Research Article - Biomedical Research (2017) Volume 28, Issue 18
Contrast-enhanced ultrasound performance of endometrioma treated with high-intensity focused ultrasound (HIFU)
Yan Zhang*, Xiaoxiang Fan, Meiwu Zhang and Dafeng Mao
Department of Interventional Therapy, Ningbo No.2 Hospital, Ningbo, Zhejiang, PR China
- *Corresponding Author:
- Yan Zhang
Department of Interventional Therapy
Ningbo No.2 Hospital, PR China
Accepted date: August 24, 2017
Abstract
Objective: This study is to investigate the application of contrast-enhanced ultrasound in assessment of High-Intensity Focused Ultrasound (HIFU) in treating endometrioma.
Methods: Totally 60 patients with endometrioma were included in this study, who underwent HIFU in our hospital from June 2014 to July 2016. At 3 d before treatment, and at 1, 3, 6, and 12 months after treatment, contrast-enhanced ultrasound were performed to assess the HIFU treatment effects.
Results: Contrast-enhanced ultrasound showed that, 49 (81.67%) and 11 (18.4%) cases exhibited dendritic and gradual enhancement patterns, respectively. After peak, there was no clear boundary between adenomyoma lesion and surrounding tissue. Totally 38 (63.34%) and 22 (36.67%) cases exhibited non-uniform and uniform enhancement, respectively. Along with contrast agent dissipation, the lesion area and surrounding myometrium showed synchronous regression in 60 cases (100%). Timeintensity curve analysis showed that, there was no significant difference in AT, while compared with control, TTP and RT were significantly shorter, and Imax was significantly higher, in the adenomyoma group. ROC analysis showed that, AUC values for TTP, Imax, and RT were 0.88, 0.89, and 0.87, respectively. Compared with 3 d before treatment, the maximum diameter was decreased, while the nonperfusion volume was significantly increased, at 6 and 12 months post-operation. In the follow-up period, significant differences were observed in the tumor ablation rate and tumor residual rate.
Conclusion: Real-time perfusion monitoring during HIFU with contrast-enhanced ultrasound contributes to diseases diagnosis and ablation assessment in the disease treatment.
Keywords
Contrast-enhanced ultrasound, Endometrioma, High-intensity focused ultrasound (HIFU), Therapeutic efficiency
Introduction
Adenomyosis could be divided into the diffuse and localized categories according to the depth and range of muscle invasion. The former diffuse type refers to the lesions diffused throughout the myometrium, and the latter localized type refers to the lesions localized as nodulars in the myometrium, which is also known as endometrioma. Uterine adenomyoma is a common disease in women of childbearing age [1], with the incidence rate of 8.8%-31.0%. It has been shown that the disease incidence has kept increasing over these years, which therefore gains more and more attention [2].
At present, the endometrioma has been shown to be closely linked with multiple pregnancy and childbirth, abortion, and chronic endometritis [3]. The clinical symptoms of endometrioma include menorrhea, anemia, pelvic pain, frequent micturition caused by adjacent organ oppression, constipation, and infertility, more of which need to be treated [4-6]. With the morphological similarity to uterine fibroids, the endometrioma is characterized by the nodular- or mass-like growth of the intimal tissue invading into the myometrium. Imaging analysis shows that, in endometrioma, there is always no clear boundary or only pseudocapsule between the lesions and surrounding tissues. The lesions are often enlarged unevenly, with significantly increased muscular blood flow, which shows star-like and/or streaky scattered distribution, or radial arrangement [7]. Usually accompanied by acoustic attenuation, the blood flow signal is always not abundant. That is the reason why there are still some difficulties for the twodimensional ultrasound in the differential diagnosis and prognosis after treatment. Therefore, it is of great clinical significance to improve the diagnosis and prognosis evaluation with the accurate and reasonable examinations.
Along with the improvement of ultrasound high-frequency probes and the application of color Doppler ultrasound, contrast-enhanced ultrasound has become a novel diagnostic technique, which represents the hot-spot in ultrasound medical research due to the reliable imaging performance in the ultrasound interventional treatment of endometrioma. In the past, adenomyosis is always treated with surgery, mostly the local lesion resection. However, the surgical resection cannot completely remove the lesions due to the invasive and aggressive growth in the myometrium. High-Intensity Focused Ultrasound (HIFU), an ultrasound intervention technique, is a relatively new non-invasive treatment method [8,9]. HIFU can cause the coagulation necrosis in the lesions by inducing transient high temperature, which would be dissolved and absorbed, or fiberized [10]. Moreover, HIFU could achieve in situ inactivation of target lesions, without damaging the surrounding tissues [11], and the uterus in female patients can be retained, maintaining the normal physiological functions. Because HIFU is performed under ultrasound monitoring, it is also featured by minimal invasion, effectiveness and safety, and rapid recovery after surgery [12,13], which plays an important role in the treatment of gynecological diseases.
In this study, to improve the accuracy of the diagnosis of endometrioma, the sonographic features and related parameters of contrast-enhanced ultrasound were investigated. Moreover, the contrast-enhanced ultrasound performance and efficacy before and after HIFU treatment were also evaluated and analysed.
Materials and Methods
Study patients
Totally 60 patients with endometrioma who underwent the HIFU treatment in our hospital from June 2014 to July 2016 were included in the study. The inclusion criteria included: (1) women of childbearing age (>18 y); (2) patients who refused surgery; and (3) patients with no examination contraindications. The exclusion criteria were as follows: (1) cytological biopsy indicated squamous intraepithelial lesions and cancer cells; (2) patients with other gynecological diseases; (3) gynecological examinations indicated suspicious tissue and/or organ adhesion in pelvic cavity; (4) patients in menstrual period, lactation, or with positive result for pregnancy test; and (5) patients with serious heart, kidney, and/or liver dysfunction. Meanwhile, another 60 women with the corresponding symptoms were selected as control. The main clinical symptoms of the patients with uterine adenomyosis included progressively increased dysmenorrhea, elongated menstrual cycle, increased menstrual flow, and abdominal bulge. All these subjects were in the non-menstrual period (5 to 8 d after menstruation) when undergoing ultrasonography. The basic information of these subjects was shown in Table 1. Prior written and informed consent were obtained from every patient and the study was approved by the local ethics review board.
| Uterine adenomyoma group (n=60) | Control group (n=60) | t | p | |
|---|---|---|---|---|
| Age, years | 43. 8 ± 6.78 | 42. 3 ± 11.76 | 0.75 | 0.58 |
| Disease course, years | 2.36 ± 0.98 | 2. 83 ± 1.76 | 0.62 | 0.62 |
| Pregnancy history | ||||
| Yes | 55 (91.66%) | 35 (58.34%) | 6.35 | 0.04* |
| No | 5 (8.34%) | 25 (41.67%) | ||
| Abortion history | ||||
| Yes | 42 (70.00%) | 21 (35.00%) | 5.36 | 0.03* |
| No | 18 (30.00%) | 39 (65.00%) | ||
| Clinical symptoms | ||||
| Increased dysmenorrhea | 60 (100%) | 60 (100%) | 4.31 | 0.01* |
| Excessive menstrual volume | 54 (90.0%) | 57 (95.5%) | ||
| Lower abdomen discomfort | 51 (85.0%) | 55 (91.6%) | ||
| Menostaxis | 46 (76.6%) | 50 (83.3%) |
Table 1. Basic information of uterine adenomyoma patients and normal control subjects.
Ultrasound contrast examination
Ultrasound contrast examination was performed with the Doppler Ultrasound Diagnostic Apparatus, with the convex array probe of 2-4 MHz and mechanical index of 0.15-0.20. The contrast agent was Sono Vue (Bracco, Italy), the main component of which was the SF8 gas. The contrast agent was prepared into microbubble suspension with 50 ml 0.9% sodium chloride, which was rapidly injected as bolus into the anterior elbow vein. Totally 10 ml 0.9% sodium chloride was used to wash the tube, with 3.0 ml each time. The myoma morphology, location, size, echo, blood flow, and resistance index were observed by ordinary ultrasound. Then the ultrasound contrast mode was started, and the observation persisted for more than 3 min after injecting the contrast agent. The images were analysed by two experienced ultrasound analysts.
HIFU operation
HIFU was performed with the Focal Ultrasound Ablation Apparatus (Model JC200; Chongqing HIFU, Chongqing, China). After sedation and analgesia, the patient was in the supine position, and the skin in the treatment region was soaked in deaerated water. The ultrasound probe and laser light were positioned, and the treatment started after the accurate positioning of the built-in ultrasonic probe.
The treatment parameters were as follows: ultrasound frequency, 1.0 MHz; sound intensity, 0-3000 W/cm2; focal distance, 16.5 cm; focal region, 3 mm × 3 mm × 8 mm; single emission time, 0.15 s; interval time, 0.25 s; emission times, 10-12; transmitter number, 3-6; and acoustic power, 70%-100% of the total power. The treatment region covered the entire tumor tissue.
Ultrasonic parameter evaluation
Patients were subjected to contrast-enhanced ultrasonography before and after HIFU. The following ultrasonic parameters were recorded and evaluated: the Arrival Time (AT), Time to Peak (TTP), maximum intensity (Imax), and Rise Time (RT). AT referred to time from the beginning of contrast injection to when the perfusion was observed in the lesion area. The TTP referred to the time when the peak average intensity was observed from the contrast injection to lesion perfusion. Imax referred to the maximum average intensity in the observation lesion area. RT referred to the time during which the intensity risen from 10% to 90% of the Imax.
Patient follow-up
Patients subjected to HIFU were followed up at 1, 3, 6, and 12 months after treatment. The maximum diameter and volume of the non-perfusion lesions were observed. The volume was calculated according to the following formulation: V=(1/6) π × vertical diameter ×horizontal diameter × thickness [9]. The lesion blood supply was described as abundant, moderate, rare, or no blood flow. RFA evaluation was performed based on the clinical symptoms, signs, and ultrasound follow-up results 3-6 months after treatment: cure, in which the adenomyoma completely disappeared, with no clinical symptoms; improvement, in which the conventional ultrasound examination tumor shrinkage ≥ 60%, with no clinical symptoms; effective, in which the tumor shrinkage was observed in the conventional ultrasound review, with alleviated clinical symptoms; and invalid, in which the no change, or increased tumor, was observed, with no clinical symptom improvement.
Adverse reaction monitoring and evaluation
Treatment-related adverse reactions included the sacrococcygeal pain, buttock pain, pain in the treatment area, and skin hot. Immediate evaluation of adverse effects after treatment included observing vital signs, skin condition in the treatment area, vaginal discharge volume and color, and limb activity. Follow-up observation for adverse reactions was performed within 1 month after treatment, which could be extended according to the actual situation.
Statistical analysis
Data were expressed as mean ± SD. Statistical software 17.0 was used for statistical analysis. The T and χ2 tests were used for the comparison. Receiver Operating Characteristic (ROC) curve was obtained, and the Area Under the Curve (AUC) was calculated. The diagnostic value of related parameters and the Youden index were determined. P<0.05 was considered statistically significant.
Result
Contrast-enhanced ultrasound performance of adenomyoma and control groups
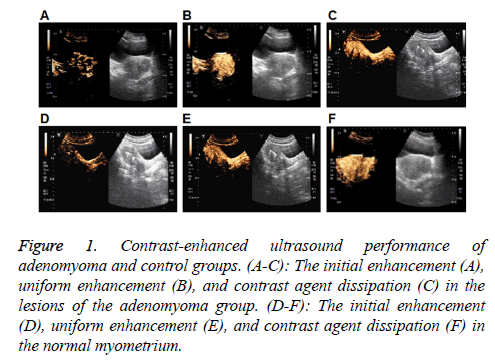
The contrast-enhanced ultrasound performance of the adenomyoma group was first investigated. As shown in Figures 1A-1C and Table 2, in the early stage of contrastenhanced ultrasound, dendritic enhancement was observed in 49 cases (49/60, 81.67%), and the gradual enhancement from the surrounding area towards the center in an irregular nodular morphology was noted in 11 cases (11/60, 18.4%). After the peak, there was no clear boundary between the adenomyoma lesion and the surrounding tissue. Totally 38 cases (38/60, 63.34%) exhibited non-uniform enhancement in the lesion area, while 22 cases (22/60, 36.67%) displayed uniform enhancement in the lesion region. Hyperechoic enhancement was observed in 34 cases (34/60, 56.67%), hypoechoic enhancement was noted in 18 cases (18/60, 30.00%), and isoechoic enhancement was observed in 8 cases (8/60, 13.34%), in the lesion areas, respectively. Along with the contrast agent dissipation, the lesion area and surrounding myometrium showed synchronous regression in these 60 cases (60/60, 100%).
Figure 1: Contrast-enhanced ultrasound performance of adenomyoma and control groups. (A-C): The initial enhancement (A), uniform enhancement (B), and contrast agent dissipation (C) in the lesions of the adenomyoma group. (D-F): The initial enhancement (D), uniform enhancement (E), and contrast agent dissipation (F) in the normal myometrium.
| Uterine adenomyoma group | Control group | |
|---|---|---|
| Initial enhancement pattern | Dendritic enhancement in 49 cases | Dendritic enhancement was observed from the myometrium towards the contrast-enhanced ultrasonography |
| Gradual enhancement from the surrounding area towards the center in an irregular nodular morphology in 11 cases | ||
| Boundary after peak | No clear boundaries | No clear boundaries |
| Peak intensity uniformity | Non-uniform enhancement in 38 cases | All uniform enhancement |
| Uniform enhancement in 22 cases | ||
| Peak intensity | Hyperechoic enhancement in 34 cases | All isoechoic enhancement |
| Hypoechoic enhancement in 18 cases | ||
| Isoechoic enhancement in 8 cases | ||
| Regression pattern | Synchronous regression with myometrium | Regression from endometrium towards periphery myometrium |
Table 2. Contrast-enhanced ultrasound performance of uterine adenomyoma and control groups.
For the contrast-enhanced ultrasound performance of the control group, as shown in Figures 1D-1F and Table 2, in the early stage, dendritic enhancement was observed from the myometrium towards the contrast-enhanced ultrasonography in the normal group showed an early enhancement of contrast enhancement from the peripheral dendrites to endometrium. After reaching the peak, homogeneous enhancement was noted in the whole myometrium, with no defect in the contrast agent filling. When the contrast agent started to become weak, regression was observed in the entire myometrium from the center to surrounding area.
Contrast-enhanced ultrasound parameters of endometrioma before treatment
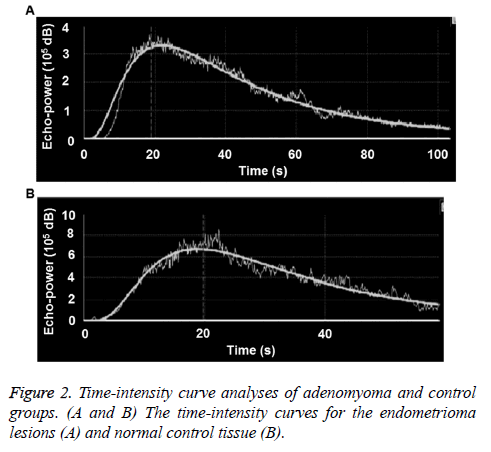
The time-intensity curves of lesions were obtained for all the patients during the contrast-enhanced ultrasonography. For the adenomyoma group, as shown in Figure 2A, the earliest AT was 8.4 s, and the latest AT was 18.5 s; and the earliest TTP was 22.8 s, and the latest TTP was 40 s. For the control group, the earliest AT was 8.9 s, and the latest AT was 17.8 s; and the earliest TTP was 25.7 s, and the latest TTP was 36 s (Figure 2B). There was no significant difference in the AT between these two groups (p>0.05). However, compared with the control group, the TTP and RT were significantly shorter, and the Imax was significantly higher, in the adenomyoma group (all p<0.05). These results suggest that the blood flow perfusion rate inside the endometrioma could provide preliminary observation data for the endometrioma perfusion evaluation with contrast-enhanced ultrasound, and contribute to the observation standard establishment, as well as future investigation and application (Table 3).
| Uterine adenomyoma group | Control group | R | p | |
|---|---|---|---|---|
| AT (s) | 10.35 ± 3.25 | 9.69 ± 2.84 | 0.89 | 0.06 |
| TTP (s) | 26.98 ± 6.43 | 28.90 ± 7.28 | 0.77 | 0 |
| Uterine adenomyoma group/Control group | 0.01 | |||
| Imax (dB) | 63.38 ± 11.91 | 61.35 ± 11.89 | 0.76 | 0.01 |
| Uterine adenomyoma group/Control group | 0 | |||
| RT (s) | 15.30 ± 3.37 | 17.22 ± 3.78 | 0.56 | 0 |
| Uterine adenomyoma group/Control group | 0.01 |
Table 3. Comparison of contrast-enhanced ultrasound parameters.
Diagnostic performance of contrast-enhanced ultrasound characteristic parameters
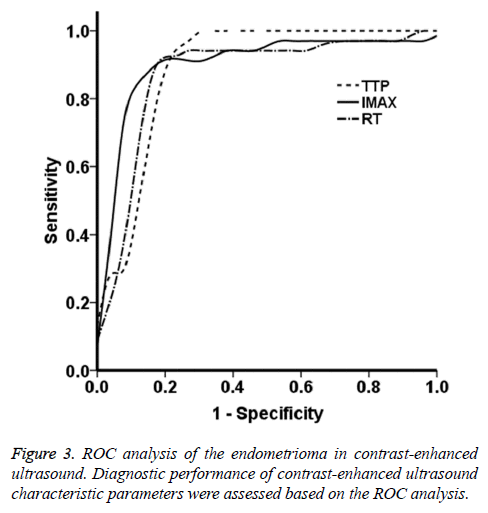
The ROC curves were obtained based on the data from the endometrioma group. As shown in Figure 3, the ROC analysis showed that, the AUC values for the parameters of TTP, Imax, and RT were 0.88, 0.89, and 0.87, respectively. These findings suggest that the contrast agent perfusion was accompanied with abundant vascular proliferation and expansion. The contrast agent quickly filled the entire lesion area, which well reflected the blood supply in fibroids. Based on the ROC curves, the Youden indices for the TTP, Imax, and RT were 24.72 s, 61.11 dB, and 13.64 s, respectively, which were the optimal diagnostic thresholds. The sensitivity and specificity of these four parameters were all >80.0%. In particular, the sensitivities and specificities for TTP were 0.941 and 0.231, for Imax were 0.912 and 0.154, and for RT were 0.882 and 0.153, respectively. These results suggest that the lesion blood supply could be evaluated in advance according to the threshold sensitivity and specificity, which might provide incidence for the clinical treatment.
Contrast-enhanced ultrasound parameter alterations in non-perfusion area of adenomyoma
The maximum diameter and volume of the non-perfusion area were investigated in the adenomyoma and control groups. In the adenomyoma group, there were no significant differences between before and after treatment. In the experiment group, compared with 3 d before treatment, no obvious differences were observed in the maximum diameter and volume of the non-perfusion area of adenomyoma at 1 month post-operation (p>0.05). However, at 6 and 12 months after treatment, the adenomyoma maximum diameter was decreased, while the non-perfusion area was significantly increased, compared before the treatment (both p<0.05). These results suggest obvious ablation effects of HIFU treatment, which has obvious effects in the clinical treatment of endometrioma (Table 4).
| Maximum diameter of non-perfusion area (cm) | p | Non-perfusion volume (cm3) | p | |
|---|---|---|---|---|
| Control group | 4.87 ± 1.23 | - | 59.23 ± 21.64 | - |
| Uterine adenomyoma group | ||||
| Three days before treatment | 4.36 ± 1.57 | 0.06 | 62.12 ± 22.43 | 0.08 |
| After treatment | ||||
| 1 month | 4.13 ± 1.36 | 0.07 | 74.76 ± 29.46 | 0.06 |
| 3 months | 3.56 ± 0.96 | 0.05 | 97.63 ± 32.47 | 0.02 |
| 6 months | 2.96 ± 0.87 | 0.02 | 108.36 ± 38.94 | 0.02 |
| 12 months | 2.35 ± 0.46 | 0.01 | 126.21 ± 40.63 | 0 |
Table 4. Uterine adenomyoma diameter and volume alterations in contrast-enhanced ultrasound.
Evaluation of adverse effects and efficiencies after HIFU treatment
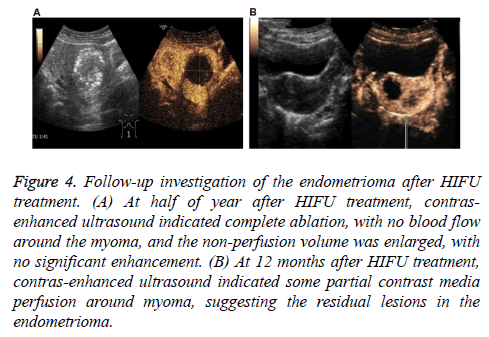
Our results showed that, in these patients, 41 cases reported sacrococcygeal pain during treatment, 7 cases reported regional radiation pain, and 38 cases reported skin hot. After adjusting the focus position, treatment rhythm, treatment intensity, and water temperature, all the symptoms were alleviated, with the pain sores below 4. After treatment, 9 cases reported abdominal pain, and 4 cases reported sacrococcygeal pain, which disappeared at 1 w post-operation. No complications occurred in all these patients. In the follow-up period, clinical and discomfort symptoms disappeared in 54 cases. No abnormal perfusion enhancement area was observed in the surrounding and inside areas of 60 lesions in contrast-enhanced ultrasound, i.e., the completely inactivated state, suggesting cure and improvement. In 6 cases, the clinical symptoms were basically relieved, and the contrast-enhanced ultrasound indicated 20 lesions that were not completely ablated, suggesting effectiveness. In all these lesions, significant differences were observed in the tumor ablation rate and tumor residual rate between these two groups (Table 5) (Figure 4), suggesting the satisfactory safety of HIFU in the treatment of endometrioma.
Figure 4: Follow-up investigation of the endometrioma after HIFU treatment. (A) At half of year after HIFU treatment, contrasenhanced ultrasound indicated complete ablation, with no blood flow around the myoma, and the non-perfusion volume was enlarged, with no significant enhancement. (B) At 12 months after HIFU treatment, contras-enhanced ultrasound indicated some partial contrast media perfusion around myoma, suggesting the residual lesions in the endometrioma.
| Lesion number | Diagnostic evaluation of contrast-enhanced ultrasound, n (%) | χ2 | p | ||
|---|---|---|---|---|---|
| Complete ablation (cure+improvement) | Incomplete ablation with residual lesion (effective) | ||||
| Single lesion | 46 | 37 | 9 | 4.24 | 0.04 |
| Multiple lesions | 34 | 23 | 11 | ||
| In total | 80 | 60 (75%) | 14 (25%) | ||
Table 5. Post-operative contrast-enhanced ultrasound of single and multiple lesions.
Discussion
In recent years, it has been more and more well accepted that the contrast-enhanced ultrasound would evaluate the treatment of endometrioma with HIFU, which could achieve monitoring in real-time for the fibroid size, tumor echo alterations, and inside blood flow [14], providing timely access to the contrast agent perfusion alterations in the treatment area in fibroid and improving the ability of lesion detection and diagnosis of ultrasound [15]. Contrast-enhanced ultrasonography can achieve real-time, continuous dynamic observation of the whole process of contrast agent perfusion in lesions [16], which could provide more accurate blood perfusion information for the clinical treatment than the basic ultrasound. In this study, 60 cases of endometrioma were subjected to the contrast-enhanced ultrasound, which exhibited a dendritic enhancement manner in the early stage; after reaching the peak, the lesion has no boundary, with uneven peak intensities, and the peak intensities were mainly hyperechoic enhancement. Moreover, synchronized regression was observed in the peripheral myometrium. These results suggest that although the endometrioma exhibits the massive echo on the two-dimensional sonogram similar to the hysteromyoma, which is not an actual tumor. This is induced by the limited distribution of the endometrial glands and the interstitial invasion in myometrium, with no clear boundary. Therefore, there was no circular or semi-circular enhancement, or clear boundaries between the lesion and surrounding tissues after peak perfusion, in the contrast-enhanced ultrasound.
It has been shown that, the contrast-enhanced ultrasound of hysteromyoma displayed the performance in which the initial enhancement was pseudocapsule enhancement with circular or semi-circular pattern, with clear boundary between the lesion and surrounding tissues. The significant differences in the contrast-enhanced ultrasound performance between hysteromyoma and adenomyoma provided evidence for the distinctive diagnostic rate [17]. The time-intensity, together with the involved various indicators, have been widely used to study the perfusion characteristics of liver, kidney, spleen, pancreas, and other organs, which especially plays an important role in the clinical distinctive diagnosis of liver tumors [18].
Up to now, there are few studies focusing on the blood supply and perfusion features of endometrioma based on the timeintensity curve. Therefore, it is of great clinical importance to investigate the perfusion-related parameter alterations to understand the blood supply distribution and contrast agent perfusion pattern in endometrioma. In this study, quantitative analyses with time-intensity were performed in both the adenomyoma and control groups. Our results showed that, there were no significant differences in the AT between these two groups (p>0.05). However, significant differences were observed in the TTP, RT, and Imax between these two groups (p<0.05). Compared with the control group, the TTP and RT were shorter, and the Imax was higher, in the limited group. This phenomenon might be caused by the fact that the contrast agent perfusion was accompanied by the abundant vascular proliferation and expansion. The contrast agent rapidly filled in the entire lesion area, leading to faster TTP and RT, as well as elevated Imax, in the lesions compared with normal control myometrium. Our results showed that, the differences in the TTP, RT, and Imax would provide quantitative evidence to understand the perfusion of endometrioma. At the meantime, The AUC values of TTP, Imax, and RT in the ROC curve were 0.88, 0.89, and 0.87, respectively, indicating potential diagnostic value. The Youden indices were 24.72 s, 61.11 dB, and 13.64 s, respectively, which could be considered as the optimal diagnostic thresholds. These results suggest that these parameters would contribute to the distinctive diagnosis of endometrioma and other benign lesions, such as uterine adenomyosis and uterine fibroids.
Contrast-enhanced ultrasound plays an important role in the treatment of endometrioma with HIFU, and the maximum diameter and volume of non-perfusion area, as well as the alleviated clinical symptoms, work as important indicators for the therapeutic efficiency of HIFU. It has been reported that, at 3, 6, and 20 months after HIFU treatment, the endometrioma volume gradually became narrowed [19], and both the internal echo enhancement and color blood flow in the tumors disappear. Contrast-enhanced ultrasound showed no perfusion of contrast agent in 18 cases of adenomyoma. In this study, our results showed that, compared with 3 d before HIFU, at 1 month after treatment, no significant differences were observed in the maximum diameter and volume of non-perfusion area (p>0.05). However, at 6 and 12 months after treatment, the maximum diameter was significantly decreased, while the volume was significantly increased, of the non-perfusion area, compared with before the treatment (both p>0.05). These results indicated that the HIFU resulted in the absence of blood supply or low blood supply status, suggesting completely or substantially inactivated tumors. These patients reported intraand postoperative complications, which would be expected for the treatment. After appropriate treatments, the symptoms were improved, and most of them disappeared at 1 w after HIFU treatment. In the follow-up contrast-enhanced ultrasound, 60 lesions were completely inactivated, and 20 lesions were not completely ablated, suggesting effectiveness. In all these lesions, there were significant differences in the tumor ablation rate and tumor residual rate between these two groups. Meanwhile, the contrast-enhanced ultrasound indicated increased non-perfusion volume, with significantly elevated ablation rate, which should be evaluated timely during operation. The residual lesions could be subjected to supplementary treatment to achieve radical treatment and improve disease prognosis.
In conclusion, our results showed that in the parameters of contrast-enhanced ultrasound, there are differences in the uniformity and altitude of peak intensities between normal uterus and other benign uterine lesions, which might contribute to the clinical diagnosis and quantitative analysis of endometrioma. Our findings suggest that, contrast-enhanced ultrasound could provide accurate evidence in the assessment of HIFU in the treatment of endometrioma. The real-time monitoring of perfusion status during HIFU treatment greatly contributed to the assessment of ablation range, safety, and effectiveness of HIFU in the disease treatment, which is of great clinical significance.
Acknowledgements
This work was supported by Scientific Research Fund Project of Zhejiang Province (no.2017KY590).
Disclosures
All authors declare no financial competing interests. All authors declare no non-financial competing interests.
References
- Yang Y, Zhang J, Han ZY, Ma X, Hao YL, Xu CT, Xu RF, Zhang BS. Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep 2015; 5: 10034.
- Bupathy, Arunachalam, Jayasree M.A clinico-pathological study of adenomyosis. J Clin Diagn Res 2012; 6: 428-430.
- Shrestha A, Shrestha R, Sedhai LB. Adenomyosis at hysterectomy: prevalence, patient characteristics, clinical profile and histopatholgical findings. Kathmandu Univ Med J (KUMJ) 2012; 10: 53-56.
- Carrafiello G, Recaldini C, Fontana F, Ghezzi F, Cuffari S, Lagana D, Fugazzola C. Ultrasound guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Interv Radiol 2010, 33: 113-119.
- Ankem K. Information-seeking behaviour of women in their path to an innovative alternate treatment for symptomatic uterine fibroids. JMLA 2007; 95: 164-172.
- Luo S, He M. Pregnancy outcome after high intensity focused ultrasound (HIFU) treatment for patients with adenomyosis. Chongqing Med 2014; 43: 454-458.
- Nepomnachhikh LM, Lushnikova EL, Pekarev OG, Lushnikova AK, Nikitenko EV. Pathomorphological analysis of internal endometriosis. Bull Exp Biol Med 2012; 153: 109-113.
- Liu X, Wang W, Wang Y, Wang Y, Li Q, Tang J. Clinical predictors of long-term success in ultrasound-guided high-intensity focused ultrasound ablation treatment for adenomyosis: a retrospective study. Med (Baltimore) 2016 ; 95: 1-6.
- Uchida T, Tomonaga T, Kim H, Nakano M, Shoji S, Nagata Y, Terachi T. Improved outcomes with advancements in high intensity focused ultrasound devices for the treatment of localized prostate cancer. J Urol 2015; 193: 103-110.
- Wu R, Hu B. Values of imaging methods in the assessment of therapeutic effects of HIFU in bone tumors. Chinese J Interv Imag Ther 2008; 5: 79-82.
- Yao J, Lu F, Li M. Efficacy evaluation of high-intensity focused ultrasound in treatment of uterine adenomyoma by color Doppler ultrasonography. Shanghai Med Imag 2006; 15: 22-24.
- Wang W, Wang Y, Wang T, Wang J, Wang L. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol 2012; 22: 2553-2558.
- Kim CH, Kim SR, Lee HA, Kim SH, Chae HD. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod 2011; 26: 559-563.
- Kim HS, Baik JH, Pham LD, Jacobs MA. MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol 2011; 18: 970-976.
- Bernatik T, Strobel D, Hahn RG, Becker D. Detection of liver metastases: comparison of contrast-enhanced wide-band harmonic imaging with conventional ultrasonography. J Ultrasound Med 2001; 20: 509-515.
- Quaia E, Calliada F, Beertolotto M. Characterization of focal lesions with contrast-specific US modes and a sulfur hexafluoride filled mircobubble contrast agent: diagnostic performance and confidence. Radiology 2004; 232: 420-430.
- Wang H, Nan C. Diagnostic value of time-intensity curve for hepatic space-occupying lesions. J Clin Hepatol 2014; 30: 1193-1197.
- Marret H, Tranquart F, Sauget S, Alonso AM, Cottier JP, Herbreteau D. Contrast-enhanced sonography during uterine artery embolization for the treatment of leiomyomas. Ultrasound Obstet Gynecol 2004; 23: 77-79.
- Wei C, Hu B. Evaluation of uterine adenomyoma treatment with high intensity focused ultrasound by contrast-enhanced ultrasound. Chin J Med Ultrasound 2010; 7: 54-59.