinical and pathological features | ||||||||||
| In Table 1, X2 test showed that the difference of the density of CD163 and frequency distribution of the ratio of CD163 of lymph node metastasis group was statistically significant in different FIGO stage (P<0.01). The difference of frequency distribution of the ratio of CD68 was statistically significant in different FIGO staging group (P<0.01). But in different lymph node metastasis group, the difference of frequency distribution of the ratio of CD68 has no statistically significant (P>0.05). | ||||||||||
| Non-parametric test Spearman correlation analysis and partial correlation coefficient suggest that the correlativity of different FIGO stage, lymph node metastasis, CD163 count and the CD163 ratio were statistically significant (P<0.01), which means that there were statistically significance in these parameters. While there were no statistically significant correlations between lymph node metastasis and CD68 count. | ||||||||||
| COX regression analysis of ovarian cancer prognosis | ||||||||||
| Multivariate analysis of prognostic factors by Cox regression model showed that, the ratio of CD163/CD68 is a negative prognostic relevant factor. While the density of CD68 and CD163has nothing to do with prognosis (Table 2). | ||||||||||
| Kaplan-Meier analysis and survival curves | ||||||||||
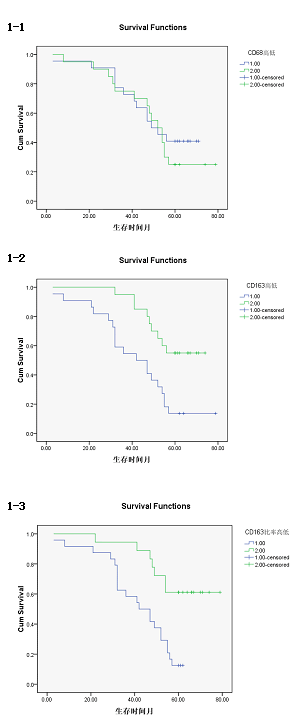
| The survival time of CD163 high density group was (42.77 ± 4.35) months, 95% confidence interval was (34.25-51.29). The survival time of CD163 low density group was (61.70 ± 3.22) months, 95% confidence interval was (55.38-68.02), there was a statistically significant difference (P<0.01). [Log rank test X2= 9.504, P=0.002 <0.01; Breslow test X2= 9.621, P=0.002 <0.01; Tarone-Wrae test: X2= 9.651, P=0.002 <0.01], the survival curves of the CD163, high/low density group was shown in Figure 3-1. | ||||||||||
| The survival time of CD163/CD68 high ratio group was (51.32 ± 3.25) months, 95% confidence interval was (35.08-47.84). The survival time of CD163/CD68 low ratio was (65.78 ± 4.19) months, 95% confidence interval was (57.57-73.99), there was significant statistical difference (P<0.01). [Log rank test X2=10.75, P=0.02<0.05; Breslow test X2=9.66, P=0.01<0.05; Tarone-Wrae test: X2=10.27, P=0.01<0.05]. The survival curves of the CD163/CD68 high/low survival curves was shown in Figure 3-2. | ||||||||||
| The survival time of CD68 high density group was (51.32 ± 4.154) months, 95% confidence interval was (43.18-59.46), The survival time of CD68 low density group was (51.45 ± 4.49) months, 95% confidence interval was (42.65-60.25), there was no significant difference (P>0.05). [Log rank test X2=0.463, P=0.496>0.05; Breslow test X2=0.120, P= 0.730>0.05; Tarone-Wrae test: X2=0.248, P=0.618>0.05]. The survival curves of the CD68 high/low density group was shown in Figure 3-3. | ||||||||||
Discussion |
||||||||||
| In this study, we investigated the relationship between the macrophage infiltration and prognosis of human ovarian cancer according to the tumor-associated inflammation. Cancer related inflammation is regarded as the seventh feature of cancer, its theories mainly contain two aspects: a) Tumor cells raised inflammatory cells to regional tumor and formatted the inflammatory microenvironment; b) Macrophages would be differentiation and polarization induced by the tumor microenvironment, became the tumor-associated macrophages (TAMs)-M2 phenotype macrophages and promoted tumor development [1]. | ||||||||||
| Our study found that a large amount of macrophages (CD68 marker) were infiltrated in human ovarian cancer tissue sections, the macrophage density in ovarian cancer tissue was significantly higher than that in benign tumor in the infiltration density (P <0.01). The M2 macrophage proportion (CD163/ CD68) in advanced human ovarian cancer (III+IV period) tissue sections was significantly higher than that in benign tumor (I+II period) (P<0.05). Benign tumors were rarely infiltrated by macrophage cells, and the macrophages were CD163 negative, suggesting that these macrophages do not belong to M2 type. Our findings and conclusions were consistent with previous reference report [6]. In figure 2-3, immunofluorescence colocalization promoted that some macrophages in ovarian cancer tissues were co-expressed the CD68 and CD163, suggesting that these macrophages were polarized to M2 phenotype, while some macrophages were not polarized. | ||||||||||
| Similar to the drifting of cell factor Th1 to Th2, was differentiated to Th2 in the systemic environment, in malignant ovarian tumor microenvironment, macrophages were differentiated to M2. The above results demonstrated that in human ovarian cancer tissue, there is the inflammation based on the macrophage infiltration and macrophage differentiation. | ||||||||||
| We believe that the macrophage differentiation status is related with the clinical stage of the tumor. In the advanced stage of tumor, the macrophages in tumor tissue were mainly M2-type, while in the early stage of tumor the macrophages were mainly M1 type. In our study, we found the proportion of M2 macrophage (ratio of D163/CD68) was associated with tumor stage. The proportion of the M2 type macrophage in the advanced human ovarian cancer tissue sections (III+IV) (CD163/CD68) 79.2 ± 18.2%, was higher than that in early stage(I+II) which was only 48.2 ± 12.1%. The difference was statistically significant (P<0.05). | ||||||||||
| The explanation of the phenomenon above is based on the tumor-associated inflammation theory [7]. In this theory, the characteristics of the tumor microenvironment are hypoxia, acidosis, interstitial high pressure and immune suppression, and the tumor-associated inflammation is established base on this. In the process of tumor development, macrophages are differentiated and polarized to tumor-associated macrophage induced by the tumor microenvironment, while the latter one can promote the tumor development. It is speculated that in the early stage of the tumor, the monocytes has just raised to the tumor from blood, the tumor micro-environment is not yet fully formed, thus there is just a little of the macrophages are differentiated, the differentiation stage was still in the initial stage. And the macrophage cells are M1 type mainly and the anti-tumor function is still survived. In advanced stage of tumor, with the increase in the absolute number of the tumor cells, systemic or local immunosuppressive environment and accumulation of inflammatory cytokines and hypoxia factors, the characteristics of tumor microenvironment with hypoxia, acidosis, interstitial hypertension and immune suppression are finally formed. Tumor-associated inflammatory microenvironment are finally perfected, macrophages are mainly differentiated to the M2 type. | ||||||||||
| Our data showed that the density of CD68-positive macrophages is unconcerned with prognosis. The ratio of M2 macrophages is the independent risk factor in the prognosis of ovarian cancer. The macrophage is a kind of cell with heterogeneity and diversity. , and can be divided into M1, M2 subtype at least [8]. CD68 is only a generic marker of human macrophages and it cannot distinguish the subtypes. CD163 is a specific marker of the M2 macrophages [5]. In this study, we used CD68 as a marker for human macrophages and CD163 for M2 macrophages, and re-examine the significance of macrophages in human ovarian cancer prognosis based on tumor-associated inflammation theory and macrophage differentiation. | ||||||||||
| Results showed that the density of CD68-positive macrophage was unrelated with the prognosis of ovarian cancer. The COX Analysis promoted that the ratio of CD163/CD68 also unrelated with the prognosis. Accordingly, we regarded the M2 macrophage as an independent risk factor for ovarian cancer prognosis. | ||||||||||
| Basic research has confirmed that the M2 type macrophage were the particular macrophage which could promote the tumor development differentiated and polarized by tumor micro-environment, it promoted the tumor progression by weakening the inflammation response in acute phase, suppressing the immune response, promoting tumor cells proliferation, metastasis, angiogenesis, and inducing immune tolerance in a local state [1], and this was consistent with our results. | ||||||||||
| The number of CD163 cells was different in the analysis of the survival curves, but the CD163 ratio rather than absolute count of CD163 cells was an independent risk factor in COX regression. | ||||||||||
| It can be explained from the following aspects: | ||||||||||
| • Macrophages are a group of cell with highly heterogeneous population. It is known that the M1 type can inhibit the development of tumor, and M2 type can promote the development of tumor. But they are just distinguished by the phenotype, and macrophages have not only these two phenotypes. M1 macrophage and M2 macrophage are the two extremes polarization endpoint of macrophage. There was debate about whether there are intermediate state macrophages, if any, how the number, function and the role of these intermediate state macrophages in prognosis could be determined. However, these intermediate states of macrophages have been preliminarily confirmed in a mouse model with breast cancer. | ||||||||||
| • The T cells, NK cells and other immune cells in the tumor microenvironment might disturb the results. | ||||||||||
| We found that the density of CD68-positive macrophages cell had no relation with ovarian cancer prognosis, which was inconsistent with the report of Wan et al. [2] And there are the inconsistence about the prognostic significance of TAMs in ovarian cancer indeed [2-4]. Some researchers try to explain the contradiction by the "double-edged sword" hypothesis, but it is not convincing. | ||||||||||
| It was considered that one reason for the above inconsistent reports was that they have not considered the differentiation and functional differences of macrophage in the tumor microenvironment. The reasons are as follows: | ||||||||||
| CD68 is only labeled on human macrophages, and it cannot distinguish the M1 and M2 macrophages. While in the tumor microenvironment, the macrophages trend to differentiate to M2 type. So there are at least two phenotypes CD68 positive cells in tumor tissue including M1 and M2 type. In this study, we found that the functions of M1 and M2 macrophages in prognostic significance were almost opposite [8]: M1 macrophages were classically activated macrophages (caMphi) and belong to important anti-tumor cells; while M2 macrophages were the promoting-tumor cells formed by the differentiation and polarization induced by the tumor microenvironment. This study also showed that the density of M2 macrophages was the negative correlation factor for the survival of some solid tumors, while the density of M1 macrophages was the positive correlation factor for improving the survival rate of patients with solid tumors (non-small cell lung cancer, NSCLC) [5]. It is suggested elsewhere that in ovarian cancer, the number and proportion of M2-type macrophages will affect the prognosis. And our results confirmed this hypothesis. Wan [2] considered that the density of TAMs was negatively correlated with prognosis, the cases with ovarian cancer in his study were all in advanced phase, a total of 68 cases included 54 cases in phase III and 13 in phase IV, the cases in phase III and IV took up to 98.5%; Shah et al [3] believed that density of TAMs was unconcerned with the survival rate, the cases in his study contained 79 cases (66%) in phase III and 17 (14%) in phase IV, the cases in III and IV stage were 96/119 (80.7%), and 23/119 (19.3%) in I-II period; Tanaka et al. [4] considered that TAMs prompted a good prognosis, and there were 67/89 cases (75.3%) in Phase III, the remaining cases were all in phase I-II. The reason caused differences in conclusions might be due to the different baseline in the different clinical stage ratio included in the study. | ||||||||||
| In summary, this study verified the presence of tumor-associated inflammation and macrophage differentiation in human ovarian tumor. We re-analyzed the prognostic value of macrophages from the view of tumor-associated inflammation - macrophage differentiation, and confirmed that the ratio of M2 macrophages in in human ovarian cancer were the predictive factors of poor prognosis. | ||||||||||
| Limitations of this study: In accordance with the theory of evidence-based medicine, the evidences to confirm the prognostic survive are large sample with multi-center and prospective and randomized control study RCT or prospective cohort study, but such studies are limited by a number of objective conditions such as time-consuming and fundconsuming. This study was retrospective study with the small sample size, and the conclusions were only on overall survival and could not aim at the disease-specific survival. So, our conclusion had only implication effect, it needs large sample with multi-center and prospective cohort study for further demonstration. | ||||||||||
Tables at a glance |
||||||||||
|
||||||||||
Figures at a glance |
||||||||||
|
||||||||||
References |
||||||||||
|