- Biomedical Research (2016) Volume 27, Issue 2
Cantharidin content determination and its inhibitory effect on human hepatoma HepG2 cells.
| Yisheng Lin1,2, Yanhao Lia1*, Zhenpeng Peng3, Shilong Yu4, Shuangfeng Lin5 and Xiaoshu Huc3* 1Department of Interventional Radiology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong Province, PR. China 2Department of Interventional Radiology, the First Affiliated Hospital Of Guangzhou University Of Traditional Chinese Medicine, Guangzhou, Guangdong Province, PR. China 3Department of Radiology, the First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, Guangdong, PR. China 4Jilin Cancer Hospital, Changchun, Jilin province, PR. China 5Laboratory Animal Center, the First Affiliated Hospital Of Guangzhou University Of Traditional Chinese Medicine, Guangzhou, Guangdong Province, PR. China |
| Corresponding Authors: Yanhao Lia, Department of Interventional Radiology, Nanfang Hospital, Southern Medical University, PR. China Xiaoshu Huc, Department of Radiology, The First Affiliated Hospital, Sun Yat-Sen University, PR. China |
| Accepted: February 16, 2016 |
Abstract
To establish a method for determination of cantharidin content in Mylabris phalerata. Pallas, and to study its inhibitory and apoptosis-inducing effects on human hepatoma cell line HepG2. Agilent column (TC-C18, 250 mm × 4.6 mm, 5 μm); mobile phase: methanol-water (volume ratio: 20:80); flow rate: 1.0 ml/min; UV detection wavelength: 230 nm; and column temperature: 30°C. MTT assay was used to determine cell viability, and flow cytometry was used to detect the effects of cantharidin on cell cycle arrest and apoptosis of HepG2 cells. Cantharidin had a good linearity (R=0.9993) within a 0.056~0.504 μg range, and had an average recovery of 98.55%. Different concentrations of cantharidin all markedly induced the apoptosis of human hepatoma HepG2 cells. After treatment for 24, 36 and 48 h, respectively, HepG2 cells were inhibited to varying degrees in a dose and time dependent manner. PI staining with flow cytometry showed that the test concentrations of cantharidin could induce HepG2 cell apoptosis, and impact the cell cycle to varying degrees by increasing the proportion of G0/G1 phase cells. Compared with the control group, apoptosis was positively correlated with drug concentration, presenting a marked dose-dependence. The method proposed is simple, fast, accurate and reproducible, which can be used for the determination of cantharidin content in Mylabris phalerata. Pallas. Cantharidin has an inhibitory effect on HepG2 cells.
Keywords |
||||||
| Cantharidin, Flow cytometry, HepG2 cell. | ||||||
Introduction |
||||||
| Banmao is the dried body of Meloidae insects Mylabris phalerata. Pallas or Mylabris cichorii. Linnaeus [1]. It is pungent, hot, and strongly toxic, which enters the liver, stomach and kidney meridians, and has blood stasiseliminating, mass-resolving, swelling-subsiding, toxicitycounteracting and sore-removing effects [2]. Originally recorded in the Shen Nong's Herbal Classic, Banmao belongs to the family Meloidae of the order Coleoptera, which is one of the earliest discovered medicinal materials with anti-tumor action. Medicinal value of Banmao lies in its ability to effectively remove dead skins, and be applied on malignant sores and scabies [3]. | ||||||
| Modern research has shown that cantharidin contained in Banma has a strong anti-tumor activity, which can be used for carbuncles, gangrenes, malignant sores, scrofula, rabies, as well as abortion; domestic and foreign scholars have done extensive research on the mechanism of action of cantharidin [4-8]. As the major active constituent of Banmao [9-10], cantharidin also has strong toxicity, so rational application of cantharidin and strict control of its dose has become the focus of research and development of cantharidin and its derivatives. | ||||||
Material and Methods |
||||||
Instruments and reagents |
||||||
| Agilent 1200 HPLC system; ELX 800 microplate reader (BioTEK, USA); FACSAria flow cytometer (BD, USA); CO-150 CO2 incubator (NBS, USA); RPMI1640 (Gibco); FBS (Hangzhou Sijiqing Bioengineering Materials Co., Ltd.); MTT (Sigma). | ||||||
Drug and cell line |
||||||
| Banmao medicinal material was purchased from Anguo market, which was identified as Mylabris phalerata. Pallas. Human HepG2 cell line was purchased from the Cell Bank at SIBCB.Mylabris phalerata. Pallas | ||||||
Chromatographic conditions |
||||||
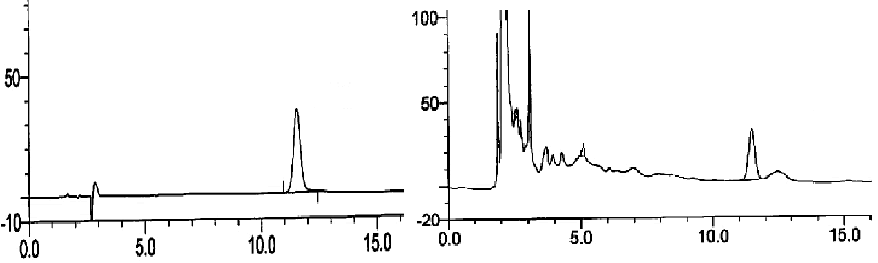
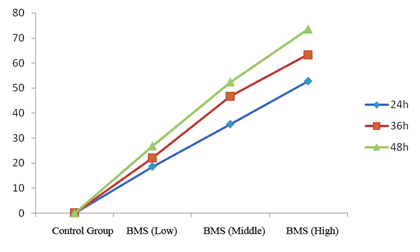
| Agilent column (TC-C18, 250 mm × 4.6 mm, 5 μm); mobile phase: methanol-water (volume ratio: 20:80); flow rate: 1.0 ml/ min; UV detection wavelength: 230 nm; and column temperature: 30°C [11,12]. The results are shown in figure 1. | ||||||
Linearity range |
||||||
| Preparation of reference solution: Cantharidin reference substance, which was dried to constant weight under vacuum at 45°C, was accurately weighed, and added with methanol to prepare a 0.056 mg/ml reference solution. | ||||||
| Preparation of test solution: About 2 g of Mylabris phalerata Pallas medicinal powder (sifted through a 20 mesh sieve) was accurately weighed, placed in a stoppered conical flask, added precisely with 50 mL of HCl/acetone solution, weighed, ultrasonicated for 45 min, then removed and let cool. After replenishing the lost weight, the solution was filtered through a 0.45 μm microporous membrane to give the test solution. | ||||||
| Linearity range: 1, 3, 5, 7 and 9 μL of cantharidin reference solution was accurately drawn, and injected into HPLC system for measurement of peak area. Standard curve was plotted with mass concentration (X) versus peak area (Y), and regression equation was y = 7853.9 × -254.346, r = 0.999 3, suggesting good linearity of cantharidin within a 0.056~0.504 μg range. | ||||||
Stability test |
||||||
| 100 μL of the same test solution was taken, and injected separately at 0, 1, 2, 3, 4, 5 and 24 h for measurement. The results showed that RSD=1.12%, indicating that the sample solution was stable within 24 h. | ||||||
Accuracy test |
||||||
| The same test solution was precisely drawn and injected six times repeatedly for measurement of peak area. RSD was found to be 1.14%, demonstrating good accuracy of the instrument. | ||||||
Recovery test |
||||||
| Mylabris phalerata Pallas medicinal materials with known contents of samples were accurately weighed, added separately with appropriate amount of cantharidin, prepared as per the method under preparation of test solution, and determined by the method under content determination, followed by calculation of recovery. The results revealed an average recovery of 98.55%, indicating that the method has good recovery. The results are shown in table 1. | ||||||
Reproducibility test |
||||||
| Five aliquots of the same batch of medicinal materials were accurately weighed, and prepared into samples as per the method under preparation of test solution. 5 μL of test solution was precisely drawn, and injected repeatedly into HPLC for determination under chromatographic conditions described above. The results found that RSD was 0.38%, indicating good reproducibility of the method. | ||||||
Content determination |
||||||
| Three batches of Mylabris medicinal materials with different origins were taken, prepared into samples as per the test solution preparation method, and determined under the above chromatographic conditions, followed by calculation of cantharidin contents. The results are shown in table 2. | ||||||
Results |
||||||
| Inhibitory effect of cantharidin on HepG2 cell growth | ||||||
Cell culturing |
||||||
| HepG2 cells were cultured in a 37°C, 5% CO2 incubator with 10% FBS-containing DMEM medium. The medium was replaced once in every 3 days. Logarithmic phase cells were harvested for the experiment. | ||||||
MTT assay of HepG2 cell growth inhibition rate by cantharidin |
||||||
| Well grown exponential phase cells were prepared into a 5 × 104/ml cell suspension, and seeded in a 96 well plate at 200 μl per well. The cells were treatment with different concentrations of cantharidin (10, 20, 40 μg/ml), and three replicate wells were set up for each concentration. After culturing for 24, 36 and 48 h, each well was added with 20 μL of 5 mg/ml MTT solution, and incubated for an additional 4 h then supernatant was discarded. Each well was added with 150 μL of DMSO, shaken, and then A value was measured at 570 nm. The experiment was repeated three times, and growth inhibition rate was calculated. | ||||||
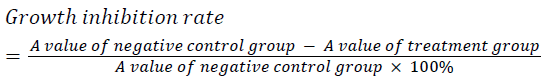 |
||||||
| Different concentrations of cantharidin could all markedly induce HepG2 cell apoptosis. After treating for 24, 36 and 48 h, HepG2 cells were inhibited to varying degrees in a dose and time dependent manner. | ||||||
Determination of cell cycle and apoptosis rate |
||||||
| Logarithmic phase HepG2 cells were taken, trypsinized, prepared into a 5 × 106 cell suspension, added with different concentrations of cantharidin samples (10, 20, 40 μg/ml), and cultured for 24 h. After trypsinization, centrifugation and supernatant removal, the cells were added with 2 mL of sodium citrate buffer, water bathed at 37°C for 30 min, then stained with 1.5 mL of PI, and kept in a dark place for 30 min. Afterwards, the cells were passed through 300-mesh nylon net six times, and analyzed for cell cycle distribution by flow cytometry. | ||||||
| The results are shown in table 3. PI staining with flow cytometry revealed that the test concentrations of cantharidin could induce HepG2 cell apoptosis. Cell cycle was impacted to varying degrees, with relative increase in the proportion of G0/G1 phase cells. Compared with the control group, apoptosis was positively correlated with the drug concentration, presenting a marked dose-dependence. | ||||||
Discussion |
||||||
| Liver cancer is a major killer threatening human health among malignancies. Most liver cancers are diagnosed at advanced stages, where the best time for treatment has been lost, thus tremendously affecting the patients and their families. At present, the major treatment for cancer is chemotherapy, especially for advanced cancer, where chemotherapy has almost become a mandatory clinical therapy. However, the majority of chemotherapeutics have strong cytotoxicity on normal cells, which suppress the development of cancer cells while causing enormous damage to the patients' bodies. | ||||||
| Primary liver cancer ranks top among malignancies in terms of mortality. How to effectively prevent liver cancer is a crucial issue faced by the medical and pharmaceutical industries. Chinese medicine generally believes that one major cause of liver cancer is the pathogenic toxins, how to effectively dispel the pathogenic toxins is an important way of treating cancer. | ||||||
| Pang Jie et al., [13] confirmed by experimental study that the disodium cantharidinate vitamin B6 injection directly inhibited H22 xenograft tumor, killed H22 cells in vitro, induced apoptosis and prolonged the survival of ascetic tumor-bearing mice; furthermore, these effects were enhanced with increasing dose. The anti-hepatoma mechanism of disodium cantharidinate vitamin B6 injection is believed to be exerted not only by inhibiting and killing tumor cells, but also by regulating the immune function. | ||||||
| Cao Yongyan et al., [14] prepared compound Banmao capsule serum by serum pharmacological approach, and used it to treat human hepatoma SMMC-7721 cells. They determined the proteins in cancer cells influenced by compound Banmao capsule using proteomic techniques such as two-dimensional electrophoresis, image analysis and mass spectrometry, and found that the anti-cancer mechanism of compound Banmao capsule is a process jointly participated by multiple channels, which involves multiple targets in the proliferative, immunologic and apoptotic aspects of tumors. | ||||||
Conclusion |
||||||
| In this study, the anti-cancer activity of Chinese medicine Banmao is explored centering on cantharidin. MTT assay and flow cytometry results demonstrate that the test concentrations of cantharidin can induce HepG2 cell apoptosis, and influence cell cycle to varying degrees, indicating that cantharidin has a growth inhibitory effect on tumor cells. But whether it can regulate immune function still needs further confirmation. | ||||||
Tables at a glance |
||||||
|
||||||
Figures at a glance |
||||||
|
||||||
References |
||||||
|