Research Article - Archives of General Internal Medicine (2023) Volume 7, Issue 5
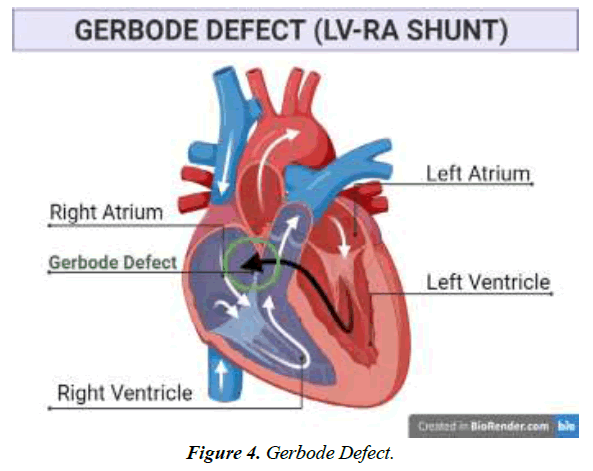
Communication anomaly among left ventricle and right atrium; Gerbode defect.
Roopeessh Vempati1*, Sabah Afroze2, Sanjay Bethanabotla3, Prerna Chandra4, Ayushi Mishal5, Yashaswi Patel6, Ihab Sheikh Hanafi7, Sweta Sahu8, Adhvithi Pingili9, Vikash Jaiswal10
1Department of Internal Medicine, Gandhi Medical College And Hospital, Secunderabad, Telangana, India.
2Department of Internal Medicine, Shadan Institute Of Medical Sciences, Teaching Hospital And Research Centre, Hyderabad, Telangana.
3Department of Internal Medicine, Osmania Medical College, Hyderabad, Telangana, India
4Department of Internal Medicine, Deccan College Of Medical Sciences, Hyderabad
5Department of Internal Medicine, Christian Medical College, Vellore, Tamil Nadu, India.
6Department of Internal Medicine, Government Medical College, Surat, India
7Department of Internal Medicine, Spartan Health Sciences University, School Of Medicine, Vieux Fort, Saint Lucia
8Department of Internal Medicine, JJM Medical College, Davanagere, karnataka, India
9Department of Internal Medicine, MedStar Health, Baltimore, Maryland, USA
10Department of Cardiovascular Research, Larkin Community Hospital, South Miami, Florida, USA
- *Corresponding Author:
- Roopeessh Vempati
Department of Internal Medicine
Gandhi Medical College And Hospital
Secunderabad, Telangana, India
E-mail: roopeshgupta666666@gmail.com
Received: 24-Sept-2023, Manuscript No. AAAGIM-23-113517; Editor assigned: 28-Sept-2023, PreQC No. AAAGIM-23-113517(PQ); Reviewed: 11-Oct-2023, QC No. AAAGIM-23-113517; Revised: 16-Oct-2023, Manuscript No. AAAGIM-23-113517(R); Published: 22-Oct-2023, DOI:10.35841/aaagim-7.5.194
Citation: Vempati R, Afroze S, Bethanabotla S, et al. Communication anomaly among left ventricle and right atrium; Gerbode defect. Arch Gen Intern Med. 2023;7(5):194
Abstract
Gerbode defect is a rare cardiovascular defect occurring as a septal abnormality among the left ventricle and right atrium. Acquired Gerbode defect had surpassed the genetic defect rate with increasing prevalence in old individuals. It can also occur as a rare complication or secondary condition due to repeated replacement of heart valves. The overwhelming majority of cases are thought to be caused by iatrogenic injury, endocarditis, myocardial infarction in the right coronary artery distribution, or blunt cardiac trauma. Dyspnea, congestive heart failure, orthopnea, and pedal edema are prevalent symptoms experienced after the mitral or aortic valve replacement procedure. Transesophageal Echocardiography (TEE), Transoesophageal Echocardiogram (TOE), and Echocardiography (ECG) are used along with Doppler for the diagnosis. Elevated atrial volumes, irregular heartbeats, and atrial fibrillation are the identifying factors for the septal defect. It is a high-risk condition that requires immediate care, such as surgical management. The pericardial patch is opted prevalently during the surgical treatment of defects.
Keywords
Gerbode defect, Left Ventricle Right Atrium communication, Mitral Valve Replacement, Aortic Valve Replacement, Mitral Stenosis.
Introduction
Rare ventricular septal abnormalities known as the Gerbode defect include Left Ventricular (LV) to Right Atrial (RA) connections [1]. Thurman J. originally wrote about this defect in 1838. However, when Professor Frank Gerbode documented a pioneering case series that included five successful operative repairs, it was given his name [2]. Abnormal communication between the left and right atrium occurs, which can be due to several causative factors such as cardiovascular diseases like endocarditis, myocardial infarction, and stroke or as a result of genetic abnormalities [3]. According to Gerbode et al., the flaws in the communication of left and right ventricles are typically congenital but can be acquired as well in rare cases.
There are two types: either an absence of the atrioventricular membranous septum or shunting that first occurs due to a ventricular septal defect, with atrial shunting resulting from an error that affects the tricuspid valve's septal leaflet [4].
Cause
Congenital defects are relatively rare (0.08%) and can be caused by a membranous Ventricular Septal Defect (VSD) and an anomaly in the leaflet of the tricuspid valve, or they might develop indirectly (1). Gerbode abnormalities are uncommon, and while some instances' pathophysiology has been linked to sequence variants in the NK2 Homeobox 2 (NKX2-5), GATA4, and T-box transcription factor 5 (TBX5) genes, the bulk of cases' causes are still unknown [5].
The condition was initially thought to be a rare genetic defect, but recent research indicates that acquired defects are now more common than congenital ones. The overwhelming majority of cases are thought to be caused by iatrogenic injury, endocarditis, myocardial infarction in the right coronary artery distribution, or blunt cardiac trauma [6].
Adults may infrequently experience acquired LV-RA shunts due to a defect in the ventricular septum's upper section [7]. It was also presented as a rare complication or secondary condition due to repeated replacement of heart valves [8]. There have been reported presentations of communication errors between valves due to the injury that occurred during surgery, which caused a shortcoming in the membrane of the atrioventricular septum [9]. A defect in the membrane interventricular septum, which is located below the crista supraventricularis, is the cause of both types of LV-RA connections. Earlier cardiac surgical procedures (mitral valve replacement, aortic valve replacement, VSD-ASD (atrial septal defect) repair, and percutaneous cardiac interventions (AV node ablation, endomyocardial biopsy, and tricuspid annuloplasty), which account for the majority of the rise in over the past 20 years, are the two primary contributors of acquired iatrogenic Gerbode defects.
Anatomy
The septal leaflet of the TV typically lies 5 to 10 mm higher towards the apex than the anterior leaflet of the mitral valve, considering the annulus of the tricuspid valve occupies a position more apically than the mitral annulus. Due to this structural configuration, the supravalvular (also known as the atrioventricular) and intra-valvular (also known as the interventricular) sections of the membrane ventricular septum are separated by the septal leaflet. A direct LV-RA connection is the outcome of a supravalvular section defect. Connectivity between the right ventricle and the left ventricle would typically emerge from a peri-membranous ventricular septal defect in the intra-valvular region [10].
Symptoms
When there are Gerbode deficiencies, the RA experiences pressure as well as volume overexertion, which is accompanied by symptoms such as dyspnea and heart failure. Physical examination reveals a noticeable pan systolic murmuring at the left sternal border [11]. An LV-RA shunt can be identified physically by a loud, harsh, pan-systolic murmur that is frequently accompanied by a thrill across the left side sternal border. However, a diagnosis cannot typically be made only on the grounds of the results of a physical examination.
Edema and shortness of breath or dyspnea are the prevalent physical symptoms observed among patients with communication defects in the chambers of the heart, mainly the left ventricle and right atrium. Heart murmur is also identified as a symptom after the mitral or aortic valve replacement surgery, such as pansystolic murmur as well as holosystolic was observed. Edema in lower limbs, pedal edema, asities, and congestive heart failure or endocarditis are also listed as symptoms [12].
Diagnosis
The preferred diagnostic method is TEE. The flow of color Doppler will show the presence of, while attenuated throughout diastole, a high-velocity systolic jet with flow directed toward the Right Atrium (RA). The advancement of innovative methods for imaging has increased the reliability in the identification of acquired Gerbode defects, especially those that arise from adverse effects of cardiac procedures and percutaneous coronary interventions involving the interventricular septum or adjacent structures, such as cardiac magnetic resonance and multidimensional Transesophageal Cardiac Echocardiography (TOE).
It's crucial to differentiate between tricuspid regurgitation and LV-RA communication. Unusual jet direction, continuous shunt flow into diastole, insufficient ventricular septal flattening, lack in right hypertrophy of the ventricular walls, and normal diastolic pulmonary artery pressure as determined by the pulmonic regurgitant velocity are some of the most significant echocardiographic clues that point to the presence of the Gerbode defect [13]. Elevated atrial volumes can cause structural alterations that lead to irregular heartbeats, including atrial fibrillation [14].
Treatment plan
The reduction of vascular resistance in the system, an increase in the output of the heart, and a decrease in lung congestion constitute the hemodynamic objectives for the defect [15].
Physiology
As a consequence of the substantial blood pressure gradient that prevails among these heart chambers, switching from LV to RA happens physiologically. The passage of blood into the Right Atrium (RA) is enhanced as a result of tunneling, which enlarges the right side of the heart compartment.
Management of disease
In patients with these abnormalities, maintaining native or temporarily assisting atrioventricular pumping is crucial to promoting appropriate LV filling, volume of stroke, and outward stream in the presence of significant regurgitant circulation and heart failure-related symptoms.
An Amplatzer occluder apparatus can be employed in the cardiovascular interventional unit to provide definitive treatment [16]. Alternately, an extremely risky invasive surgical alternative known as the commando method, which combines mitral and aortic valve replacement alongside intervalvular fibrous repairs to the body, could be performed [17].
Mitral valve replacement
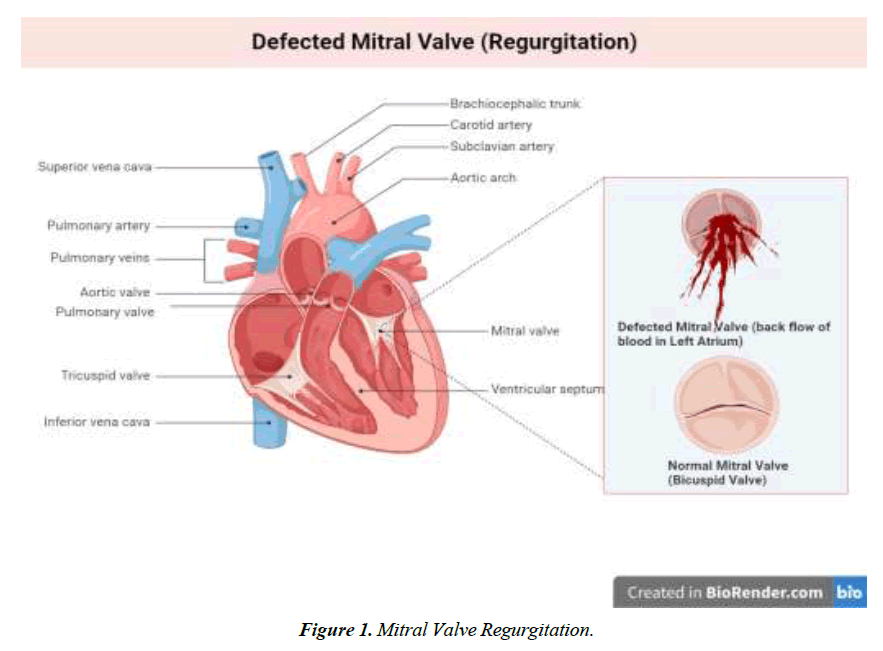
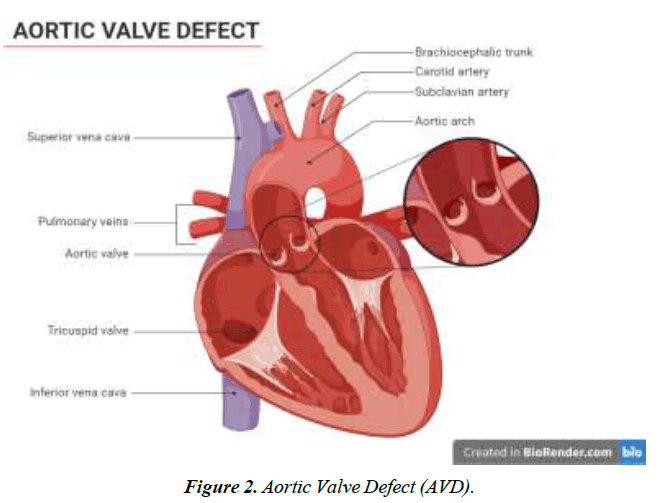
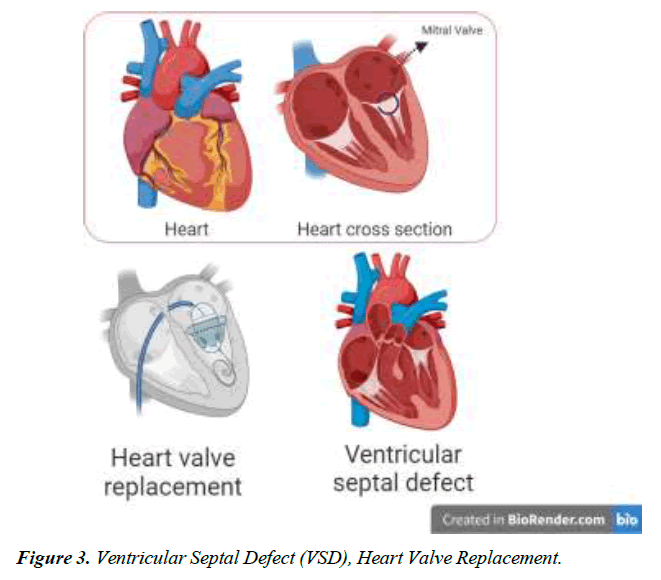
We conducted a literature-based analysis of case reports research to identify the characteristics, clinical impact, symptoms, diagnostic procedure, management, and intervention for the defect. The majority of patients diagnosed with Gerbode defect are older adults except for two individuals aged 23 and 25. A twenty-five-year-old male patient with arterial septal defect and cleft mitral valve had undergone a surgical procedure for the treatment of the condition at 16 years. CV-LA communication deficit was reported after nine years of surgery and diagnosed with the help of ECG and color flow. The second patient was 23 years old with a history of mitral and tricuspid valve replacement due to iatrogenic factors. Hematuria, hemolysis, and pan systolic murmur were reported after three months of surgery. Surgical management of defects was employed for optimal outcomes [18]. Dyspnea was one of the prevalent symptoms among all the patients, along with murmur, chest pain, and edema. Congestive heart failure and orthopnea were typically present in 4 of 16 patients reported in this study Figure 1-4.
Age
Occurrence of Gerbode defect was more prevalent in the population above 40 years, with almost 50% of patients in the age bracket of 51-60 years and above 70 years. The maximum age of patients suffering from LV-RA defect among communication was 81 years male, as reported by Sunderland et al. After five years of surgery for aortic valve replacement. 2 patients, making up 11% of research patients reported in this study, are in their twenties, indicating infrequent occurrence of acquired Gerbode defect in young individuals. A study reported a case of a 30-year-old female patient with mitral valve replacement in the past with infected endocarditis diagnosed by utilizing TOE and ECG. The Gerbode defect occurring secondary to any disease is an acquired defect in cardiovascular chambers.
Diagnosis
Myocardial stenosis was the prevalent causative agent among patients with Gerbode defect. There was also an incidence of myocardial stenosis along with tricuspid regurgitation. Aortic stenosis occurred in two patients, as reported by the research authors. There was also a diagnosis of endocarditis in 3 patients. An incident of iatrogenic occurrence of Gerbode defect was also presented by the researcher [19-22].
Diagnostic method
ECG was utilized in almost all the cases of Gerbode defect diagnosis. Many studies used TOE, which stands for transoesophageal echocardiogram, for the purpose of identification of the LV-RA communication impediment in the heart of patients. TOE is conducted via the probe. The probe is inserted via the esophageal route of the patients to find a clear and precise image of the heart for a better understanding of the disease. Left ventriculography was used by Aoyagi et al. only as the patient was suffering from tricuspid regurgitation (TR). 2 case reports included the Doppler color method as a diagnostic tool for obtaining a colorful and reliable image of the thoracic cavity [22-27] (Table 1).
| Age Range | No of patients n (%) | Reference |
|---|---|---|
| >40 | 3 (18.76%) | |
| 41-50 | 3 (18.76%) | [12, 16] |
| 51-60 | 3 (18.76%) | [1, 22, 24] |
| 61-70 | 2 (12.16%) | [26, 28] …. |
| 71< | 5 (31.2%) | [10, 20, 23, 27, 28] |
Table 1. Age Range of Participants.
Causative agent
Myocardial valve replacement surgery was identified as one of the potential causative agents for communication defects acquired in the heart after surgery between two chambers, the left ventricle and right atrium. Myocardial replacement surgery was also conducted in conjunction with tricuspid annuloplasty, which was also reported in the two patients of the research case report. Aortic Valve Replacement was reported by Fanari et al. in one patient and Sunderland et al. in two patients, whereas Dzwonczyk & Davidson reported Replacement of both aortic and mitral valve in a patient.
Symptoms
Edema and shortness of breath or dyspnea were the most frequently reported symptoms by the patients. Heart murmur of pansystolic as well as holosystolic was also observed. Edema was observed mainly in the lower extremities of the body, mainly pedal edema among three patients suffering from Gerbode defect or communication barrier between the chamber of heart LV and RA. Congestive heart failure with prominently acute presentation was also documented in the studies [28].
Type of Gerbode Defect
All the case studies reported Gerbode defect as an acquired deficit in the ventriculoarterial chamber communication.
Time duration
Patients within higher age brackets demonstrated reported heart defects within a short period of time, mainly months, such as a 77-year female presentation of the defect within two months, and the same occurrence of the brief time period was observed with the increasing age except for Sunderland et al. that reported time period of 5 years after the aortic valve surgery and history of Coronary Artery Bypass Grafting (CABG) at the age of 81 in a male patient.
Comorbidities
Pulmonary hypertension was significantly reported to be greater than other comorbidities among the patients. Congestive hepatomegaly was present in a 51-year-old female patient, reported by Moaref et al., and Hodgkin lymphoma was present in a 54-year-old male patient suffering from acquired Gerbode defect and acute heart failure along with hemolytic anemia, acute kidney injury, and aortic stenosis. Non-metastatic cancer of the large intestine, particularly the colon (Duke’s carcinoma), was presented by Collis et al. with a history of right hemicolectomy in 73-year-old male patients suffering from mitral valve regurgitation.
Management of disease
Surgical management of disease was opted by the majority of studies with methods such as pericardial patch closure, with two studies opting for interventional procedures like catheterization as a less invasive approach owing to the condition of their patient as surgery is a high-risk procedure for this disease.
Limitation and recommendation
We gathered the case reports for the rare condition of Gerbode defect characterized by LV-RA communication defect. Our main focus was the acquired Gerbode defect, as it had surpassed the genetic occurrence of the condition. It would have been better to gather and compare the genetic and acquired defect characteristics and clinical implications along with the difference in their management and intervention for treatment. Such a study would be beneficial for future perspectives, acting as contributing documentation for researchers and healthcare professionals to determine and recommend an optimal treatment plan for better outcomes. Interventional strategies utilized by researchers for the treatment of this condition, along with an assessment of overall survival rates, should also be added in future studies. A study assessing the quality of life among patients of Gerbode defect, as well as a study to determine the dietary causative agents, is advised to explore in future Table 2.
| Study | Year | Age | Sex | Diagnosis | Diagnostic Method | Causative Agent (Surgery, disease) | Symptoms | Type of Gerbode Defect | History of surgeries | Time | Comorbidities | Management |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uwabe et al. | 2022 | 77 | F | MS, TR | ECG | MVR, TAP | Pan systolic murmur, edema | A | 2 months | Pulmonary Hypertension | Surgical pericardial patch | |
| Rebecca et al. | 2021 | 54 | M | MS, TR9 | TOE | MVR & TVR | Acute Heart Failure, weight gain, SOB, orthopnea | A | Pacemaker surgery, Surgery for MS, and tricuspid regurgitation | One month | Hodgkin lymphoma, Pulmonary Hypertension, Arterial septal defect, hemolytic anemia, Acute Kidney Injury, | pursue transcatheter device closure |
| Moaref et al. | 2008 | 51 | F | MS | TOE | MVR | Edema, pain in lower limbs, ascites | A | Moderate tricuspid regurgitation | 1 week | Congestive hepatomegaly, | Surgical operation |
| Aoyagi et al. | 2008 | 71 | F | TR | ECG, left ventriculography | MVR, TAP | Heart murmur, anemia | A | 8 months | Surgical operation by mattress suture & bicuspid annuloplasty | ||
| Fanari et al. | 2015 | 50 | F | AS | TOE, CT | AVR | Congestive heart failure | A | 12 years | Pursue Amplatzer muscular ventricular septal defect occlude. | ||
| Amirghofran&Emaminia | 2009 | 51 | F | MS | TOE | MVR | Dyspnia, lower limb edema, ascities | A | Few weeks | Surgical management | ||
| Peñalver et al. | 2017 | 30 | F | Infective endocarditis | TOE, ECG | MVR | A | Surgical management | ||||
| Paolini et al. | 1993 | 69 | F | Infective endocarditis | TOE, ECG, Doppler color | MVR | Holysytolic murmur, congestive heart failure, SOB, orthopenia | A | 6 months | Pulmonary HTN | Surgical management | |
| Miller et al. | 1993 | 41 | F | MS | MVR | congestive heart failure | A | Closed mitral commissurotomy, mitral regurgitation, tricuspid insufficiency | 8 years | Pulmonary HTN | Surgical management | |
| Usle et al. [1] | 2007 | 54 | M | MS | Doppler color | MVR | Respiratory distress, aortic and tricuspid regurgitation | A | 2 months | Surgical management | ||
| Collis et al. [27] | 2017 | 73 | M | MR | TTE | MVR | Dyspnnea. chest pain | A | Right hemicolectomy, | Non-metastatic Duke’s (cecal carcinoma), stroke | Surgical patch closure | |
| Abdi et al. [19] | 2015 | 23 | M | iatrogenic | ECG, TEE | MVR& TVR | Hematuria, hemolysis, pan systolic murmur | A | 3 months | anemia | Surgical management | |
| Dzwonczyk & davidson [10] | 1995 | 74 | F | ECG | MVR | Holosystolic murmur, pedal edema | A | Chronic atrial fibrillation, HTN, acute MI | Surgical management | |||
| Dzwonczyk & davidson [10] | 1995 | 25 | M | Arterial septal defect, cleft mitral valve | ECG, color flow | AVR, MVR | Holosystolic Murmur | A | 9 years | Surgical management | ||
| Sunderland et al. [28] | 2020 | 64 | M | AS, CAD, infective endocarditis | ECG | AVR | SOB, pansystolic and diastolic murmur | CABG | HTN, DM-II, Hypercholesterolemia, nephrotic syndrome, pulmonary embolism | Surgical management | ||
| Sunderland et al. [28] | 2020 | 81 | M | AS, CAD | ECG, | AVR | Delirious, SOB, pyrexial | CABG | 5 years | Surgical management |
Table 2. Characteristics of studies.
Conclusion
Mitral valve replacement procedures are conducted in cardiovascular events according to the requirement of disease treatment criteria. It has been reported that there is a greater incidence of Gerbode defect, which can be characterized as a communication barrier or hindrance among the chambers of the heart, which are the right atrium and left ventricle, due to various conditions and surgical progression. Moreover, Gerbode defect is a rare event reported scarcely in the documentation of cases. Acquired Gerbode rate occurrence had surpassed genetic Gerbode defects required surgical intervention, but it is a high-risk procedure. The pericardial patch opted for the surgical plan to fill the communication between heart chambers.
References
- Uslu N, Kayacioglu I, Ates M, et al. ‘Acquired’left ventricular to right atrial shunt after mitral valve replacement: Detection by transthoracic colour Doppler echocardiography. Can J Cardiol. 2007;23(9):735.
- Gerbode F, Hultgren H, Melrose D, et al. Syndrome of left ventricular-right atrial shunt: successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg. 1958;148(3):433.
- Newman Jr JN, Rozanski L, Kreulen T. Acquired left ventricular to right atrial intracardiac shunt after myocardial infarction: a case report and review of the literature. J Am Soc Echocardiogr. 1996;9(5):716-20.
- Kelle AM, Young L, Kaushal S, et al. The Gerbode defect: The significance of a left ventricular to right atrial shunt. Cardiol Young. 2009;19(S2):96-9.
- Borkar Y, Nayak K, Shetty RK, et al. Gerbode Ventricular Septal Defect–A Rare Cardiac Anomaly Associated with Genetic Variants in Indian Population-A Case Series. J Clin Diagn Res. 2017;11(3):GR01.
- Saker E, Bahri GN, Montalbano MJ, et al. Gerbode defect: A comprehensive review of its history, anatomy, embryology, pathophysiology, diagnosis, and treatment. J Saudi Heart Assoc. 2017;29(4):283-92.
- Wasserman SM, Fann JI, Atwood JE, et al. Acquired left ventricular-right atrial communication gerbode-type defect. Echocardiogr. 2002;19(1):67-72.
- Morritt GN, Jamieson MP, Irving JB, et al. Development of left ventricular-coronary sinus fistula following replacement of mitral valve prosthesis. J Thorac Cardiovasc Surg. 1978;76(3):381-4.
- Yokoyama M, Yamada T, Nakahara H, et al. Left ventricular-right atrial communication following mitral valve replacement. Nihon Kyobu Geka Gakkai. 1990;38(9):1483-7.
- Dzwonczyk T, Davidson Jr WR. The spectrum of left ventricular-right atrial communications in the adult: Essentials of echocardiographic assessment. J Am Soc Echocardiogr. 1995;8(3):263-9.
- Yuan SM. A systematic review of acquired left ventricle to right atrium shunts (Gerbode defects). Hellenic J Cardiol. 2015;56(5):357-72.
- Miller DC, Schapira JN, Stinson EB, et ak. Left ventricular-coronary sinus fistula following repeated mitral valve replacements. J Thorac Cardiovasc Surg. 1978;76(1):43-5.
- Silbiger JJ, Kamran M, Handwerker S, et al. The Gerbode defect: left ventricular to right atrial communication—anatomic, hemodynamic, and echocardiographic features. Echocardiogr. 2009;26(8):993-8.
- Chou H, Chen H, Xie J, et al. Higher incidence of atrial fibrillation in left ventricular-to-right atrial shunt patients. Front physiol. 2020;11:580624.
- Sasayama SH, Takahashi MA, Osakada G, et al. Dynamic geometry of the left atrium and left ventricle in acute mitral regurgitation. Circulation. 1979;60(1):177-86.
- Fanari Z, Barekatain A, Abraham N, et al. Percutaneous closure of acquired Gerbode defect: management of a rare complication of cardiac surgery. Interact Cardiovasc Thorac Surg. 2015;21(1):127-8.
- Uchida T, Kuroda Y, Kobayashi K, et al. Surgical treatment for left ventricular–aortic discontinuity and Gerbode defect with endocarditis. Interact Cardiovasc Thorac Surg. 2020;30(3):439-42.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1-9.
- Abdi S, Momtahen M, Shafe O. Transcatheter closure of iatrogenic Gerbode defect with an Amplatzer duct occluder in a 23-year-old patient. J Cardiol Cases. 2015;12(2):45-7.
- Uwabe K, Okuzono Y, Masuda N, et al. Acquired left ventricular-right atrial communication after mitral valve replacement and tricuspid annuloplasty. J Card Surg. 2022;37(11):3922-4.
- Haraf RH, Karnib M, El Amm C, et al. Gerbode defect following surgical mitral valve replacement and tricuspid valve repair: a case report. Eur Heart J Case Rep. 2021;5(2):ytaa534.
- Moaref AR, Aslani A, Zamirian M, et al. Left ventricular to right atrial communication (Gerbode-type defect) after mitral valve replacement. J Am Soc Echocardiogr. 2008;21(4):408-e1.
- Aoyagi S, Arinaga K, Oda T, et al. Left ventricular–right atrial communication following tricuspid annuloplasty. Eur J Cardiothorac Surg. 2008;34(3):680-1.
- Amirghofran AA, Emaminia A. Left Ventricular-Right Atrial Communication (Gerbode-Type Defect) Following Mitral Valve Replacement. J Card Surg. 2009;24(4):474-6.
- Peñalver J, Shatila W, Silva GV. Percutaneous closure of 2 paravalvular leaks and a Gerbode defect after mitral valve replacement for infective endocarditis. Tex Heart Inst J. 2017;44(2):153-6.
- Paolini G, Gallorini C, Triggiani M, et al. Mitral valve prosthetic endocarditis: development of left ventricular-coronary sinus fistula following replacement. Eur J Cardiothorac Surg. 1993;7(12):663-4.
- Collis R, Afoke J, McGregor CG. An acquired Gerbode defect from the left ventricle to the coronary sinus following mitral valve replacement. J Card Surg. 2017;32(6):361-3.
- Sunderland N, El-Medany A, Temporal J, et al. The Gerbode defect: a case series. Eur Heart JCase Rep. 2021;5(2):ytaa548.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at,Google Scholar,Cross Ref
Indexed at, Google Scholar,Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref