Review Article - Journal of Pain Management and Therapy (2022) Volume 6, Issue 4
Choice and optimization of therapy for pain associated with chronic vascular ulcers.
Gaia Degli Angeli, Greta Loss, Myriam Maritan, Rolando Tasinato*, Sebastiano Antonio PillironeDepartment of General and Vascular Surgery, Mirano General Hospital, Venezia, Italy
- *Corresponding Author:
- Rolando Tasinato
Department of General and Vascular Surgery
Mirano General Hospital, Venezia, Italy
E-mail: rolandotasinato@libero.it
Received: 05-Jul-2022, Manuscript No. AAPMT-22-68806; Editor assigned: 06-Jul-2022, PreQC No. AAPMT-22-68806(PQ); Reviewed: 21-Jul-2022, QC No. AAPMT-22-68806; Revised: 22-Jul-2022, Manuscript No. AAPMT-22-68806(R); Published: 29-Jul-2022, DOI: 10.35841/aapmt-6.4.118
Citation: Angeli DG, Loss G, Maritan M, et al., Choice and optimization of therapy for pain associated with chronic vascular ulcers. J Pain Manage Ther 2022;6(4):118
Abstract
Pain is found in 46-8,45% of patients with vascular ulcers of the lower limbs, varying according to the records considered and the etiology of the ulcer. Vascular ulcers associated with arterial insufficiency are generally more painful than vascular ulcers associated with venous insufficiency. An algorithm for the management of chronic cancer pain in patients is provided by the WHO analgesic ladder, which is commonly used by physicians. In clinical practice, the ladder is also applied to chronic non-cancer pain, even though this kind of application is limited by the absence of an evaluation of the pathogenetic mechanisms of pain. The WHO analgesic ladder drives therapy solely depending on the severity and persistence of pain, recommending progressive “step by step” pharmacologic therapy, starting with non-opioid medications (NSAIDs and Paracetamol) for mild pain, followed by mild (e.g. tramadol) and strong opioids (e.g. morphine) for moderate to severe pain. It must be noted that addiction to long-term therapy may occur even with non-opioid drugs. Short-term therapy with opioids has been associated with adverse reactions (nausea, constipation, sleepiness, dizziness and itching) in nearly 50% of the patients. Our revision of the literature on this subject analyzes the issues of analgesic chronic therapy with opioids, providing directions on how to optimize it for patients with chronic pain associated with the presence of vascular ulcers of the legs.
Keywords
Opioids, Chronic non-cancer pain, Vascular skin ulcers, Wound care.
Introduction
Chronic non-cancer pain is one of the main causes of morbidity in developed countries, with an estimated prevalence varying from 9% to 62%. It is frequently found in patients with nonrevascularizable leg arteriopathy or severe chronic venous insufficiency associated with ulcerative lesions of the lower limbs. Chronic pain associated with vascular ulcers of the leg often results in a meaningful expenditure both for the patient and medical services and has a big impact on the quality of life and mental health of the affected subjects [1-3]. In patients with diabetes mellitus, even though the presence of sensitive and autonomic neuropathy of the lower limbs reduces pain perception, it is frequently associated with higher morbidity and a higher number of amputations. This review focuses on the role of opioids, which not only are regarded as essential drugs for the treatment of moderate to severe cancer pain according to 1979’s WHO analgesic ladder, but in the last 20 years have been used widely even for chronic non-cancer pain, especially that of orthopedic or rheumatic origin. Opioids and their derivatives offer great means for the treatment of moderate and severe pain in patients with vascular ulcers of the lower limbs, as both their dosage and their frequency of administration can be adequately adjusted during the day [4]. On the other hand, many clinical trials did not confirm the efficacy of long-term opioid administration for chronic noncancer pain and there remains a risk for abuse or overdose. Cochrane’s analysis found that long-term opioid therapy was ineffective or scarcely tolerated in about one third of patients with chronic non-cancer pain. As a result, European and North American centers’ most recent guidelines for the management and prevention of diseases acknowledge that non-opioid medications represent the first step for the treatment of chronic pain, whereas the use of opioids should only be considered when analgesic and functional benefits are not achieved through NSAIDs [5].
Opioids
Opiate medications are currently available in different formulations and doses. In particular, Morphine and its derivatives (especially Oxycodone and Fentanyl) can be administered via both parenteral and oral routes. Topical formulations and transdermal or intrathecal administration systems are also available. These solutions amplify the routes of administration offering a series of therapeutic schemes that are greatly varied and adaptable to each patient [6,7]. Opioids exert their analgesic effects in humans via agonist, partial agonist or antagonist activity on δ (DOP), κ (KOP) and μ (MOP) opioid receptors. DOP, KOP and MOP opioid receptors are coupled to G proteins in the membrane and are widely distributed throughout the nervous system and, to a lesser extent, the periphery. Multiple prohormone-derived endogenous ligands act on these receptors. The different level of affinity of the opioid for the receptor can affect both analgesic and side effects.
Weak opioids such as Tramadol and Codeine, but also Tapentadol and Buprenorphine, due to their lower affinity for MOP receptors than Morphine, have a reduced impact on respiratory depression and fewer gastrointestinal side effects. Methadone too is safer and more tolerated than Morphine, because of its lower affinity for MOP receptors. Prolonged opioid therapy can contribute to pharmacological tolerance to opioids, augmented sensibility to pain (expressed as opioidinduced hyperalgesia) or both and leads to a need to increase the administered dose. Prolonged opioid therapy can also lead to hormonal changes and alterations in the immune response. Opioids with high affinity for MOP receptors, such as Morphine, Fentanyl and Oxycodone, are more responsible for side effects such as itchiness, constipation, sleepiness, nausea or vomit [8,9].
Morphine derivatives raise susceptibility to infections, accelerate infection progression and increase one’s mortality risk. Experimental studies demonstrated that opioids lower neutrophil recruitment to the infected area in vitro. Recent findings suggest that Morphine alters the gut microbiome and reduces pathogen clearance, favoring bacterial translocation through the gut barrier. The great interpersonal variability of both analgesic and side effects represents another issue associated with long-term opioid therapy. This can be partly explained by genetics, as genetically-determined variability in pharmacodynamics can take part in different phases of the pharmacologic action. Genes coding for primary pharmacological targets, such as ion channels or receptors, and intracellular targets, such as second messengers, can all present variations in pharmacodynamics. Many pharmacokinetics processes are subjected to genetic variability, yet genetic variants of opioid-metabolizing enzymes are studied most [8- 10].
Interpersonal variability when using opioids like Codeine, Tramadol and Oxycodone can be explained by the polymorphisms of the P450 cytocrome enzymes (CYP2D6). Besides genetic variants, other variables, such as age, disease, comorbidities, concurrent assumption of other drugs, organ function and compliance can all have an impact on pharmacotherapy and must be considered when choosing the right medication for the treatment of pain. Despite the frequent limitations to their use, many international revues show that low to medium-dosage opioids, whether in association with NSAIDs or not, remain a fundamental and irreplaceable cornerstone in the long-term therapy of chronic cancer and non-cancer pain.
Nonsteroidal anti-inflammatory drugs (nsaids)
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are greatly used for the treatment of chronic non-cancer pain. Most NSAIDs act by inhibiting the activity of cyclooxygenase enzymes (COX-1 and COX-2), which are key enzymes to the formation of prostaglandins, prostacyclins and tromboxanes, mediators responsible for the inflammatory response and pain.
Both single-dose and multiple-dose NSAIDs are effective for the treatment of chronic non-cancer pain. NSAID-based therapy main side effects are represented by gastrolesivity, gastrointestinal bleeding and documented cardiac damage in case of long-term treatment, especially with COX-2 inhibitors. Whether treatment with NSAIDs should be limited to the first step of the analgesic ladder or can be applied to moderate and severe pain (second and third step) is still uncertain. Many guidelines associate NSAIDs to opioids in order to use reduced doses of opioids and thus get fewer side effects from both drugs [11,12].
How to optimize analgesic therapy for the vascular patient
The many alerts on NSAIDs and COX-2 inhibitors cardiovascular effects in the latest years led physicians to limit their use in patients with increased cardiovascular risk. The first step in optimizing opioid-based chronic therapy resides in deciding when one can start it and who can start it. Many guidelines suggest starting it when moderate to severe pain is present and associated with functional impairment or reduced quality of life, and as part of a multimodal strategy that integrates its use with the use of NSAIDs or adjuvant medications such as corticosteroids, tricyclic antidepressants or anti-epileptic agents [13].
According to most guidelines on the treatment of mild to moderate chronic pain (steps from 1 to 5 of the NRS) published in the latest 15 years, non-pharmacological therapy and nonopioid pharmacological therapy should be preferred for the treatment of chronic pain. Physicians should only consider opioid-based therapy when benefits both in terms of pain and function are expected to be higher than risks [14].
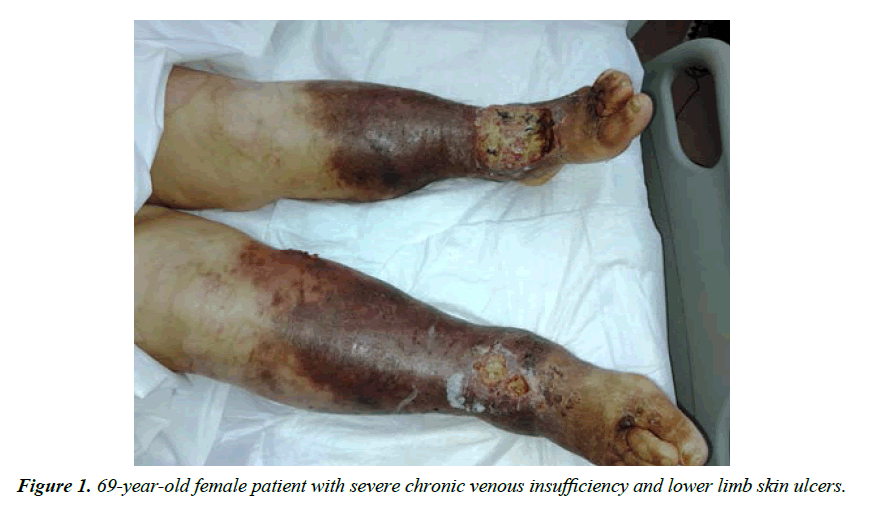
Patients should be adequately selected and monitored, in order to avoid therapy abuse. For patient selection and risk stratification, physicians should rely on anamnesis, physical examination and adequate tests. Having a personal or family history of alcohol or drug abuse is the most predictive factor for the risk of opioid abuse, inappropriate use or other inadequate behaviours related to the drug [15]. Young age and psychiatric conditions too can predict aberrant drug taking regarding opioid medications (Figure 1).
Physicians always need to inform patients on the risks and potential benefits associated with COT before starting a therapeutic experiment. Patients should be informed about opioid-related common adverse reactions (constipation, nausea, sedation), other greater risks (abuse, addiction, overdose) and potential long-term damage (hyperalgesia and endocrine or sexual dysfunction). Besides informed consent, a plan for the management of COT is needed in order to define pain and function targets, how opioids are going to be prescribed and administered, clinical follow-up expectations and monitoring of concurrent therapies. To avoid unrealistic expectations about the potential benefits related to opioid assumption, patients should be informed that complete pain relief is rare; studies suggest an improvement of 3 to 4 points on the NRS on average [16-18].
Lastly, how opioids are administered is also important. The right way consists in starting off with a low dose and increasing it slowly. For naïve patients, i.e. subjects with no previous therapeutic exposure to opioids, the starting dose should be low and titrated slowly, in order to minimize the risk of side effects.
A starting dose of MED 5-10 mg four times per day at last is suggested, increasing it by no more than 5-10 mg per week. Frail elders and patients with comorbidities can benefit from cautious initiation and titration of therapy.
According to the latest CDC’s guidelines, if opioids are used for chronic non-cancer pain, they should be combined with non-pharmacological therapy and pharmacological nonopioid therapy, in order to improve the analgesic effects of the treatment and reduce the risks associated with COT. As regards the maximum daily dose, CDC’s guidelines suggest that physicians be cautious when prescribing opioids in any dose: they should prescribe the minimum effective dose and re-evaluate carefully the evidence of individual risks and benefits when a dose higher than MED 50 mg is reached; they should also avoid reaching a dose higher than or equal to MED 90 mg. According to Canadian guidelines, the optimal dose is the one which improves function or reduces pain by at least 30%. For most patients, this dose is much lower than MED 200 mg.
As for formulations, there is not enough evidence, but neither was proven otherwise, that long-acting opioids are relatively more efficient than short-acting opioids for the management of chronic pain. There is no proof about LAOs causing less adverse reactions than SAOs, but the latter are probably safer as a starting therapy as they have a shorter half-life and lower involuntary overdose risk. The benefits of transitioning towards long-acting opioids include more constant pain control, better adherence and lower risk of addiction or abuse, but finely carried-out studies have not demonstrated these benefits. Transition to LAOs is a reasonable objective for most patients, but there is no valid reason to request low-risk patients assuming stable opioid doses to change their regimen. Combinations of long-acting and short-acting opioids have a higher chance of causing opioid abuse and increase the risk for severe side effects and events in patients with chronic non-cancer pain. Some authors suggest that in equivalent doses, LAOs have a significantly higher chance than SAOs of inducing androgen deficit in men. Moreover, risk for pneumonia was found to be higher in patients treated with long-acting opioids (OR 3,43; IC 95% 1,44-8,21) than in those treated with short-acting opioids (OR 1,27; IC 95% 0,98-1,64).
Patients who did not respond to or had side effects with an opioid can benefit from transitioning to a different opioid. Because of an unpredictable and incomplete cross-tolerance, the new opioid starting dose must not be higher than 50% of the previous dose if this latter was high (i.e. higher than MED 75 mg), or than 60 to 75% of the previous dose if this latter was moderate (i.e. lower than MED 75 mg). Regular monitoring is recommended for all patients under COT. In patients with low adverse reaction risk who are assuming a stable dose of opioid, monitoring at least once every 3-6 months is sufficient. Monitoring should systematically include an evaluation of the four A’s: Analgesia, Activities of daily living, adverse effects, and aberrant drug taking [19-20]. When no benefit is achieved through the use of different opioid medications, opioids should be reduced and then discontinued. Observational studies demonstrated that patients with severe pain, despite high opioid doses, undergo a reduction of pain and mood improvement after reducing the dose. Gradual suspension might act by relieving hyperalgesia and withdrawal symptoms (withdrawal after discontinuation is more serious when using high-dose opioids). Reduction can also improve one’s mood, decreasing opioid-induced sedation and dysphoria. When opioids are reduced or discontinued, a quite slow protocol needs to be used, in order to reduce withdrawal signs and symptoms to the slightest. A 10% reduction of the original dose per week is a reasonable starting point; discontinuation plans can be personalized and get even slower (i.e. 10% reduction per month) when one has been using opioids for 6 years.
Conclusion
Chronic pain associated with chronic vascular ulcers requires a multidisciplinary approach, including various pharmacological and non-pharmacological treatment strategies, although every option needs to be optimized according to the single patient. Revascularization (surgical or endovascular) for the arteriopathic patient and venous reflux correction for the phlebopathic patient remain the treatment options of choice. Nevertheless, these options are not applicable to a relevant number of patients; for these subjects, as a matter of fact, medical therapy and pain control are the only applicable therapeutic strategies left. The choice of the pharmacological treatment of pain is particularly important, especially when considering chronic opioid-based therapy, because choosing the right opioid and its way of administration and selecting the patients is essential.
When choosing an opioid for a single patient, an evaluation of his potential risks and benefits must be carried out correctly both before and during the course of the treatment. Appropriate COT initiation and titration, regular and complete monitoring during COT and foresight and management of opioid-related adverse reactions can lead to better results and optimization of the therapy itself. Choosing the correct daily dose, together with the right formulation, makes it possible to avoid high doses and the risk of abuse or aberrant drug taking. The three T’s, as for Titration, Tailoring (personalization) and Tapering (gradual discontinuation), are useful concepts and guidelines for a rational, safe and appropriate prescription of opioids for patients with chronic non-cancer pain, especially for those having chronic vascular wounds.
References
- Gethin G, Probst S, Stryja J, et al. Evidence for person-centred care in chronic wound care: a systematic review and recommendations for practice. J Wound Care. 2020;29(Sup9b):S1-22.
- Jones J, Williams H. Wound management should not be a pain. Br J Community Nurs. 2017 ;22(Sup9):S38-46.
- Els C, Jackson TD, Kunyk D, et al. Adverse events associated with medium‐and long‐term use of opioids for chronic non‐cancer pain: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017; 10(10).
- Cooper TE, Fisher E, Gray AL, et al. Opioids for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev. 2017; 7 (7).
- Mathieson S, Maher CG, Ferreira GE, et al. Deprescribing Opioids in chronic non-cancer pain: systematic review of randomised trials. Drugs. 2020; 80 (15): 1563-1576.
- Abbott LI, Fiala CA, Rakel BA . Factors associated with high pain intensity during wound care procedures: A model. Wound Repair Regen. 2017; 25 (4): 558-563.
- Busse J.W., Wang L., Kamaleldin M., et al. Opioids for chronic noncancer pain. JAMA 320, 2448. 2018
- Sommer C, Klose P, Welsch P, et al. Opioids for chronic non-cancer neuropathic pain. An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration. Eur J Pain. 2020; 24 (1): 3-18.
- Goto T, Tamai N, Nakagami G, et al. Can Wound Exudate from Venous Leg Ulcers Measure Wound Pain Status? A Pilot Study. PLoS One. 2016; 11 (12): e0167478.
- Arroyo-Novoa CM, Figueroa-Ramos MI, et al. Acute wound pain: gaining a better understanding. Adv Skin Wound Care. 2009; 22 (8): 373-80; 381-2.
- Palop Larrea V, Martinez-Mir I et al. Use of opioids for chronic non-cancer pain. Med Clin (Barc). 2021; 156 (2): 96-98.
- Currow DC, Phillips J, Clark K. Using opioids in general practice for chronic non-cancer pain: an overview of current evidence. Med J Aust. 2016; 204 (8): 305-9.
- Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound Bed Preparation 2021. Adv Skin Wound Care. 2021; 34 (4): 183-195.
- Woo KY, Sibbald RG. Chronic wound pain: a conceptual model. Adv Skin Wound Care. 2008; 21 (4): 175-88; quiz 189-90.
- Paul J. A cross-sectional study of chronic wound-related pain and itching. Ostomy Wound Manage. 2013; 59 (7): 28-34. PMID: 23846004.
- Taub CJ, Sturgeon JA, Chahal MK, et al. Self-reported traumatic etiology of pain and psychological function in tertiary care pain clinic patients: a collaborative health outcomes information registry (CHOIR) study. Scand J Pain. 2020; 20 (3): 499-509.
- Labianca R, Sarzi-Puttini P, Zuccaro SM, et al. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig. 2012; 32 (1): 53-63.
- Gallagher R. The management of wound-related procedural pain (volitional incident pain) in advanced illness. Curr Opin Support Palliat Care. 2013; 7 (1): 80-5.
- Chalhoub RM, Kalivas PW. Non-Opioid Treatments for Opioid Use Disorder: Rationales and Data to Date. Drugs. 2020; 80 (15): 1509-1524.
- Nijs J, Leysen L, Vanlauwe J, Logghe T, et al. Treatment of central sensitization in patients with chronic pain: time for change? Expert Opin Pharmacother. 2019; 20 (16): 1961-1970.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref