The radix of Asiasarum heterotropoides var. mandshuricum F. Maekawa (A. radix) is called seshin in Korean, saishin in Japanese, xì x?n in Chinese and Chinese wild ginger in English and is widely used to treat various diseases. The aim of this study was to determine the dose-dependent anti- inflammatory and anticancer effects using methanol, ethanol and water extracts of A. radix. Methanol, ethanol and water extracts of Asiasarum heterotropoides were obtained. A mouse leukaemic monocyte macrophage cell line (RAW 264.7) was exposed to A. radix at final concentrations that ranged from 25 to 200 μg/ml. The production of nitric oxide (NO) in lipopolysaccharide-induced RAW 264.7 cells was quantified. The inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), interleukin-6 (IL-6) and cycloxygenase-2 (COX-2) mRNA expressions were evaluated using lipopolysaccharide (LPS)-stimulated RAW 264.7 cells with semi-quantitative reverse transcription-polymerase chain reaction. Human lung carcinoma cell lines (A549 cells) were treated with A. radix and superoxide dismutase-2 (SOD-2), and caspase-3 expression was tested. Higher cytotoxicity was noted after treatment with methanol, ethanol and water extracts of A. radix. Stimulation with LPS for 24 h led to a robust increase in NO production, but A. radix significantly suppressed NO by the LPS-stimulated RAW 264.7 cells in all groups. Pretreatment with absolute ethanol extract of A. radix suppressed the LPS-stimulated iNOS, IL-1β, IL-6, and COX-2 expressions. The results showed that A. radix extract showed significantly higher cytotoxicity when compared with the untreated control. The expression of SOD-2 and caspase-3 increased with the increase of exposure time to A. radix extracts. Within the limits of this study, A. radix showed an anti-inflammatory effect using RAW 264.7 cell lines and anticancer effects on A549 cells. These effects were influenced by extraction methods. Absolute ethanol extract showed the highest anti-inflammatory and anticancer effects in these experimental settings.
Keywords |
| Anti-inflammatory agents, Antineoplastic agents, Herbal medicine, Plant roots |
Introduction |
| Medicinal herbs used in traditional oriental medicine are
attractive sources for developing novel therapeutics or
preventives
since they have been used for thousands of
years in clinics [1]. The radix of Asiasarum heterotropoides
var. mandshuricum F. Maekawa (A. radix) is called seshin
in Korean, saishin in Japanese, xì xin in Chinese and
Chinese wild ginger in English and is widely used to treat various diseases [2]. A. radix has been used for its antiinflammatory,
antiallergy, antibacterial, and anticancer
effects [1, 3-5]. |
| Inflammatory responses are recognized as natural defense
mechanisms critical for the recruitment of a variety of
immune cells and molecules to sites with infectious
microbes or injured tissues [6]. Inflammation is a complex
process characterized by the contributions of mediators, including nitric oxide (NO) and free radicals [7]. Blocking
the expression of cyclooxygenase-2 (COX-2) and
inducible nitric oxide synthase (iNOS) can restrain the
production of high-output NO, and inhibiting COX-2 and
iNOS expressions have been used as functional criteria for
developing anti-inflammatory agents [6, 7]. |
| In cancer, mutations in progenitor cells most likely undergo
uncontrolled proliferation [8]. Apoptosis is important for
controlling cell numbers and proliferation, but cancer
cells do not undergo apoptosis [9]. Thus, drugs and agents
that can restore the normal apoptotic pathway may have
potential for treating cancers. |
| The aim of this study was to determine the dose-dependent
anti-inflammatory and anticancer effects using methanol,
ethanol and water extracts of A. radix. To our knowledge,
this investigation is the first to elucidate the comparative
effects of methanol, ethanol and water extracts of A. radix
on mouse leukaemic monocyte macrophage cell lines
(RAW 264.7) and human lung carcinoma cell lines (A549
cells). |
Materials and Methods |
Chemicals and Reagents |
| All chemicals and reagents used in this study were
purchased from Sigma (St. Louis, MO, USA) unless
otherwise specified. |
Plant Material and Preparation of the Extracts |
| The dry roots of Asiasarum heterotropoides were obtained
from Chungju Hospital of Korean Medicine (Jecheon,
Korea). The roots of A. radix were chopped to a small
size of 0.5 cm long, dried in the shade and powdered in a
mechanical grinder. The pulverized roots were extracted
for absolute ethanol, 70% ethanol, absolute methanol,
70% methanol, water and boiling water for 3 hours, and
finally the extractions were dried under a vacuum rotary
evaporator (CCA-1110, Eyela, Tokyo, Japan). Twenty
grams of dry roots in each group were used, and 1.544,
3.704, 1.575, 3.852, 4.105, and 5.181 g were obtained for
the absolute ethanol extract, 70% ethanol extract, absolute
methanol extract, 70% methanol extract, water extract, and
boiling water extract, respectively. The yields were 7.72,
18.52, 7.88, 19.26, 20.53, and 25.91% (w/w), respectively. |
Anti-inflammatory Assay |
Cell line and cell culture |
| A RAW 264.7 cell line was purchased from the Korean
Cell Line Bank (Seoul, Korea). RAW 264.7 cells were
maintained in RPMI 1640 medium supplemented with
10% FBS, 100 U/ml of penicillin, and 100 μg/ml of
streptomycin. The cells were incubated at 37°C in a
humidified atmosphere of 95% air and 5% CO2. |
Cytotoxicity assay |
| The cytotoxicity of samples of RAW 264.7 cells was
tested. Cells were seeded into 96-well plates at a density of 1 Ã 105 cells/well. After incubation for 18 h, cells
were exposed to medium along with samples at different
concentrations for 24 h. The supernatant was removed
from each well, and 10 μl of MTT solution (5 mg/ml in
phosphate-buffered saline) and 90 μl of FBS-free medium
were added to each well and incubated for 4 h at 37°C.
Then, the supernatant was sucked out and 200 μl of
DMSO were added to each well. The plate was vibrated
slightly for 10 min, and the amount of MTT formazan was
quantified by measuring the absorbance at 490 nm using
an enzyme-linked immunosorbent assay (ELISA) plate
reader (ELx800TM, Bio-Tek, Winooski, VT, USA). The
optical density of formazan formed in the control cells was
considered 100% viability. Cell viability was expressed as
a percentage of the control culture value. |
Quantification of NO production in lipopolysaccharide
(LPS)-induced RAW 264.7 cells |
| The inhibitory effect of NO production in LPS-induced
RAW 264.7 cells was determined according to the method
of Jiang et al. [10]. RAW 264.7 cells were plated in 96-
well cell plates and incubated for 18 h. Then, cells were
stimulated with LPS (2 μg/ml) in the presence or absence
of samples with various concentrations for 24 h. Aliquots
of 100 μl of cell culture medium were mixed with 100
μl of Griess reagent [0.1% aqueous solution of naphthylethylenediamine
dihydrochloride, 50 μl; 1% sulfanilamide
(in 5% phosphoric acid), 50 μl]. The absorbance was
determined at 550 nm using an ELISA plate reader
(ELx800TM). |
Reverse transcription-polymerase chain reaction (RTPCR)
analysis |
| RAW 264.7 cells (1 Ã 106) were grown in 6-well plates for
18 h. Then, cells were treated with various concentrations
of samples for 30 min, and LPS (2 μg/ml) was added. After
incubation for 24 h, the total RNA of the cells was isolated
with a Trizol RNA isolation kit (Invitrogen, Carlsbad, CA,
USA). The total RNA was reverse-transcribed to cDNA
and used as the template for PCR amplification. The
iNOS, IL-1β, and COX-2 primers (Table 1) were used in the PCR. The amplified PCR products were separated
on 1% agarose gel, and the gel was stained with ethidium
bromide. The gel was photographed with a Mini BIS
Image Analysis System (DNR Bio-Imaging Systems Ltd.,
Jerusalem, Israel). |
 |
Anticancer Assay |
Cell line and cell culture |
| A lung cancer A549 cell line was purchased from the Korean
Cell Line Bank (Seoul, Korea). Cells were maintained in
DMEM medium supplemented with 10% FBS, 100 U/ml
of penicillin, and 100 μg/ml of streptomycin. The cells
were incubated at 37°C in a humidified atmosphere of
95% air and 5% CO2. |
Cytotoxicity assay |
| The cytotoxicity of samples on A549 cells was detected
by MTT assay. Cells were seeded into 96-well plates
and incubated with samples for 24, 48 or 72 h. Then, the
supernatant was removed and 100 μl of MTT solution
were added to each well and incubated for 4 h at 37°C. The
supernatant was sucked out, and 200 μl of DMSO were
added to each well. The amount of MTT formazan was
quantified by measuring absorbance at 550 nm. |
RT-PCR analysis |
| A549 cells (1 Ã 106) were grown in 6-well plates for 24
h. Then, cells were treated with samples for different
amounts of time (0, 3, 6, 12, 24 and 48 h). The total
RNA of the cells was isolated with a Trizol RNA isolation
kit (Invitrogen, Carlsbad, CA, USA). The total RNA
was reverse-transcribed to cDNA and used as the template
for PCR amplification. The forward and reverse
primers were as follows: 5?-TGTTACCAACTGGGACGACA-
3? and 5?-CTCTCAGCTGTGGTGGTGAA-3?
for β-actin; 5?-TGGTGGAGAACCCAAAGG-3? and 5?-GTCAAAGGAACCAAAGTCACG-3?for superoxide
dismutase-2 (SOD-2); and 5?AGTGGAGGCCGACTTCTTGT-
3? and 5?-CTGTTGCCACCTTTCGGTTA-
3? for caspase-3. The amplified PCR products
obtained by PCR were separated on 1% agarose gel
electrophoresis, and the gel was stained with ethidium
bromide. The gel was photographed with a Mini BIS
Image Analysis System (DNR Bio-Imaging Systems
Ltd.). |
Statistical Analysis |
| The data are represented as means ± standard deviations
of the experiments. A one-way analysis of variance
(ANOVA) with a post hoc test was performed to
determine the differences between the groups using
a commercially available program (SPSS 12 for
Windows, SPSS Inc., Chicago,mIL, USA). The level of
significance was 0.05. |
Results |
Anti-inflammatory Assay |
| Cytotoxicity assay: The effects of A. radix on RAW 264.7
cells are presented in Figure 1. The results showed that
the 70% ethanol extract and the boiling water extract
did not exhibit any statistically significant toxicity on
RAW 264.7 cells (P>0.05). The absolute ethanol extract
at concentrations of 25, 50, 100, and 200 μg/ml reduced
cell viability to 90.3 ± 1.3%, 80.3 ± 1.4%, 73.8 ± 0.9%,
and 71.8 ± 0.2 %, respectively, when the control was
considered 100% (100.0 ± 0.8 %) (P<0.05). |
| Quantification of NO production in LPS-induced RAW
264.7 cells: Stimulation with LPS for 24 h led to a robust
increase in NO production. However, A. radix significantly
suppressed NO in the LPS-stimulated RAW 264.7 cells in
all groups (70% ethanol, ethanol, 70% methanol, methanol, water and boiling water extracts) (Figure 2A). The boiling
water extract showed an 80.9 ± 0.8 % reduction of NO
production at 25 μg/ml. The highest reduction of NO
production was achieved in the ethanol extract group at
200 μg/ml with 98.9 ± 0.4 %. |
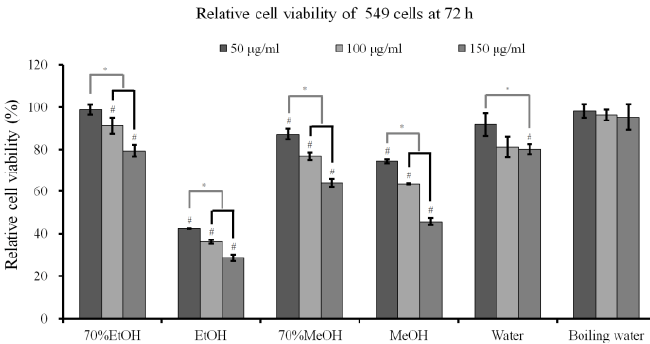 |
Figure 1. Cell viability of RAW 264.7 cells after incubation in the presence of different extracts from A. radix for 24 h. Each value
is expressed as the mean ± SD (n=3)
# A statistically significant difference was seen when compared with the control (non-treated group) at 24 h (P<0.05)
* There was a statistically significant difference when compared with the 25 μg/ml group for each extraction method
EtOH: ethanol
MeOH: methanol |
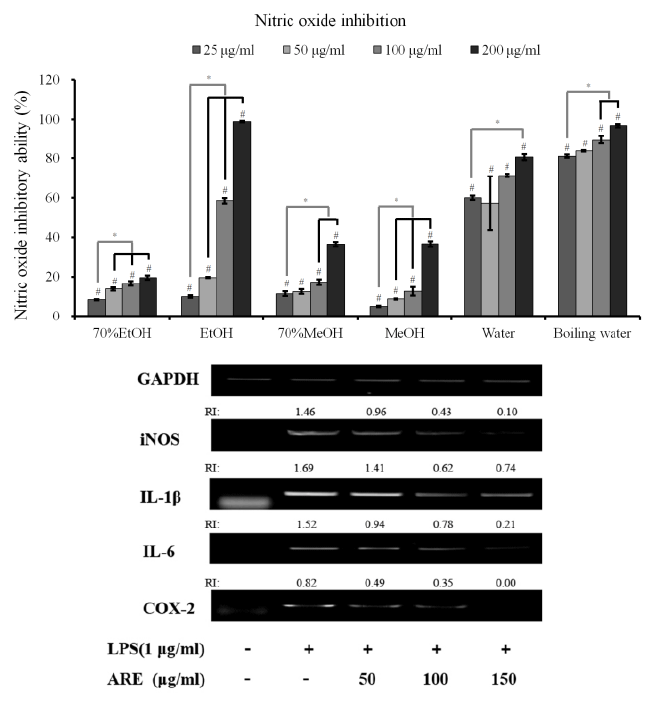 |
Figure 2. (A) Nitric oxide (NO) inhibition of different extracts from A. radix on LPS-stimulated RAW 264.7 cells. Each value is
expressed as the mean ± SD (n=3)
#A statistically significant difference was seen when compared with the control at 24 h (P<0.05)
* A significant difference was seen when compared to the control (nonloaded group) (P<0.05)
EtOH: ethanol
MeOH: methanol
(B) Effects of absolute ethanol extract of A. radix (AHE) on nitric oxide synthase (iNOS), interleukin 1β (IL-1β), interleukin 6 (IL-6) and cyclooxygenase-2 (COX-2) mRNA expression in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells |
RT-PCR analysis |
| The iNOS, IL-1β, IL-6 and COX-2 mRNA expressions in
the unstimulated RAW 264.7 cells were minimal, but their
mRNAs were profoundly induced after the treatment with
LPS (Figure 2B). Pretreatment with the absolute ethanol
extract of A. radix suppressed the LPS-stimulated iNOS,
IL-1β, IL-6 and COX-2 expression. The suppression of
inflammatory-related genes increased with the increasing
concentration of A. radix extract. |
Anticancer assay |
| Cytotoxicity assay and RT-PCR analysis: The results
of cell viability of A549 cells after incubation in the presence of different extracts from A. radix for 24 h, 48
h and 72 h are shown in Figures 3A-3C. The results at
24 h showed that A. radix extracts significantly reduced
cellular viability when compared with the untreated
control (P<0.05) (Figure 3A). The results at 48 h and
72 h showed similar trends with 24 h data (Figures 3B
and 3C). The absolute ethanol extract showed the most
powerful effects of 42.3 ± 0.1%, 36.1 ± 1.0%, and 28.5
± 1.5% with concentrations of 50, 100, and 150 μg/ml,
respectively, at 72 h when the control was considered
100 (100.0 ± 2.7)% (P<0.05). The expression of SOD-2
and caspase-3 increased with the increase of exposure
time to the absolute ethanol extract (Figure 3D). |
Discussion |
| In this study, we examined the effects of different
extracts of A. radix on RAW 264.7 and A549 cells under
predetermined concentrations. The absolute ethanol extract
showed the highest anti-inflammatory and anticancer effects in these experimental settings. |
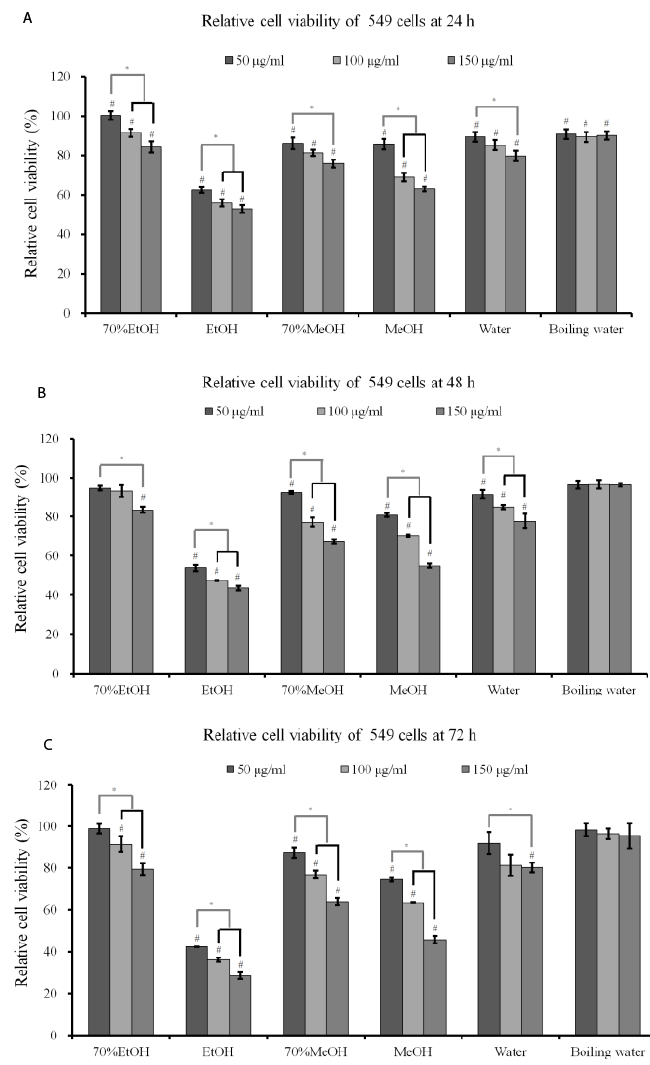 |
Figure 3. (A) Cell viability of A549 cells after incubation in the presence of different extracts from A. radix for 24 h. Each value
is expressed as the mean ± SD (n=3)
#A statistically significant difference was seen when compared with the control (nontreated group) at 24 h (P<0.05)
* There was a statistically significant difference when compared with 25 μg/ml group in each extraction method.
EtOH: ethanol
MeOH: methanol
(B) Cell viability of A549 cells after incubation in the presence of different extracts from A. radix for 48 h. Each value is expressed
as the mean ± SD (n=3)
#A statistically significant difference was seen when compared with the control (nontreated group) at 48 h (P<0.05).
*There was a statistically significant difference when compared with 25 μg/ml group in each extraction method.
(C) Cell viability of A549 cells after incubation in the presence of different extracts from A. radix for 72 h. Each value is expressed
as the mean ± SD (n=3)
#A statistically significant difference was seen when compared with the control (nontreated group) at 72 h (P<0.05)
*There was a statistically significant difference when compared with 25 μg/ml group in each extraction method.
(D) A549 cancer cells were plated in 6-well plates and then exposed to absolute ethanol extract (AHE) for the indicated times.
Caspase-3 and superoxide dismutase-2 (SOD-2) mRNA levels in A. radix-stimulated cells were detected by reverse transcriptionpolymerase
chain reaction (RT-PCR) |
| The RAW 264.7 macrophage model is considered a useful
model for evaluating anti-inflammatory agents because
a number of different inflammatory mediators, including
NO, prostaglandin E2 and tumor necrosis factor-a,
are generated by the macrophages upon stimulation
with LPS (a primary component of the Gram-negative
bacteria cell wall) [6]. Increased anti-inflammatory
effects of the ethanol extract may be explained by the
phenolic and flavonoid compounds in A. radix because
with typical extraction procedures phenolic compounds
are mostly carried out using organic solvents [11,12].
These compounds have reportedly shown various healthpromoting
biological actions, including anti-inflammatory,
anticarcinogenic, and anti-atherosclerotic functions [7]. It
was unclear whether the A. radix-mediated inhibition of
NO production was the consequence of inhibiting iNOS,
COX-2, IL-1β, and IL-6 at the transcriptional level or due
to some other mechanism. This study clearly suggested
that the suppressive activity of A. radix extracts on iNOS,
COX-2, IL-1β and IL-6 was mediated via transcriptional
levels. |
| Caspases are crucial mediators of programmed cell
death (apoptosis), and caspase-3 is a frequently activated
protease in mammalian cell apoptosis [13]. Cytotoxic
effects on lung carcinoma cell lines are partly explained
by the expression of caspase-3. The transcriptional level
of caspase-3 was increased with longer exposure to
A. radix extracts in this study. Additionally, this study
showed that the transcriptional level of SOD-2 was greater
with an increase in exposure time. The role of SOD in
carcinogenesis has been widely studied but remains
ambiguous and controversial [16]. A major intracellular
form of the SOD enzyme is considered a tumor suppressor
[9,14]. A previous report showed that increased manganese
SOD expression suppresses the malignant phenotype of
human melanoma cells [15]. |
| Within the limits of this study, A. radix showed antiinflammatory
effects using a RAW 264.7 cell line and
anticancer effects on A549 cells. These effects were
influenced by extraction methods. The absolute ethanol
extract showed the highest anti-inflammatory and
anticancer effects in these experimental settings. |
Acknowledgement |
| The authors report no conflicts of interest related to this
study. The author does not have any financial interest
in the companies whose materials are included in the
article. |
References |
- Oh SM, Kim J, Lee J, Yi JM, Oh DS, Bang OS. Anticancer potential of an ethanol extract of Asiasari radix against HCT-116 human colon cancer cells in vitro. Oncol Lett 2013; 5: 305-310.
- Jeong SH, Lee JE, Jin SH, Ko Y, Park JB. Effects of Asiasari radix on the morphology and viability of mesenchymal stem cells derived from the gingiva. Mol Med Rep 2014; 10: 3315-3319.
- Kamei T, Kondoh T, Nagura S, Toriumi Y, Kumano H, Tomioka H. Improvement of C-reactive protein levels and body temperature of an elderly patient infected with Pseudomonas aeruginosa on treatment with Mao-bushi-saishin-to. J Altern Complement Med 2000; 6: 235-239.
- Kim HM, Moon YS. Asiasari radix inhibits immunoglobulin E production on experimental models in vitro and in vivo. Immunopharmacol Immunotoxicol 1999; 21: 469-481.
- Jang JY, Lee JH, Shin HK, Choi YH, Lee JD, Choi BT. Partially purified Asiasari radix inhibits melanogenesis through extracellular signal-regulated kinase signaling in B16F10 cells. Int J Mol Med 2010; 25: 287-292.
- Jiang Y, Wang MH. Ethanol extract of Synurus deltoides (Aiton) Nakai suppresses in vitro LPS-induced cytokine production in RAW 264.7 macrophages and in vivo acute inflammatory symptoms. Nutr Res Pract 2014; 8: 11-19.
- Jiang Y, Wang MH. Different Solvent Fractions of Acanthopanax senticosus Harms Exert Antioxidant and Anti-Inflammatory Activities and Inhibit the Human Kv1.3 Channel. J Med Food 2014.
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006; 66: 4553-4557.
- Li C, Wang MH. Aristolochia debilis Sieb. et Zucc. induces apoptosis and reactive oxygen species in the HT-29 human colon cancer cell line. Cancer Biother Radiopharm 2013; 28: 717-724.
- Jiang Y, Hu W, Han W, Yeo JH, Wang MH. Antioxidant and nitric oxide production inhibitory activities of scouring rush (Equisetum hyemale L.). Food Science and Biotechnology 2012; 21: 1037-1044.
- Reis SF, Rai DK, Abu-Ghannam N. Water at room temperature as a solvent for the extraction of apple pomace phenolic compounds. Food Chem 2012; 135: 1991-1998.
- Mahugo Santana C, Sosa Ferrera Z, Esther Torres Padron M, Juan Santana Rodriguez J. Methodologies for the extraction of phenolic compounds from environmental samples: new approaches. Molecules 2009; 14: 298-320.
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999; 6: 99-104.
- Bravard A, Sabatier L, Hoffschir F, Ricoul M, Luccioni C, Dutrillaux B. SOD2: a new type of tumor-suppressor gene? Int J Cancer 1992; 51: 476-480.
- Church SL, Grant JW, Ridnour LA, Oberley LW, Swanson PE, Meltzer PS. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci USA 1993; 90: 3113-3117.
- Liu X, Wang A, Lo Muzio L, Kolokythas A, Sheng S, Rubini C. Deregulation of manganese superoxide dismutase (SOD2) expression and lymph node metastasis in tongue squamous cell carcinoma. BMC Cancer 2010; 10: 365.
|



