Research Article - Journal of Biochemistry and Biotechnology (2022) Volume 5, Issue 3
Anti-hyperglycemic and anti-hyperlipidemic activities of Radix Astragali and Panax notoginseng extract in human participants: A randomized, double-blind, crossover clinical trial.
Shih-Chien Huang12, Ching-Pin Lin34, Jing-Yi Hu1, and You-Cheng Shen12*
1 Department of Health Industry Technology Management, Chung Shan Medical University, Taichung, Taiwan
2Department of Internal Medicine and Division of Gastroenterology, Chung Shan Medical University Hospital, Taichung, Taiwan
3Department of Nutrition, Chung Shan Medical University Hospital, Taichung, Taiwan
4Institute of Biochemistry, Microbiology, and Immunology, Chung Shan Medical University, Taichung, Taiwan
- *Corresponding Author:
- You-Cheng Shen
Department of Health Industry Technology Management
Chung Shan Medical University
Taichung
Taiwan
E-mail: youcheng@csmu.edu.tw
Received: 28-Apr-2022, Manuscript No. AABB-22-62129; Editor assigned: 30-Apr-2022, PreQC No. AABB-22-62129(PQ); Reviewed: 14-May-2022, QC No AABB-22-62129; Revised: 18-May-2022, Manuscript No. AABB-22-62129(R); Published: 25-May-2022, DOI:10.35841/aabb-5.3.111
Citation: Shen YC, Huang SC, Lin CP, et al. Anti-hyperglycemic and anti-hyperlipidemic activities of Radix Astragali and Panax notoginseng extract in human participants: A randomized, double-blind, crossover clinical trial. J Biochem Biotech 2022;5(3):111
Abstract
To the best of our knowledge, there is no clinical trial conducted with a standardized Astragalus and notoginseng extract (ANS) in human subjects. The goal of the current study is to investigate the effect of ANS on hyperglycemia and dyslipidemia regulation and its mechanism of action in pre-diabetic with hyperlipidemia human subjects. This randomized, double-blinded crossover trial was conducted in participants aged >20 years, with fasting blood glucose levels 100–125 mg/dL, glycosylated hemoglobin (HbA1C) 5.8–6.4%, and cholesterol 200–249 mg/dL. Eligible participants were asked to take five ANS or placebo capsules daily for 6 weeks. Changes in blood glucose and lipids levels were measured every three weeks. Fasting blood glucose and lipids, oralglucose- tolerance-test, small dense low-density lipoprotein-cholesterol (sdLDL-C), high-density lipoprotein-cholesterol (HDL-C), adiponectin, HbA1C, and adenosine monophosphate-activated protease kinase (AMPK) were measured. Renal and hepatic functions were analyzed for any adverse effects. After 6 weeks of ANS supplementation, fasting blood glucose and glucose area under the curve (AUC) were significantly decreased by 10.1% and 12.69% (p<0.05), respectively. Triglycerides and sdLDL-C were significantly decreased by 31.06% and 19.96% (p<0.05), respectively, in the ANS group than in the placebo group. HDL-C, adiponectin, and AMPK were significantly increased by 11.78%, 22.07%, and 12.72% (p<0.05), respectively in the ANS group than in the placebo group. The above results indicate that oral supplementation with ANS reduces hyperglycemia and dyslipidemia without adverse events.
Keywords
Astragalus, Notoginseng, Fasting blood glucose, Adiponectin, AMPK.
Introduction
Hyperglycemia and hyperlipidemia can lead to metabolic disorders if not controlled. Metabolic syndrome is a cluster of cardiovascular risk factors that is an important cause of morbidity and mortality worldwide [1]. The components of metabolic syndrome, including hyperglycemia, high blood pressure, high triglyceride concentrations, low HDL cholesterol concentrations, and central obesity [2], are also frequently observed in individuals with type 2 diabetes [3]. Obesity is a medical condition in which changes in physiological and biochemical functions in the human body cause an excessive accumulation of body fat [4-6]. Obesity is related to the top 8 of 10 causes of death in Taiwan [7]. Long-term use of medication can cause side effects, including nausea, gas, bloating, diarrhea, low blood sugar, and an upset stomach. Functional foods are effective alternatives for patients with diabetes mellitus to regulate blood glucose levels and for those with obesity to regulate fat metabolism with no or lesser adverse effects. Many studies have demonstrated that certain Chinese medicinal plants may regulate lipogenesis gene expression, inhibit lipase synthesis enzyme activity, and promote lipolysis [8-11]. Radix Astragali and Panax notoginseng have been shown to reduce fat accumulation in adipocytes and animals [12-18]. The major components of Panax notoginseng are ginsenosides. It is also rich in alkaloids, volatile oils, flavonoids, polysaccharides, and dencichine. The major components of Radix Astragali (Astragalus) are astragalosides. It also contains saponins, flavonoids, and other ingredients [19]. Astragalus is widely used in China to treat various ailments, such as type 2 diabetes, kidney disease, and autoimmune diseases [20-22]. Two studies found that Astragalosides reduced blood cholesterol, triglycerides (TG), and low-density lipoprotein-cholesterol in mice fed with a high fat diet or fructose (LDL-C) [23,24]. Panax notoginseng is one of the most widely used herbs in traditional and complementary medicine. Previous studies showed that Ginsenosides Rb1 and Rg1decreased blood glucose, liver cholesterol, triglycerides, and free fatty acids in mice [25,26].
Adiponectin, produced and secreted by the adipocytes [27], activates adenosine monophosphate-activated protease kinase (AMPK) [28]. The activation of the AMPK pathway inhibits gluconeogenesis and maintains the metabolic balance of blood glucose and lipids in humans [29]. Conversely, a decrease in the adiponectin concentration in the blood causes a decrease in insulin sensitivity and increases the risk of obesity [30]. Individuals with obesity shows lower levels of adiponectin in circulation [31], which leads to abnormal blood lipid levels, affecting LDL-C, increasing small dense lowdensity lipoprotein-cholesterol (sdLDL-C), and accelerating metabolic syndrome development and atherosclerosis [32]. Astragalosides or ginsenosides can increase the production of adiponectin in 3T3L1 adipocytes in animals and prevent obesity and related complications, such as insulin resistance and type 2 diabetes [33-35]. There is no research on blood glucose and blood lipid regulation in humans when Aastragalosides and ginsenosides are administered simultaneously. Therefore, the current study aimed at investigating whether a standardized Astragalus and notoginseng extract (ANS) can regulate blood glucose and blood lipids levels in participants with hyperglycemia and hyperlipidemia.
Materials and Methods
Samples and chemicals
Standardized ANS samples and placebo were provided by NuLiv Science USA Inc (Brea, CA, USA). Each ANS capsule contains a 50mg mixture of equal amounts of Astragalus membranaceus (10:1 hydroethanolic extract) and Panax notoginseng (50:1 aqueous extract) that is rich in saponins. The total saponin content is approximately 1.25 mg (2.5%), and the participants were required to consume five capsules (total saponins 6.25 mg) per day. The other component of the ANS capsule is maltodextrin. The placebo was replaced with maltodextrin, which is similar in color and appearance and the same weight as the ANS capsule.
Participants and Study design
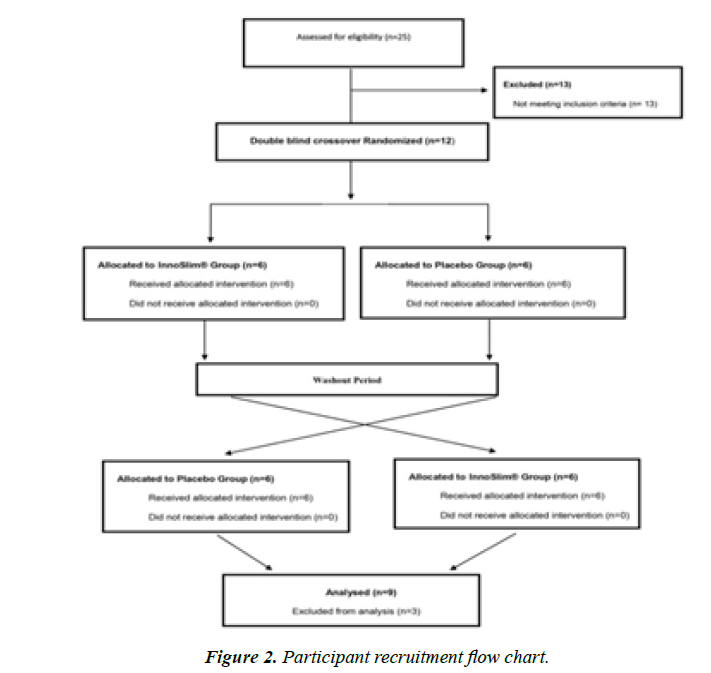
This study was conducted in October 2019 with 25 recruited individuals aged >20 years from the Chung Shan Medical University (Taichung City, Taiwan).Twelve participants were enrolled in this 16 week, double-blinded, randomized, crossover trial. Participants were required to take five ANS or placebo capsules daily for 6 weeks in the first phase of the study (two capsules before breakfast and three before dinner). The washout period was four weeks, during which the participants stopped taking ANS and placebo capsules and maintained their lifestyle and dietary habits (no caloric restrictions). Then, the second phase was conducted for another 6 weeks (with the same protocol, except the participants were crossed over). The inclusion criteria were participants aged >20 years, who voluntarily participated in this study with fasting blood glucose of 100–125 mg/dL, glycosylated hemoglobin (HbA1C) between 5.7% and 6.4%, and total cholesterol between 200 and 249 mg/dL. The exclusion criteria were participants with chronic diseases (cardiovascular, kidney or liver disease, cancer, and uncontrolled diabetes mellitus). We excluded participants who smoked, consumed alcohol regularly, were pregnant or lactating, were participating in another human trial during the first 30 days of the study, or were taking any health products or drugs that might interfere with the research (health foods that regulate blood glucose or blood lipids or anti-diabetes drugs and anti-hyperlipidemic agents (statins)). A participant was considered to reach the clinical endpoint when any of the following conditions were met: completion of the protocol and required follow-up, the occurrence of an adverse event, loss of contact, non-compliance, use of medication, medical contraindication, withdrawal of consent, death, or other reasons.
Ethics
The protocol for this study received ethics approval from the Chung Shan Medical University Hospital IRB (Protocol No. CS2-19061) and has been registered at ClinicalTrials. gov under reference number NCT04201314. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All participants signed the informed consent form before the trial.
Intervention
This study spanned over 16 weeks (6 weeks first phase, 4 weeks washout period, and 6 weeks second phase).The principal investigator and the researcher tossed a coin to randomly assign participants. The ANS sample and placebo were filled in the pot by the manufacturer. During the investigation, the researcher and the participants were blinded. Out of 12 participants, only 9 completed the study, and their data was analyzed. The remaining 3 participants withdrew due to personal and other issues.
Blood samples collected
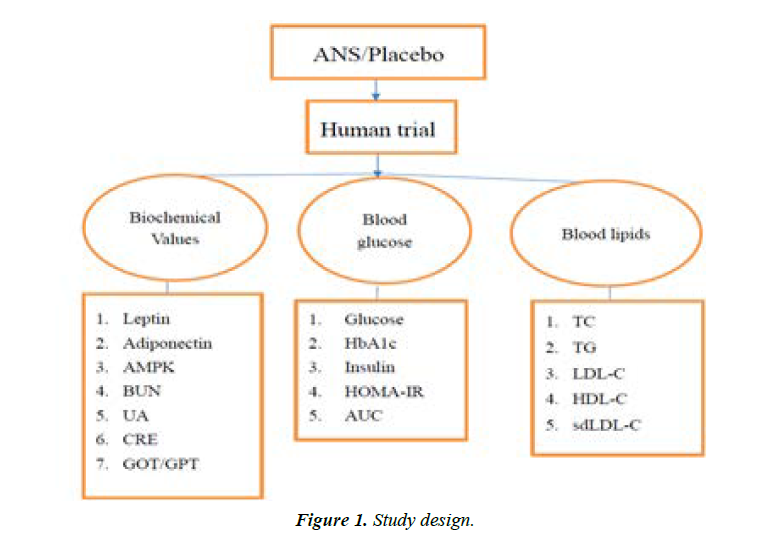
The primary outcome measures were blood glucose level and lipid profile, and the secondary outcome measures were AMPK levels. Fasting blood samples were collected at baseline (0 weeks), and in the 3rd, 6th, 10th, 13th, and 16th weeks, and various biochemical parameters including lipid profile (total cholesterol, TG, LDL-C, high-density lipoprotein-cholesterol (HDL-C), sdLDL-C), renal function (urea nitrogen, creatinine, and uric acid), liver function (GOT and GPT), fasting blood glucose, insulin, adiponectin, HbA1C, and AMPK were measured using commercial kits (Figure 1).
Compliance
The researchers sent text messages or conducted telephone follow-up to track the participants who participated in the study. The study intervention compliance was measured by counting the amount of remaining capsules in the bottle Participants were considered compliant with the study intervention if their adherence to the protocol during the trial period was more than 80%.
Statistical analysis
All the experimental data are expressed as mean ± standard deviation (SD) and compared by one-way repeated measure repeated measures ANOVA using SigmPlot software (version 14.0 for Windows, USA). Similarly, the differences between the ANS and placebo groups at different time intervals (3rd or 6th weeks) were analyzed using Student’s t-test. Also, the paired t test was used to compare the difference within the same group. The analysis was performed using SPSS software (version 18.0 for Windows, USA). A p-value of less than 0.05 was deemed statistically significant.
Abbreviations: ANS, astragalus and notoginseng extract; HbA1C, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance; AUC, area under curve; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoproteincholesterol; sdLDL-C, small dense low-density lipoproteincholesterol; AMPK, adenosine monophosphate-activated protease kinase; BUN, blood urea nitrogen; UA, uric acid; CRE, creatinine; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase.
Results
Demographic data of the participants
A total of 25 participants were recruited in this study, wherein 13 were excluded because they did not meet the inclusion criteria, and 12 completed the study (Figure 2). Excluding three participants with poor compliance, the data of nine participants were analyzed. The cohort comprised four men and five women aged 44–71 years, with an average age of 57.5 years, as shown in Table 1.
| Gender (Men/Women) | ||||
|---|---|---|---|---|
| Age group (years) | 40–50 | 43.5 ± 2.8 | 1 | 1 |
| 50–60 | 53.6 ± 2.8 | 2 | 3 | |
| = 60 | 67.5 ± 3.5 | 1 | 1 | |
Table 1. Age and sex of participants.
The anthropometric measurement results shown in Table 2.The anthropometric changes of the participants are shown in Table 2. After supplementation with ANS for six weeks, there was no significant difference in anthropometric. The researchers sent a reminder to them each week via text message or telephone to ensure that the participants followed the protocol. As a result, the total average compliance of the participants for 6 weeks was 87.5%.This indicates that the participants took the ANS or placebo as directed.
| Week | Body weight (Kg) | Body fat (%) | Offal fat | Waistline | BMI |
|---|---|---|---|---|---|
| ANS | |||||
| 0 | 73.76 ± 6.52a | 34.32 ± 5.74a | 14.11 ± 4.34a | 93.36 ± 4.62a | 28.27 ± 2.65a |
| 3 | 73.34 ± 6.87a | 33.76 ± 6.28a | 13.78 ± 4.49a | 93.33 ± 6.58a | 28.10 ± 2.63a |
| 6 | 73.50 ± 6.57a | 33.53 ± 6.14a | 13.78 ± 4.35a | 92.88 ± 7.08a | 28.16 ± 2.63a |
| Placebo | |||||
| 0 | 73.26 ± 5.19a | 33.76 ± 5.74a | 13.89 ± 3.66a | 93.36 ± 5.19a | 28.12 ± 2.35a |
| 3 | 73.24 ± 5.59a | 34.44 ± 5.51a | 13.78 ± 3.90a | 93.00 ± 4.39a | 28.08 ± 2.55a |
| 6 | 73.29 ± 6.45a | 33.96 ± 6.01a | 13.67 ± 4.18a | 93.56 ± 5.03a | 28.08 ± 2.55a |
| P(D3-0) | 0.25 | 0.66 | 0.57 | 0.83 | 0.24 |
| P(D6-0) | 0.68 | 0.54 | 0.42 | 0.86 | 0.86 |
| Data is expressed as mean ± SD. Data within the same column bearing different superscripts were significantly different (p<0.05). P(D3-0): comparison of D3-0 between the two groups; P(D6-0): comparison ofD6-0 between the two groups.“*” indicates p<0.05. BMI, Body Mass Index | |||||
Table 2. Anthropometric measurement of participants.
Comparison of blood glucose-related levels in study groups
Table 3 shows the blood glucose levels at the three-time points (0, 3rd, 6th week).
| Week | Glucose (mg/dl) | HbA1c (%) | Insulin (mIU/L) | HOMA-IR | AUC (mg/dl.min) |
|---|---|---|---|---|---|
| ANS | |||||
| 0 | 123.0 ± 17.4a | 6.18 ± 0.49a | 16.99 ± 9.09a | 5.3 ± 3.1a | 11,395.8 ± 4,854.2a |
| 3 | 119.0 ± 22.1a | 6.26 ± 0.56a | 16.24 ± 8.69a | 4.9 ± 3.0a | 9,806.1 ± 4,357.2b |
| 6 | 113.0 ± 21.8b | 6.20 ± 0.47a | 13.72 ± 8.35a | 4.0 ± 2.9a | 9,835.2 ± 4,036.7b |
| Placebo | |||||
| 0 | 116.7 ± 12.4a | 6.33 ± 0.75a | 19.80 ± 11.45a | 5.9 ± 3.8a | 12,201.0 ± 5,314.1a |
| 3 | 117.7 ± 12.4a | 6.38 ± 0.70a | 18.77 ± 10.99a | 5.6 ± 3.6a | 12,140.5 ± 5,429.3a |
| 6 | 119.0 ± 13.8a | 6.33 ± 0.59a | 19.76 ± 16.22a | 5.8 ± 5.0a | 12,078.4 ± 5,158.7a |
| P(D3-0) | 0.27 | 0.78 | 0.9 | 0.97 | 0.16 |
| P(D6-0) | 0.04* | 0.89 | 0.4 | 0.38 | 0.04* |
Table 3. Comparison of blood glucose levels between the study groups.
Data is expressed as mean ±SD. Data within the same column bearing different superscripts were significantly different (p<0.05). P(3-0): comparison of 3-0 between the two groups; P(6-0): comparison of 6-0 between the two groups.“*” indicates p<0.05. AUC, area under the curve
After 6 weeks of oral supplementation with ANS, the fasting blood glucose, insulin and HOMIR-IR were considerably decreased by 10.01%, 19.05% and 22.84%, respectively, compared to those in the placebo group. HbA1C in each group showed no change from baseline. This may be because HbA1C reflects the average levels of blood glucose over the past 3 months and the duration of the trail is only 6 weeks. If the duration of supplementation with ANS is prolonged, we may see changes in HbA1C. This trial also tested oral glucose tolerance test (OGTT) and found that the area under the curve (AUC) after supplementation with ANS for 6 weeks decreased by 13.69%, while that in the placebo group only decreased by 1.00%. The AUC in the ANS group was significantly lower by 12.69% (p <0.05) as compared with the placebo group.
Comparison of blood lipid levels between the study groups
The changes in blood lipid levels are shown in Table 4.
| Week | TC (mg/dl) | TG (mg/dl) | LDL-C (mg/dl) | HDL-C(mg/dl) | sdLDL-C (mg/dl) |
|---|---|---|---|---|---|
| ANS | |||||
| 0 | 207.2 ± 14.8a | 128.1 ± 53.8a | 131.0 ± 17.5a | 44.9 ± 7.7a | 53.2 ± 16.1a |
| 3 | 201.7 ± 23.3a | 115.8 ± 59.6a | 124.9 ± 15.9a | 45.4 ± 7.8a | 47.1 ± 17.1b |
| 6 | 197.6 ± 25.2a | 91.3 ± 36.3b | 120.9 ± 19.2a | 48.6 ± 9.2b | 42.0 ± 15.9c |
| Placebo | |||||
| 0 | 206.1 ± 27.7a | 111.2 ± 44.9a | 135.3 ± 23.3a | 48.0 ± 10.1a | 45.8 ± 19.7a |
| 3 | 205.8 ± 26.1a | 106.3 ± 27.8a | 133.8 ± 22.9a | 47.2 ± 6.8a | 46.1 ± 20.0a |
| 6 | 206.5 ± 27.1a | 113.8 ± 38.7 a | 133.1 ± 24.1 a | 46.3 ± 8.1a | 45.3 ± 20.0a |
| P(Ñ3-0) | 0.47 | 0.7 | 0.59 | 0.53 | 0.02* |
| P(Ñ6-0) | 0.16 | 0.01* | 0.42 | 0.03* | 0.002* |
Table 4. Comparison of blood lipid levels between the study groups.
Data for each group are expressed as mean ± SD. Data within the same column bearing different superscripts were significantly different (p<0.05). P(3-0): Comparison of 3-0 between the two groups; P(6-0): Comparison of 6-0 between the two groups. “*”indicates p<0.05.
After six weeks of oral supplementation with ANS, the TG and sdLDL-C were significantly lowered by 31.06% and 19.96%, respectively, in the ANS group as compared with the placebo group (p<0.05). In addition, HDL-C was significantly elevated by 11.78% as compared with that in the placebo group (P<0.05). The total cholesterol and LDL-C levels in the ANS group were decreased by 4.82% and 6.08% as compared with the placebo group. However, there was no significant difference.
Comparison of adiponectin and AMPK levelsbetween the study groups
Table 5 shows that when the participants took ANS oral supplementation for three weeks, adiponectin increased significantly by 22.53% (p<0.05), and the effect lasted until the 6th week (22.07%, p<0.05), while there was no change in the placebo group.
| Week | Adiponectin (µg/mL) | AMPK (ng/mL) |
|---|---|---|
| ANS | ||
| 0 | 2.13 ± 1.39a | 904.4 ± 401.2a |
| 3 | 2.61 ± 1.77b | 930.8 ± 386.3a |
| 6 | 2.60 ± 1.75b | 940.4 ± 381.8a |
| Placebo | ||
| 0 | 2.45 ± 1.51a | 964.3 ± 399.5a |
| 3 | 2.45 ± 1.49a | 931.1 ± 309.4a |
| 6 | 2.45 ± 1.47a | 880.0 ± 303.6 a |
| P(Ñ3-0) | 0.005* | 0.43 |
| P(Ñ6-0) | 0.01* | 0.04* |
Table 5. Comparison of adiponectin and AMPK between the study groups.
Data for each group are expressed as mean ± SD. Data within the same column bearing different superscripts were significantly different (p<0.05). P(3-0): comparison of 3-0between the two groups; P(6-0): comparison of 6-0 between the two groups.“*”indicates p<0.05).
AMPK was significantly increased by 12.72%in the ANS group as compared with the placebo group (p<0.05). Adiponectin and AMPK levels in the ANS group were significantly higher than those in the placebo group after 6 weeks of oral supplementation of ANS. The increase in adiponectin activated AMPK, thereby increased lipolysis, reduced gluconeogenesis, and increased muscle utilization of blood glucose.
Safety assessment
The safety evaluation indicators of this study include kidney and liver function, as shown in Table 6.
| Week | BUN (mg/dL) | UA (mg/dL) | CRE (mg/dL) | GOT (IU/ L) | GPT (IU/ L) |
|---|---|---|---|---|---|
| ANS | |||||
| 0 | 15.0 ± 3.1a | 5.7 ± 1.0a | 0.8 ± 0.2a | 29.1 ± 16.6a | 33.8 ± 21.8a |
| 3 | 15.3 ± 4.2a | 5.8 ± 1.1a | 0.8 ± 0.2a | 26.9 ± 13.1a | 32.6 ± 18.4a |
| 6 | 15.1 ± 4.2a | 5.7 ± 1.0a | 0.9 ± 0.2a | 23.3 ± 6.8a | 28.6 ± 11.3a |
| Placebo | |||||
| 0 | 15.6 ± 4.3a | 5.9 ± 1.1a | 0.8 ± 0.1a | 27.9 ± 13.9a | 34.8 ± 16.6a |
| 3 | 16.1 ± 3.5a | 6.0 ± 1.2a | 0.9 ± 0.1a | 28.9 ± 12.8a | 37.8 ± 20.5a |
| 6 | 14.6 ± 2.9a | 5.8 ± 1.0 a | 0.8 ± 0.2 a | 30.0 ± 14.6a | 34.9 ± 19.8a |
| P(Ñ3-0) | 0.88 | 0.88 | 0.17 | 0.21 | 0.21 |
| P(Ñ6-0) | 0.43 | 0.84 | 0.58 | 0.1 | 0.3 |
Table 6. Comparison of the safety evaluation between the study groups.
Data for each group are expressed as mean ± SD. Data within the same column bearing different superscripts were significantly different (p<0.05). P(3-0): comparison of 3-0 between the two groups; P(6-0): comparison of 6-0 between the two groups. “*” indicates p<0.05.
After 6 weeks of oral supplementation with ANS or placebo, the renal function markers, including blood urea nitrogen, uric acid, creatinine, and liver function markers, including GOT and GPT, showed no significant change. This indicates that oral supplementation with ANS has no adverse effects.
Discussion
To the best of our knowledge, this is the first human trial that examined the effect of a standardized ANS on blood glucose and lipid levels in human subjects. The current study showed ANS increased adiponectin level in circulation, up-regulated AMPK activity, decreased glucose and glucose area under the curve (AUC) through gluconeogenesis inhibition by reduced insulin and HOMA-IR levels, decreased triglycerides and sdLDL-c, and increased HDL-c. Similar results were reported by Yamauchi et al. [36] in a mouse model. In that study, abnormal blood glucose was induced by intraperitoneal injection of LacZ gene and DN-α1AMPK to mice. However, with administration of adiponectin, the glucose levels in DN- α1AMPK mice were significantly lowered via altering AMPK signaling pathway. Furthermore, Zhou et al. [26] showed that after intraperitoneal injection of 60mg/kg ginsenoside Rb1 for 12 days, fasting blood glucose levels were significantly reduced and glucose tolerance improved in high-fat dietinduced abnormal blood glucose male rats. These results are similar to the results found in the current study. In addition, in Du et al.'s study [37], in which 100 mg/kg astragalin IV was added to a high-fat diet to mice for 14 days, there was a significant improvement in glucose tolerance, and an increase in the AMPK activation that promoted the metabolic utilization of blood glucose. These results again are similar to the current study. Moreover, in Lee et al. study [38], a 20 μM ginsenoside Rg1 was added to C2C12 muscle cells for 3 hours and the study found that the insulin resistance (IR) was improved as a result of activating the AMPK pathway.
In the current study, TG and sdLDL-C were significantly decreased while HDL-C was considerably increased after 6 weeks of oral supplementation with ANS. We attribute these changes to the activation of AMPK by the increased adiponectin levels in circulation [39]. There are similar studies by Liu et al. [25], in which male mice were fed a high-fat diet and followed by the administration of 20 mg/ kg of ginsenoside Rg1 for 4-week, and by Wu et al. [40], in which C57BL/6 mice were fed a high-fat diet and injected intravenously (IV) with 25 mg/kg/d of astragalin IV for 13 weeks. The results of these two studies showed a significant decrease in TG and total cholesterol. In addition, a study by Zhou et al. [26], in which C57BL/6 mice were fed a highfat diet and co-administered with intraperitoneal injection of 60mg/kg ginsenoside Rb1 for 12 days, found TG and total cholesterol levels were decreased significantly, while HDL-C level increased significantly. Furthermore, Yeo et al. [34] cultured 3T3-L1 adipocytes with 10μg/mL ginsenoside Rg1 and found TG was significantly lowered and adiponectin expression was significantly increased. Jiang et al. [41] used TNFα to induce insulin resistance in 3T3-L1 adipocytes. After adding 100μmol/L astragalin IV for 48 hours, TG decreased significantly.
sdLDL-C is a small and very low-density cholesterol. It is the end product of LDL-C oxidation. When sdLDL-C level in human body is high, it intrudes the vascular endothelial cells and induces phagocytosis of macrophages, leading to atherosclerosis. ANS decreased sdLDL-C significantly after 6 weeks of oral supplementation, indicating ANS was capable of reducing the production of LDL-C oxides. This result suggests ANS may have an anti-atherosclerotic effect, which requires further validation.
In summary, ANS effectively regulates the glucose and lipids metabolism by increasing the adiponectin levels and activating the AMPK pathway in participants who took ANS for 6 weeks. The results of the current study help elucidate that the ANS regulates glucose and fatty acid metabolism through upregulation of adiponectin. To the best of our knowledge, this is the first human trial that focused its study on the validation of this mechanism and the first human trial that administered astragalus and notoginseng simultaneously to the participants.
The limitation of this study was the small sample size and shorter duration. As a result, while insulin and cholesterol levels showed a downward trend, HbA1C showed no significant change. Therefore, the duration of oral supplementation with ANS should be increased in future studies to investigate whether ANS can improve HbA1C, as well as the long-term effect of ANS on blood glucose. In addition, there is a need to administer the extract to participants without diabetes as normal controls and to those with diabetes along with or without any antidiabetic medications (no ANS). This would better elucidate the effects of the ANS.
Conclusion
The present study was the first human trial to investigate the effect of ANS on hyperglycemia and hyperlipidemia. After 6 weeks of oral supplementation with ANS, fasting blood glucose, AUC, TG, and sdLDL-C were significantly decreased in the ANS group as compared with the placebo group. In addition, HDL-C, adiponectin, and AMPK were significantly higher in the ANS group as compared with the placebo group. ANS helped regulate glucose and lipid metabolism by increasing adiponectin levels and activated AMPK signaling pathway and thereby reduced blood glucose and blood lipids levels with no adverse effects. Based on this pilot trial, we would like to conduct an extensive clinical trial to crosscheck the effect of ANS on hyperglycemic and hyperlipdemic subjects with or without standard antidiabetic or dyslipidemia medication as well as to explore the deep mechanism behind ANS's anti-diabetic and hypolipidemic properties.
Author Contributions
The authors’ responsibilities were as follows—Y.C.S., and S.C.H.: Designed the research “Conceptualization”; J.Y.H., S.C.H, and Y.C.S.: Conducted the analyses; J.Y.H., C.P.L., and Y.C.S.: Wrote the manuscript; S.C.H., and C.P.L.: Critically reviewed manuscript; Y.C.S.: Had primary responsibility for final content.
Institutional Review Board Statement
The protocol for this study received ethics approval from the Chung Shan Medical University Hospital IRB (Protocol No. CS2-19061) and has been registered at ClinicalTrials. gov under reference number NCT04201314. This study was conducted in accordance with the ethical principles expressed in the Declaration of Helsinki.
Informed Consent Statement
Before the trial, the researchers clearly explained the contents and execution methods of the study phase to the participants. In addition, all participants signed the informed consent form before the trial.
Acknowledgements
The authors would like to thank NuLiv Science USA Inc (Brea, CA, USA) for providing the study with ANS (InnoSlim®) and placebo capsules. This trial was funded by Chung Shan Medical University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):1-8,
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640-5.
- Tkac I. Metabolic syndrome in relationship to type 2 diabetes and atherosclerosis. Diabetes Res Clin Pract. 2005;68:S2-9.
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242-56.
- Kadowaki T, Hara K, Yamauchi T, et al. Molecular mechanism of insulin resistance and obesity. Exp Biol Med. 2003;228(10):1111-7.
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. cell. 2001 Feb 23;104(4):531-43.
- Welfare, M.o.H.a. Ministry of Health and Welfare: 2014.
- Yamamoto M, Shimura S, Itoh Y, et al. Anti-obesity effects of lipase inhibitor CT-II, an extract from edible herbs, Nomame Herba, on rats fed a high-fat diet. Int J Obes. 2000;24(6):758-64.
- Karu N, Reifen R, Kerem Z. Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J Agric Food Chem. 2007 Apr 18;55(8):2824-8.
- Abidov MT, Del Rio MJ, Ramazanov TZ, et al. Effects of Aralia mandshurica and Engelhardtia chrysolepis extracts on some parameters of lipid metabolism in women with nondiabetic obesity. Bull Exp Biol Med. 2006;141(3):343-6.
- Uysal KT, Wiesbrock SM, Marino MW, et al. Protection from obesity-induced insulin resistance in mice lacking TNF-a function. Nature. 1997;389(6651):610-4.
- Xiong Y, Shen L, Liu KJ, et al. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010 Oct 1;59(10):2505-12.
- Kim MS, Lee MS, Kim SH, et al. Anti-obesity effects of ginsenoside Rd via AMPK and PPAR gamma. KSBB Journal. 2007;22(5):341-4.
- Koh EJ, Kim KJ, Choi J, et al. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J Ginseng Res. 2017;41(1):23-30.
- Wang S, Zhai C, Liu Q, et al. Cycloastragenol, a triterpene aglycone derived from Radix astragali, suppresses the accumulation of cytoplasmic lipid droplet in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2014;450(1):306-11.
- Wang D, Song Y, Li SL, et al. Simultaneous analysis of seven astragalosides in Radix Astragali and related preparations by liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. J Sep Sci. 2006;29(13):2012-22.
- Wu H, Gao Y, Shi HL, et al. Astragaloside IV improves lipid metabolism in obese mice by alleviation of leptin resistance and regulation of thermogenic network. Sci Rep. 2016 Jul 22;6(1):1-1.
- Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48(5):511-3.
- Ren S, Zhang H, Mu Y, et al. Pharmacological effects of Astragaloside IV: a literature review. J Tradit Chin Med. 2013;33(3):413-6.
- Zhai R, Jian G, Chen T, et al. Astragalus membranaceus and Panax notoginseng, the novel renoprotective compound, synergistically protect against podocyte injury in streptozotocin-induced diabetic rats. J Diabetes Res. 2019 Apr 16;2019.
- Liu J, Nile SH, Xu G, et al. Systematic exploration of Astragalus membranaceus and Panax ginseng as immune regulators: insights from the comparative biological and computational analysis. Phytomedicine. 2021;86:153077.
- Wang T, Zhou X, Zou W, et al. Synergistic effects of Ginseng CA Mey and Astragalus membranaceus (Fisch.) Bunge on activating mice splenic lymphocytes detected by microcalorimetry and the underlying mechanisms predicted by in silico network analysis. Journal of Thermal Analysis and Calorimetry. 2018;132(3):1933-42.
- Zhang N, Wang XH, Mao SL, et al. Astragaloside IV improves metabolic syndrome and endothelium dysfunction in fructose-fed rats. Molecules. 2011 May;16(5):3896-907.
- Qin H, Liu P, Lin S. Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE-/- mice induced by hyperlipaemia. Evidence-Based Complementary and Alternative Medicine. 2015 Oct;2015.
- Liu H, Wang J, Liu M, et al. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. 2018 Jul;10(7):830.
- Zhou P, Xie W, He S, et al. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019 Mar;8(3):204.
- Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003 Apr;46(4):459-69.
- Yamauchi T, Kamon J, Minokoshi YA, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature medicine. 2002 Nov;8(11):1288-95.
- Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proceedings of the National Academy of Sciences. 2001;98(4):2005-10.
- Miczke A, Suliburska J, Pupek-Musialik D, et al. Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with fat diet. International journal of clinical and experimental medicine. 2015;8(7):10358.
- Shimada K, Miyazaki T, Daida H. Adiponectin and atherosclerotic disease. Clinica chimica acta. 2004;344(1-2):1-2.
- Hulthe J, Hultén LM, Fagerberg B. Low adipocyte-derived plasma protein adiponectin concentrations are associated with the metabolic syndrome and small dense low-density lipoprotein particles: atherosclerosis and insulin resistance study. Metabolism. 2003;52(12):1612-4.
- Gu W, Kim KA, Kim DH. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol Pharma Bull. 2013;36(1):102-7.
- Yeo CR, Yang C, Wong TY, Popovich DG. A quantified ginseng (Panax ginseng CA Meyer) extract influences lipid acquisition and increases adiponectin expression in 3T3-L1 cells. Molecules. 2011;16(1):477-92.
- Agyemang K, Han L, Liu E, et al. Recent advances in Astragalus membranaceus anti-diabetic research: pharmacological effects of its phytochemical constituents. Evid Based Complement Alternat Med. 2013.
- Yamauchi T, Kamon J, Minokoshi YA, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat med. 2002;8(11):1288-95.
- Du Q, Zhang S, Li A, Mohammad IS, et al. Astragaloside IV inhibits adipose lipolysis and reduces hepatic glucose production via Akt dependent PDE3B expression in HFD-fed mice. Front physiol. 2018;23;9:15.
- Lee HM, Lee OH, Kim KJ, et al. Ginsenoside Rg1 promotes glucose uptake through activated AMPK pathway in insulin-resistant muscle cells. Phytother Res. 2012;26(7):1017-22.
- Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends in Endocrinology & Metabolism. 2017;28(8):545-60.
- Wu H, Gao Y, Shi HL, et al. Astragaloside IV improves lipid metabolism in obese mice by alleviation of leptin resistance and regulation of thermogenic network. Scientific reports. 2016 Jul 22;6(1):1-1.
- Jiang, B, Yang Y, Jin H, et al. Astragaloside IV attenuates lipolysis and improves insulin resistance induced by TNFalpha in 3T3-L1 adipocytes. Phytother Res. 2008; 22;1434-1439.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref