Research Article - Journal of Biochemistry and Biotechnology (2022) Volume 5, Issue 5
Anti-Hyperglycemic Effects of Aqueous Extracts of Moringa Oleifera in Streptozotocin-Induced Diabetes Wistar Rats
Rakhi Das1*, Ranju Khatiwada2, Bijay Kumar Das1
1Department of Biochemistry, Universal Engineering and Science College, Pokhara University, Lalitpur, Nepal
2Department of Chemistry, Sukuna Multiple Campus, Tribhuvan University, Morang, Nepal
- Corresponding Author:
- Rakhi Das
Universal Engineering and Science College
Department of Biochemistry, Pokhara University
Lalitpur, Nepal
E-mail: Rakhidas021@gmail.com
Received: 06-Sep-2022, Manuscript No. AABB-22-73813; Editor assigned: 07-Sep-2022, PreQC No. AABB -22-73813(PQ); Reviewed: 21-Sep-2022, QC No AABB-22-73813;Revised: 23-Sep-2022, Manuscript No. AABB-22-73813(R); Published: 30-Sep-2022, DOI:10.35841/aabb-5.5.124
Citation: Das R, Khatiwada R, Kumar Das B. Efficacy of Moringa Oleifera aqueous leaf extracts on hyperglycemia in streptozotocin-induced diabetic wistar rats. J Biochem Biotech 2022;5(5):124
Abstract
In this work, streptozotocin-induced diabetes in Wister rats was compared to the antidiabetic effects of Moringa olifera. We used 16 Wistar rats for the experiment. By chance, there were four groups of four animals each (n=4). Streptozotocin, dissolved in citrate buffer, at a dosage of 70 mg/kg body weight, was administered intraperitoneally to animals in groups 2 and 3 in order to induce diabetes (0.1 m, pH 4.5). Moringa oleifera leaf extract was administered to the animals in groups 2 and 4, respectively. When the study's time was up, the animals were put to death, and the pancreas was taken out, weighed, and homogenized before being tested for the presence of insulin, malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) using the proper diagnostic kits. The results showed that after one week 79% of the rats in the diabetic groups treated with moringa leaves after therapy turned out to be normoglycemic. Moringa's average body weight increased by 18.5% in the third week, 26.5% in the sixth week, and 20.5% in the seventh. After getting treatment for hyperglycemia with moringa, insulin levels nearly returned to normal while pancreatic SOD and GSH concentrations increased in the groups that received the extract. In light of this, it can be said that moringa possesses hypoglycemic properties that can be highly beneficial in the management of diabeties.
Keywords
Diabetes, Moringa Olifera Extracts, Pancreatic superoxide dismutase (SOD), Glutathione, Malondialdehyde, Insulin level.
Introduction
Moringa trees are in full near the end of the dry season, when most edibles are often sparse, and appear promising as a food in the tropics. There are now a lot of studies on the nutritional benefits of Moringa, both in academic and lay literature. According to reports, Moringa's leaves have a protein content that is comparable to that of milk and eggs and contain more vitamin A than carrots, Calcium than milk, iron than spinach, vitamin C than oranges, and Potassium than bananas. There seems to be little dispute about the significant wealth gain to be realized by consuming Moringa leaf powder in situations where starvation is approaching because the nutritional characteristics of the plant are now so well documented (Lowell, 1989). The advantages for the treatment, prevention, or both that may result from dietary or topical administration of Moringa preparation ( such as extracts, decoction, poultices, creams, oils, emollients, salves, powders, and porridges) are not entirely well known (Palada, 1996) (Figure 1).
Reviewing the assertions stated and evaluating the caliber of the evidence supporting the more thoroughly researched assertions is helpful. Numerous references to traditional medicine attest to its healing abilities and scientific validation of these widely used practices in underdeveloped nations, supporting at least part of the claims. In the scientific literature, Moringa preparation has been cited as having antibiotic, anti-inflammatory, hypocholesterolemic, and hypoglycaemic properties. It has also been noted to have significant efficacy in water purification through flocculation, sedimentation, antibiosis, and even a decrease in the titer of Schistosome cercariae (Olsen, 1987).
A diverse range of metabolic illnesses known as diabetes mellitus includes glucose intolerance and fasting hyperglycemia [1]. About 20 million people worldwide have type 1 diabetes, which accounts for 10% of all occurrences of diabetes and is immune-mediated [2]. 90% of people with diabetes have type II, insulin-resistant diabetes. It affects an estimated 6% of the adult population, and it is predicted that by 2010, there would be 200-300 million cases worldwide, an increase of 6% each year [3]. Diabetes is linked to debilitating and potentially fatal complications like coronary artery disease, retinopathy, nephropathy, and hepatopathy [4]. These problems have oxidative stress caused by chronic hyperglycemia as its pathogen [5]. According to earlier studies, antioxidants can shield people from the incapacitating consequences of diabetes by scavenging free oxygen and superoxide radicals [5]. Additionally, it has been noted that antioxidant depletion appears to be a significant risk factor for problems and that antioxidant supplementation reduced the risk [6]. Further, diabetes-related insulin resistance or dysfunction increases the risk of atherosclerosis and dyslipidemia [7]. There is evidence that cellular damage brought on by free radicals plays a role in the development of diabetes mellitus [4]. The goal of the study was to examine the antihyperglycemic properties of Moringa oleifera leaves in adult Wistar rats that had developed diabetes as a result of streptozotocin (STZ) induction.
Material and Methods
Collection, identification and authentication of Plant material
Mature fresh leaves of Moringa oleifera were harvested from a farm in the terai region of Nepal. The plant material was then identified and authenticated by Botanist of Natural Herbarium Department, Godawari Lalitpur Nepal.
Shade drying, Granulation and Extraction Moringa oleifera leaves
The plants leaves were taken, washed with water and dried in shade. Then the shade dried plant leaves were coarsely powdered by means of mix grinder and was filtered through sieve no. 60 to get the coarse powder. Then the coarsely powdered materials were weighed, packed in an airtight container and used for extraction with suitable solvent.
Preparation of Plant extract
About 1.5 kg of dried leaves powder of Moringa olifera was placed in 1500ml (solvent to sample ratio of 10:1 (v/w) solvent to dry weight has been used) of methanol as a solvent and then stored at room temperature for 72 hours. The extract was then filtered through Whatmann No.1 filter paper and evaporated to dryness using Rotary Evaporator at a much reduced pressure. This was followed by the dilution of the crude extract (concentrated using a rotary evaporator) with mother solvent i.e. DMSO (Dimethyl Sulpho-oxide) to obtain a stock solution of 100mg/ml from which a series of dilutions were made as required.
Grouping of Experimental Animal
There were sixteen Wistar rats utilized. The animals ranged in age from 6 to 10 weeks (150-200g). The animals were housed in cages. The animals were subjected to a 12-hour cycle of light and darkness while kept at ambient temperature. They were kept on animal diets and given unrestricted access to food and water.
Sixteen Wistar rats were divided randomly into four groups with each group containing four rats (n=4):
Group 1: Normoglycemic animals.
Group 2: the diabetic group that received Moringa extracts only.
Group 3: untreated diabetic group.
Group 4: the normoglycemic group that received Moringa extracts only.
Induction of hyperglycemia in Wistar rats
Eight overnight-fasted, randomly chosen rats were given one intraperitoneal injection of STZ at a dose of 70 mg/kg body weight to cause hyperglycemia [8,9]. Just before injection, STZ was dissolved in citrate buffer (0.1 m, pH 4.5). For 72 hours, hyperglycemia was allowed to grow [10]. Hyperglycemic animals were those whose fasting blood glucose (FBG) was 250 mg/dL or higher [11] and were included in the study. One intraperitoneal injection of 0.1 m citrate buffer (1 mL/kg bw; pH 4.5) was given to control animals.
Moringa oleifera treatments
An analysis of the literature helped to support the amount of Moringa oleifera aqueous extract that was employed in the investigation [11]. For a maximum of six weeks, rats in groups 2 and 4 received daily oral administration of 200 mg/kg body weight of Moringa oleifera aqueous extract (in physiological saline) at 9.00-10.00 each day through the oral cannula.
Measurement of blood glucose
At 9:00-10:00, overnight fasted rats' blood sugar levels were measured using a one-touch ultra 2 glucometer. Blood was drawn from the tail's dorsal vein. For six weeks, blood glucose levels were checked on the first day of treatment and once a week.
Termination of treatment
The therapies were given for six weeks to evaluate the morphological and biochemical changes that were occurring in the various groups. Animals were cervical dislocated and slaughtered after six weeks. Following a laparotomy, the pancreas was removed, homogenized in phosphate buffer solution (pH 7.4), and stored in the refrigerator for the determination of insulin, malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) using the appropriate diagnostic kits.
Statistical analysis
The results were examined using the formula mean standard error of the mean (mean SEM). To compare mean values, a one-way analysis of variance was used (ANOVA). p<0.05 was chosen as the cutoff for statistical significance.
Results
Assesment Blood glucose levels
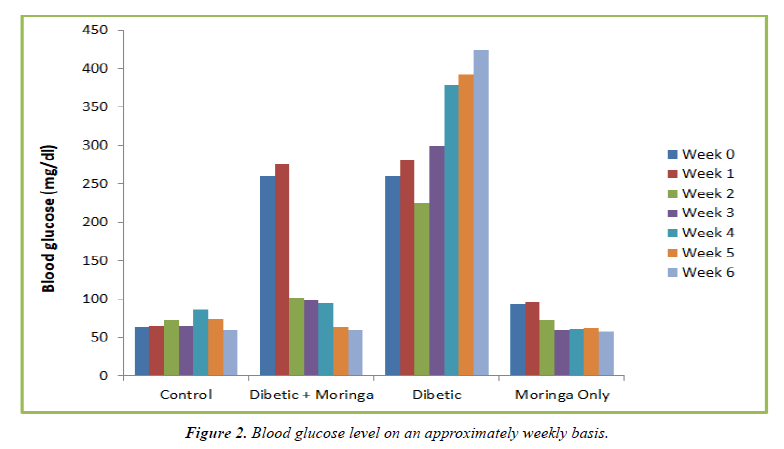
Throughout the six weeks, blood sugar levels were assessed in several groups. All animals in the untreated diabetic and the treated diabetic groups remained hyperglycemic (FBG> 250 mg/dL) at week 0, which was a week following the induction of hyperglycemia. 90.5% of the diabetic rats receiving Moringa medication after one week of treatment achieved normoglycemia, and their blood sugar levels were nearly identical to those of the control group (p>0.05). As the administration went on until the sixth week, the blood glucose level gradually returned to normal, as depicted in (Figure 1).
Assesment of Body weight
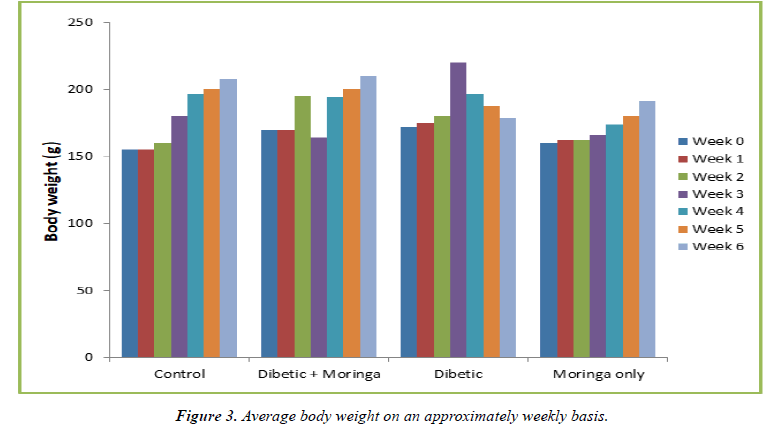
The average body weight of the animals in the untreated diabetic group increased by 32.4% by week 3, but by week 6 their average body weight had decreased by 23% from the initial average body weight at week 0. This was revealed by the weekly changes in body weight of the animals from different groups. When compared to their weight at week 0, the diabetic group receiving Moringa treatment experienced a 20.5% increase at week 3, but a 27.4% increase at week 6 (Tables 1and 2) (Figure 2).
| Group | Blood Glucose (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| Control | 94 | 95 | 73 | 65 | 86 | 74 | 60 |
| Diabetic + Moringa | 260 | 276 | 102 | 98 | 95 | 64 | 60 |
| Diabetic | 260 | 280 | 255 | 300 | 378 | 392 | 425 |
| Moringa Only | 94 | 96 | 73 | 60 | 61 | 63 | 58 |
Table 1. Blood glucose level on an approximately weekly basis.
| Group | Body Weight (g) | ||||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | |
| Control | 155 | 155 | 160 | 180 | 196 | 200 | 208 |
| Diabetic + Moringa | 170 | 170 | 195 | 194 | 194 | 200 | 210 |
| Diabetic | 172 | 175 | 180 | 220 | 196 | 188 | 179 |
| Moringa Only | 160 | 162 | 162 | 166 | 174 | 180 | 191 |
Table 2. Average body weight on an approximately weekly basis (g).
Assessment of Pancreatic insulin level
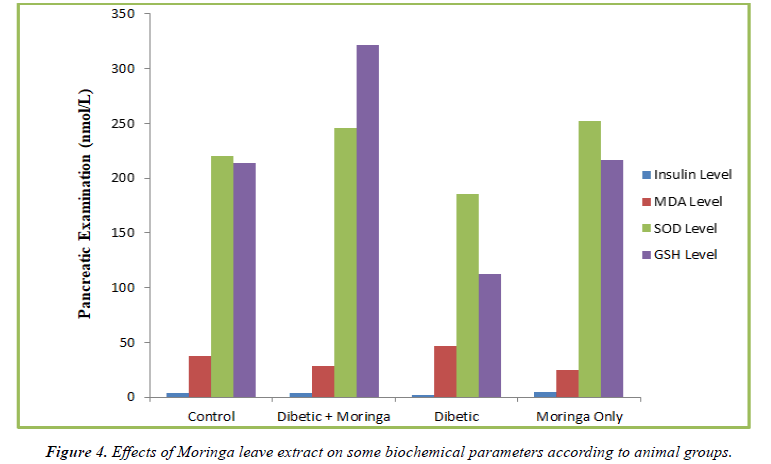
Table 3 displays the pancreatic insulin levels in the four groups at week 6. The insulin levels of the hyperglycemic groups treated with Moringa were not substantially different from the control group (p>0.05). However, the plasma insulin level in the hyperglycemic group who weren't receiving treatment was low and fell two fold from the control group. This decline differed significantly from the control (Figure 3).
| Group | Pancreatic Examination (nmol/L) | |||
|---|---|---|---|---|
| Insulin Level | MDA Level | SOD Level | GSH Level | |
| Control | 4.2 ± 1.24 | 37.64 ± 2.4 | 220.05 ± 3.32 | 213.66 ± 5.36 |
| Diabetic + Moringa | 3.8 ± 2.2 | 28.53 ± 2.42 | 245.33 ± 15.84 | 321.04 ± 10.21 |
| Diabetic | 1.8 ± 1.5 | 46.72 ± 1.72 | 185.46 ± 6.66 | 112.82 ± 4.25 |
| Moringa Only | 4.3 ± 1.05 | 24.33 ± 1.5 | 251.67 ± 6.14 | 216.34 ± 5.32 |
Table 3. Effects of Moringa leaf extracts on some biochemical parameters according to animal groups.
Assessment of Pancreatic MDA level
The Moringa-treated group had a low level of pancreatic MDA, which was statistically different from the control group (p< 0.05). When compared to the control group, the untreated diabetic group's elevated pancreatic MDA level statistically significantly (p<0.05).
Assessment of Pancreatic SOD levels
In contrast to the untreated diabetes group, where the pancreatic SOD level was low and the decline was statistically significant when compared to control (p>0.05), the diabetic groups treated with Moringa had pancreatic SOD levels that were comparable and similar to control (p> 0.05).
Assessment of Pancreatic GSH level
Table 3 displays the level of pancreatic GSH levels at week 6. At week 6, the pancreatic GSH level in the diabetic group receiving Moringa treatment increased by 50% (p<0.05). At week 6, there was a 49% drop in pancreatic GSH levels in the untreated diabetes group as compared to the control group (p<0.05) (Figure 4).
Discussion
In this study, therapy with Moringa oleifera's aqueous leaf extract at a dose of 200 mg/kg/d resulted in normoglycemia in 90.4% of the animals by the end of the first week of treatment, and all of the animals had their blood glucose levels brought to near normal by the end of the second week. Tende et al. have noted that Moringa leaves have hypoglycemic properties [11]. The enhancement of insulin release from the ß cells of the pancreatic islet of Langerhans, inhibition of gastrointestinal glucose uptake with a-glucosidase or pancreatic amylase enzyme inhibitors, inhibition of gluconeogenesis, and inhibition of glycogenolysis were used to produce hypoglycemic activity [12,13]. According to reports, the presence of a-glucosidase and pancreatic amylase enzyme inhibitors is what gives Moringa its hypoglycemic effects [14]. The inability of blood glucose to rise after ingesting glucose is due to these enzyme inhibitors which stop the digestion of glucose into an absorbable product. These inhibitors have been found in plants like Morus alba, which can display hypoglycemic activity, under reports [15]. Also, the antioxidants flavonoids, phenol, and vitamins C and E in Moringa leaf extract may be the cause of its hypoglycemic effects.
Increment in pancreatic oxidative stress is linked to disease development in STZ diabetes [16], and this investigation has verified this. Nitric Oxide (NO) donor STZ has been shown to mediate pancreatic islet cell death, most likely through DNA damage [17]. In addition to producing NO, STZ also produces ROS due to its impact on mitochondria and an increase in xanthine oxidase activity [18]. Scavengers of NO [17] and ROS [18] have therefore been found to protect against DNA damage and cell toxicity brought on by these chemicals. Hyperglycemia is typically linked to lipid peroxidation [13]. When cells are destroyed without insulin, as in the case of untreated hyperglycemic mice, the tissue switches to using fatty acids and acetyl-CoA [13]. Untreated hyperglycemic animals had higher amounts of MDA in their pancreas. In the mice treated with Moringa, the increase in MDA levels was substantially different from the control (p<0.05). Oleic acid, one of the components of Moringa leaf extract, was able to increase the release of insulin [19], and this group's early onset of hypoglycemia was able to prevent lipid peroxidation. The aforementioned explanation can be used to explain why the low MDA levels found in the pancreatic and blood of the Moringa-treated group were much lower than those of the control and untreated hyperglycemic animals.
Cells naturally produce the antioxidant SOD [20]. This study demonstrated an inverse relationship between tissue SOD levels and plasma glucose levels [16]. Animals from the untreated hyperglycemic group had low pancreatic SOD levels, and this decline differed significantly from the control group (p<0.05) in comparison. The presence of antioxidants like flavonoids and vitamins C and E in Moringa leaf extract [14] must be the cause of its antioxidant activity. The Moringa extract was able to stop the oxidative damage that hyperglycemia caused to tissues by ensuring that normoglycemia was reached within the third and second weeks, respectively. Typically, the generation of Oxygen radicals and ROS is what causes tissue to be destroyed in a hyperglycemic state [4].
γ-glutamyl-Cysteine synthase catalyzes the first step in the production of glutathione, which is the generation of γ-glutamyl-cysteine. Glutamyl synthase next adds the glycerin moiety [21-23]. Glutathione, also knowns as glutamylcysteine- glycine, is essential for the body's defense against oxidative stress [24]. The majority of glutathione in human blood is found in erythrocytes (2mmol/L), whereas plasma glutathione concentrations are very low (10mmol/L). This is consistent with reports from Dominiguez et al. [25] and Elhadd et al. That type 1 diabetes causes a 32% decrease in tissue glutathione levels and type 2 diabetes causes a 30% decrease. The depletion depends on the blood glucose levels and does not result from a drop in the rate of glutathione synthesis; rather, it happens despite unchanged or accelerated rates of glutathione production. Glutathione depletion results from diabetes' increased use of antioxidants [25]. The diabetic group that received treatment with Moringa had high levels of pancreatic GSH, and this rise stood out from the control and untreated diabetic groups (p<0.05).
Conclusion
As a result, it is determined that Moringa leaf extract in aqueous form has a hypoglycemic impact. This further emphasizes the promise of these herbal treatments for the control of diabetic hyperglycemia.
References
- Kantárová D, Buc M, Stuchlíková M, et al. Ethiopathogenesis of autoimmune diabetes mellitus in humans. A review. Central European Journal of Immunology. 2006;31(3):3-4.
- Libman I, Songer T, LaPorte R. Hew Many People in the US Have IDDM?. Diabetes Care. 1993;16(5):841-2.
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetic Med. 1997;14(S5):S7-85.
- Komolafe OA, Ofusori DA, Adewole OS, et al. Histological and histochemical studies of the aorta and pulmonary trunk in STZ-induced diabetic Wistar rats treated with Momordica charantia. Int J Morphol. 2013;31(2):716-23.
- Johansen JS, Harris AK, Rychly DJ, et al. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4(1):1-1.
- Pawar SM, Tolanur SI, Mohana LT. MDA, FRAP status in diabetic with coronary heart disease patients. J Pharm Biomed Sci. 2011;4:1-4.
- Rossetti L, Goldberg IJ. A new piece in the diabetes puzzle. Nat Med. 2002 Feb;8(2):112-4.
- Genet S, Kale RK, Baquer NZ. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: effect of vanadate and fenugreek (Trigonella foenum graecum). Mol Cell Biochem. 2002;236(1):7-12.
- Lal MA, Körner A, Matsuo Y, et al. Combined antioxidant and COMT inhibitor treatment reverses renal abnormalities in diabetic rats. Diabetes. 2000;49(8):1381-9.
- Lenzen S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216-26.
- Tende JA, Ezekiel I, Dikko AA, et al. Effect of ethanolic leaves extract of Moringa oleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic Wistar rats. British Journal of Pharmacology and Toxicology. 2011;2(1):1-4.
- Adewole SO, Caxton-Martins EA, Ojewole JA. Protective effect of quercetin on the morphology of pancreatic β-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2007;4(1):64-74.
- Abdulkarim SM, Long K, Lai OM, et al. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food chemistry. 2005;93(2):253-63.
- Sudha SS, Karthic R, Naveen JR, et al. Anti hyperlipidemic activity of Spirulina platensis in Triton x-100 induced hyperlipidemic rats. Hygeia JD Med. 2011;3(2):32-7.
- Kakkar R, Mantha SV, Radhi J, et al. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci. 1998 Jun 1;94(6):623-32.
- Kröncke KD, Fehsel K, Sommer A, et al. Nitric oxide generation during cellular metabolization of the diabetogenic N-Mefhyl-N-Nitroso-Urea streptozotozin contributes to islet cell DNA damage.
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537-46.
- MU D. Vitamin contents of the flowers and seeds of moringa oleifera.
- Meki AR, Abdel-Ghaffar SK, et al. Aflatoxin B1 induces apoptosis in rat liver: protective effect of melatonin. Neuroendocrinology Letters. 2001;22(6):417-26.
- Shi ZM, Feng P, Jiang DQ, Wang XJ. Mistletoe alkali inhibits peroxidation in rat liver and kidney. World J Gastroenterol: WJG. 2006;12(25):4052.
- Van Huyen JP, Delignat S, Kazatchkine MD, et al. Comparative study of the sensitivity of lymphoblastoid and transformed monocytic cell lines to the cytotoxic effects of Viscum album extracts of different origin. Chemotherapy. 2003;49(6):298-302.
- Darmaun D, Smith SD, Sweeten S, et al. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005;54(1):190-6.
- West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17(3):171-80.
- Domínguez C, Ruiz E, Gussinye M, et al. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes care. 1998;21(10):1736-42.
- Elhadd TA, Kennedy G, Hill A, et al. Abnormal markers of endothelial cell activation and oxidative stress in children, adolescents and young adults with type 1 diabetes with no clinical vascular disease. Diabetes Metab Res Rev. 1999;15(6):405-11.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref