Research Article - Journal of Biochemistry and Biotechnology (2022) Volume 5, Issue 4
Anticarcinogenic, antimicrobial and broad-spectrum analysis of aqueous leaf extract of Citrullu colocynthis in vitro.
Lakshmanan1, Rohini A2*, Fathima Sumaya1, P Usha3
1M.Sc Biochemistry, Department of Biochemistry, Rathnavel Subramaniam College of Arts and Science, Coimbatore, Tamil Nadu-641402, India
2Assistant Professor, Department of Biochemistry, Rathnavel Subramaniam College of Arts and Science, Coimbatore, Tamil Nadu-641402, India
3B.Sc Biochemistry, Department of Biochemistry, Rathnavel Subramaniam College of Arts and Science, Coimbatore, Tamil Nadu-641402, India
- Corresponding Author:
- Rohini A
Department of Biochemistry
Rathnavel Subramaniam College of Arts and Science
Coimbatore, Tamil Nadu-641402
E-mail: rohini@rvsgroup.com
Received: 15-Jul-2022, Manuscript No. AABB-22-69272; Editor assigned: 18-Jul-2022, PreQC No. AABB-22-69272(PQ); Reviewed: 01-Aug-2022, QC No AABB-22-69272; Revised: 05-Aug-2022, Manuscript No. AABB-22-69272(R); Published: 12-Aug-2022, DOI:10.35841/aabb-5.4.116
Citation: Rohini A, Lakshmanan, Sumaya F, et al. Anticarcinogenic, antimicrobial and broad-spectrum analysis of aqueous leaf extract of Citrullu colocynthis in vitro. J Biochem Biotech 2022;5(4):116
Abstract
Breast cancer is the leading cause of death among women around the world. Systemic toxicity, metastasis, tumor recurrence, off-target effects, and drug resistance are all severe drawbacks of current treatments. Due to the excellent anticancer potential at very low concentrations, phytochemicals such as secondary metabolites have received interest in recent years. Citrullus colocynthis is a well-known medicinal herb. Therapeutic phytochemicals abound in its leaves, fruits, and roots. saponins, phenols, tannins, flavones, flavonoids, alkaloids, glycosides, and terpenoids are among them. The plant extract proves to have promising anticancer potential by inhibiting the MCF-7 breast cancer cell line. The spectrum analysis of the aqueous leaf extract of Citrullus colocynthis showed the presence of many compounds which are responsible for the anticarcinogenic activity.
Keywords
Cytotoxicity, MTT, Phytochemical, Cancer, MCF-7 breast cancer cell line.
Introduction
Breast cancer is a heterogeneous illness, with some cases progressing rapidly while others progressing slowly and with a good prognosis. Breast cancer is one of the most common and leading causes of cancer-related deaths in women globally, with 1.7 million cases diagnosed each year. This incidence is expected to rise to about 3.2 million instances per year by 2050, according to projections. This massive occurrence has a significant influence on individuals all over the world, necessitating the development of innovative prevention and cure measures [1]. In addition to socioeconomic issues, the availability of equal screening opportunities and effective therapies for all women can help to lower the global burden of breast cancer. However, previously used chemotherapeutic medications have been linked to a variety of adverse effects, including peripheral neuropathy, bone health, fatigue, anxiety, depression, cognitive impairment, cardio toxicity, and lymphedema, as well as medication resistance and tumor recurrence [2]. As a result, one of the most significant problems in breast cancer research is to find new promising, effective, and less expensive therapy choices to reduce morbidity and death. Anticancer secondary metabolites are abundant in medicinal plants. Phytochemical research is gaining traction because they are effective anticancer agents at low doses with little adverse effects.
Citrullus colocynthis is a well-known medicinal herb. Desert gourd, egusi, colocynth, and bitter apple are some of the common names for it. It is a member of the Cucurbitaceae plant family, which is found primarily in the Mediterranean Basin and Asia, including Pakistan (Sultan et al.,). Cultivation of C. colocynths has risen in various places in the last decade due to its medicinal and nutraceutical properties. China, India, Russia, the United States, Egypt, and Iran are the top producers [3]. Its pharmacology, toxicity, and photochemistry have all been studied extensively. According to the Australian New Crop website in 2010, the number of research articles on this plant is increasing [4]. Its crude extracts therapeutic phytochemicals abound in its leaves, fruits, and roots. Saponins, phenols, tannins, flavones, flavonoids, alkaloids, glycosides, and terpenoids are among them (Ahmed et al., 2019). This plant has also been used as a traditional treatment for a variety of respiratory, cardiovascular, neurological, musculoskeletal, and gastrointestinal issues [5-8].
Medicinal Parts
The main medicinal part of the plant is the fruit pulp, and it is better to remove the pulp from the fruit when it is administered, because after the pulp is taken out of the fruit its potency is decreased. Other medicinal parts are the seed, leaf, and root.
Uses
Citrullus colocynthis is well-known for its vast variety of traditional medical applications, including diabetes, asthma, gastrointestinal ailments, and other microbial infections. The fruit of a well-known bitter, acrid, cooling, cathartic, carminative, antipyretic, and anthelmintic, the fruits of Citrullus colocynthis are used to treat hypoglycemia, tumors, asthma, urethra, dyspepsia, constipation, splenomegaly and other. Medicinal plants contain a range of active compounds of great importance and therapeutic properties as most studies indicate the increasing activities of antimicrobial plants by detecting areas targeted by antibiotics by plant extracts against drug-resistant microbial pathogens [9]. Citrullus colocynthis is used widely in different parts of the world for the treatment of a number of diseases including diabetes, constipation, leprosy, asthma, bronchitis, jaundice, joint pain, cancer and mastitis. C.colocynth is an herb. The ripe fruit and seed are used as a medicine. Despite serious safety concerns, C.colocynth is used for diabetes, high cholesterol and blood fats called triglycerides, constipation, and tuberculosis. It is also used in combination products for treating liver and gallbladder ailments [10].
Methodology
Anticarcinogenic effect
The MTT assay is used to measure cellular metabolic activity as an indicator of cell viability, proliferation and cytotoxicity. This colorimetric assay is based on the reduction of a yellow tetrazolium salt (3-(4, 5-dimethylthiazol-2-yl) 2, 5 diphenyltetrazolium bromide or MTT) to purple formazan crystals by metabolically active cells. The viable cells contain NAD(P)H dependent oxidoreductase enzymes which reduce the MTT to formazan.36 The insoluble formazan crystals are dissolved using a solubilization solution and the resultingcolored solution is quantified by measuring absorbance at 500-600 nm using a multi-well spectrophotometer. The darker the solution, the greater the number of viable, metabolically active cells.
Preparation of Plant Extracts
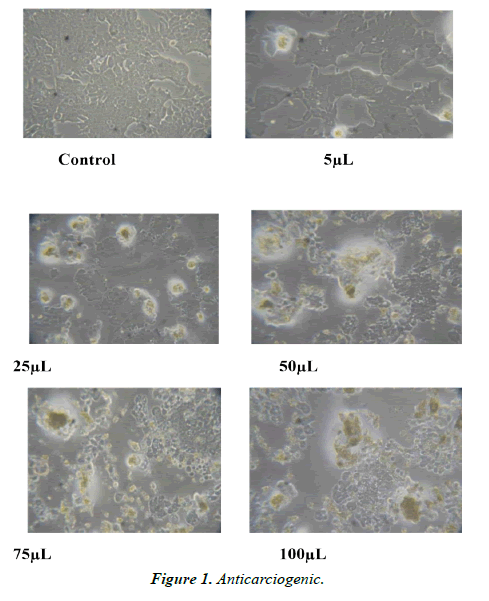
The plant materials were air dried at room temperature for about 10 days and grounded into powder. Dry powder was extracted with aqueous solution for 7 days at room temperature. Dry aqueous extracts were then dissolved in dimethyl sulphoxide (DMSO) to obtain appropriate solutions of the extracts. (Figures 1-4).
In vitro Assay for Cytotoxic Activity
Cell line and culture medium
Cells were cultured in liquid medium (RPMI1640) supplemented 10% Fetal Bovine Serum (FBS), 100 μ/ml penicillin and 100 μg/ml streptomycin, and maintained under an atmosphere of 5% CO2 and 95% air at 37°C. For testing, cells were washed with phosphate buffer saline (PBS) and harvested by Trypsinization and were plated in 96 well plates (one cells/well) and incubated under 5% CO2 and 95% air at 37°C for 24 hours. The cells were treated with different concentrations of plants extracts including 5, 25, 50, 75, 100 and 10 mg/ml. Dilution of stock solutions was made in culture medium yielding final extracts concentrations with a final DMSO concentration of 0.1%. This concentration of DMSO did not affect cell viability. Control cells were incubated in culture medium only. All concentrations of plants extracts were in triplicates on the same cell batch (Tables 1-4).
| Test Details | |
|---|---|
| Source of cell line | NCCS; Pune |
| Culture Media | MEM mediam supplemented with fetal bovine serum |
| Assay method | MTT method |
| Reagent | MTT solution (1 mg/ ml), DEMSO 100%, PBS |
| Incubation | 37 with 5% Co2 |
| Incubation of cells with MTT | 3-4 hours |
| Absorbance | 570 nm |
| Imaging | Inverted Phase Contrast Microscope |
Table 1. Materials and test details.
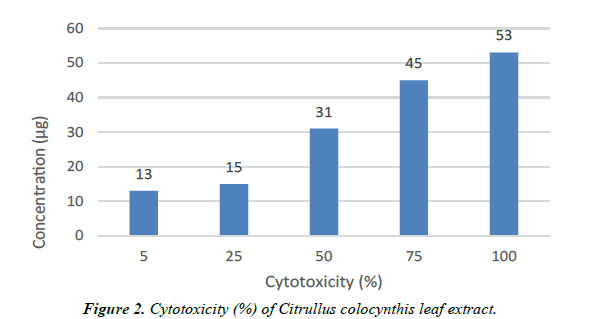
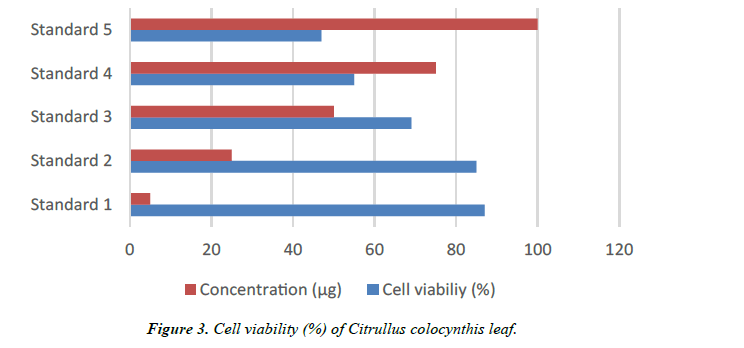
| Concentration (µg) | Cytotoxicity (%) | Cell viability (%) | Reactivity |
|---|---|---|---|
| 5 | 13 | 87 | Slight |
| 25 | 15 | 85 | Slight |
| 50 | 31 | 69 | Mild |
| 75 | 45 | 55 | Mild |
| 100 | 53 | 47 | Moderate |
Table 2. Anticarcinogenic activity.
| Organism used | Escherichia coli | ||||
|---|---|---|---|---|---|
| Dilution Volume | 20 µl | 40 µl | 60 µl | 80 µl | 100 µl |
| After 24 hours Incubation | Growth observed | Growth observe d | Growth observed | Growth observed | Growth observed |
| Zone of Diffusion | No Zone Observed | No Zone Observed | No Zone Observed | No Zone Observed | No Zone Observed |
| Diameter Zone | - | - | 5mm | 7mm | 10mm |
| Organism used | Pseudomonas salmonella | ||||
| Dilution Volume | 20 µl | 40 µl | 60 µl | 80 µl | 100 µl |
| After 24 hours Incubation | Growth observed | Growth observed | Growth observed | Growth observed | Growth observed |
| Diameter Zone | - | - | Zone | Zone | |
| Zone of Diffusion | No Zone Observed | No Zone Observed | 3mm Observed | Observed 5mm | Observed 7mm |
| Organism used | Staphylococcus aureus | ||||
| Dilution Volume | 20 µl | 40 µl | 60 µl | 80 µl | 100 µl |
| After 24 hours Incubation | Growth observed | Growth observed | Growth observed | Growth observed | Growth observed |
| Zone of Diffusion | No Zone Observed | Zone Observed | Zone Observed | Zone Observed | Zone Observed |
| Diameter Zone | - | 5 mm | 9 mm | 12 mm | 14 mm |
| Organism used | Bacillus subtilis | ||||
| Dilution Volume | 20 µl | 40 µl | 60 µl | 80 µl | 100 µl |
| After 24 hours Incubation | Growth observed | Growth observed | Growth observed | Growth observed | Growth observed |
| Zone of Diffusion | Zone Observed | Zone Observed | Zone Observed | Zone Observed | Zone Observed |
| Diameter Zone | 2 mm | 4 mm | 8 mm | 10 mm | 15 mm |
Table 3. Antimicrobial activity.
| SI NO | PEAK VALUE | COMPOUND NAME | FORMULA |
|---|---|---|---|
| 1 | 6.0773 | 5-Amino-2-methyl-2H-tetrazole | C2H5N5 |
| 2 | 7.0329 | Benz aldehyde | C7H6O |
| 3 | 7.7099 | Tetraethyl silicate | C8H20O4Si |
| 4 | 18.225 | Ethanamine,2,2’-azobis[ N,N,1,1,2,2-hexafluoro- | C4F12N4 |
| 5 | 1.3927 | 2,4- Imidazolidinedione,5- methyl-5-methyl-1H-Phrazole,4- nitro | C6H10N2O2 |
| 6 | 4.83895 | DL-Lactamide,N,O-diprophyl- | C9H19NO2 |
| 7 | 6.010048 | Decane | C10H22 |
| 8 | 1.99244 | Benzenemethanamine,N,N’-[(4 methylcyclohexlidene)di-4,1- phenylene]bis[4-methyl –N- (methylphenyl)- | C47H48N2 |
| 9 | 3.76352 | Linalool | C10H18O |
| 10 | 9.44475 | 1,4-cyclohexadine,1-methyl- | C7H10 |
| 11 | 4.00011 | 1-Propene,3-chloro-1,1,2,2,3- pentafluoro | C3CIF5 |
| 12 | 10.00309 | Phosphorichloricacid, biphenyl ester | C10H22ClO3P |
| 13 | 17.32234 | Benzene,(2-nitroprophyl)- | C9H11NO2 |
| 14 | 7.11533 | Aspirin | C9H8O4 |
| 15 | 1.35676 | Propanedintitrile, 2-[2,6-vis[2- [4- | C16H21NO |
| (diphenylamine)phenyl]ethanyl]- 4Hpyran-4-ylidene]- | |||
| 16 | 14.4734 | 6H-purin-6-one,1,7-dihydro- | C5H4NO |
| 17 | 15.9578 | 2-Flurobenzoic acid, 2- | C9H8CIFO2 |
| chloroethyl ester | |||
| 18 | 16.8408 | 4,5-Dimethyl-2-isobutyloxzole | C9H15NO |
| 19 | 16.91238 | 9H-Purine | C5H4N4 |
| 20 | 19.33453 | 3-Buten-1one,1-(4- methyl phenyl)- | C11H12O |
| 21 | 19.88789 | Benezene,1,1’,1’’’-(1,2- | C34H36O4 |
| ethenediylidene)tetrakis[4- ethoxy- | |||
| 22 | 20.21921 | 1H-Pyrazole-4,5-dione,3-amino- | C9H9N5O |
| ,4-phenylhydrazone | |||
| 23 | 21.17334 | Benzamide,2,4-dimethyl-N- isobuty- | C13H19NO |
| 24 | 21.20135 | O-Phenylenedimethylene- bis(diethyldithiocarbamate) | C15H26O |
| 25 | 21.9818 | 4-Isoprophyl-6-methyl-1- methylene-1,2,3,4- tetrahydronaphalene | C15H20 |
| 26 | 23.8023 | 2-Fluancarboxylic acid,3- fluorophenyl ester | C11H7FO3 |
| 27 | 26.1539 | -Valine,n-propargyloxycarbonyl- ,dodecyl ester | C21H37NO4 |
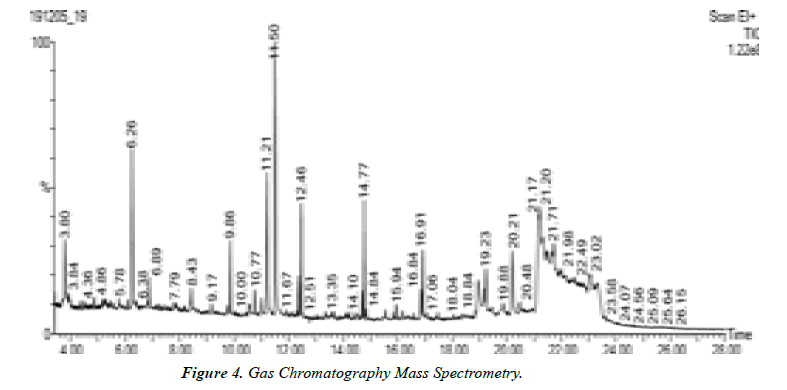
Table 4. Peak values of different compounds.
MTT assay
Growth of tumoral cells quantitated by the ability of living cells to reduce the yellow dye 3(4, 5 dimethyl-2-thiazolyl)-2,5- diphenyl-2H-terazolium bromide (MTT) to a blue formazan product. At the end of 72 hours incubation, the medium in each well was replaced by MTT solution (20 cell/well, 5 mg/ ml in phosphate-buffered saline), the plates were incubated for 4 hours under 5% CO2 and 95% air at 37ºC. MTT reagent was removed and the formazan crystals produced by viable cells were dissolved in 100 DMSO and gently shake.
Reagents
RPMI 1640 medium, Fetal bovine serum (FBS), 3-(4, 5dimethylthiazol-2-yl)-2, 5diphenyltetrazolium bromide (MTT), Phosphate Buffer Saline (PBS), Trypsin, Ethylene Diamine Tetra Acetic Acid (EDTA), antibiotic, and dimethylsulfoxide (DMSO) were purchased from Sigma.
Procedure
An anticancer test method is performed for the given test samples by selecting a culture flask with 80- 90% confluences. After trysinization and centrifugation the cancer cells are seeded in the well plate for 24 hrs at 37±1°C incubation to form a monolayer. The culture medium from the cancer cells is replaced with fresh medium. Test sample at different concentration in triplicates were added to the cells. After the incubation of sample with cells at 37±1°C for 1824 h, MTT (1 mg/ml) were added in all the wells and incubated for 4 h. After incubation, DMSO is added in the wells and read at 570 nm using photometer.
Antimicrobial Activity
Preparation of extracts
100 gm of fresh leaves of Citrullus colocynthis were dried in shade and ground. 10 gm of powder was used for extraction. Extracts were prepared separately and successively using Soxhlet apparatus with different solvents namely ethanol, chloroform, water and petroleum ether. The extracts were concentrated to dryness and the residue was dissolved in 10 ml of respective solvents and used for the assay of antibacterial activity.
Test organisms
Both gram positive and gram-negative microorganisms were used for the test. The gram-positive organisms used were Staphylococcus aureus and Bacillus subtilis. The gramnegative organisms used were Escherichia coli, Pseudomonas aeruginosa. All the bacterial strains were maintained on freshly prepared Muller Hinton agar (Hi-media) slant and stored. The bacterial strains were procured from Sri Shakthi Food Testing Laboratory, Mettupalayam Road, Thudiyalur, Coimbatore, Tamil Nadu.
Screening of antibacterial effects
Antibacterial effect was determined by disc diffusion method (Bauer et al.,). Muller Hinton agar were inoculated with 0.2 ml bacterial suspension of overnight culture of each bacterium and uniformly spread out. Then the disc was placed on the upper layer of the seeded agar plates. Triplicates were maintained. The plates were incubated at 37°C for 24 hours. The inhibition zones were measured and recorded. Positive control was used by standard antibiotic Gentamycin (0.2ml/ml) and negative controls were maintained by the respective solvents.
Procedure
1. Allowed plates to settle to room temperature before use. 2. Dipped a sterile cotton swab into the bacterial suspension to remove excess liquid, rotated the swab several times with a firm pressure on the inside wall of the tube above the fluid level. 3. Using the swab streaked the Mueller-Hinton agar plate to form a bacterial lawn. 4. To obtain uniform growth, streaked the plate with the swab in one direction, rotated the plate 90° and streak the plate again in that direction. 5. Repeated this rotation 3 times. 6. Allowed the plate to dry for approximately 5 minutes at room temperature. 7. Placed the respective culture with concentration of Citrullus colocynthis leaf Extract, Extract (20 μL, 40 μL, 60 μL, 80 μL, 100 μL) by Disc Diffusion Method. 8. Plates were incubated for 24 hrs. At an incubation temperature of 37°C. 9. Measured the zone sizes, in millimeters using a ruler or template.
Results
Anticarcinogenic, Antimicrobial and broad-spectrum analysis of aqueous leaf extract of Citrullu colocynthis In vitro
Antimicrobial Activity
Test organisms
Eschericia coli: MTCC 452, Pseudomonas salmonella: MTCC 1688 Staphylococcus aureus: MTCC 096, Bacillus subtilis MTCC 441.
Test Conditions
Incubation Temp & Period: 37°C - 24 hours (Bacteria)
Interpretation of GC-MS chromatogram
X-axis of GC-MS chromatogram shows amount of time taken for analytes pass through column, and reach mass spectrometer detector. The peaks that are shown correspond to the time at which each of components reached the detector. The y-axis or area of peaks is a Reflection of amount of analyte. When looking at GC-MS chromatogram, area will be based on the number of counts taken by Mass spectrometer detector at retention point. Unknown compounds are identified based on Retention times of known standards with other detectors. The mass spectrometer then allows Identification of compound by mass spectrum obtained at time of testing.
Gas chromatography equipment can be directly interfaced with rapid scan mass spectrometer of various types. The flow rate from capillary column in generally low enough that the column output can be fed directly into ionization chamber of MS. The simplest mass detector in GC is the Ion Trap Detector (ITD). In this instrument, ions are created from the eluted sample by electron impact or chemical ionization and stored in a radio frequency filed; the trapped ions are then ejected from the storage area to electron multiplier detector. The ejection is controlled so that scanning on the basis of mass-to charge ratio is possible. The ion trap detector is remarkably compact and less expensive than quadrapole instruments. GC MS instrument have been used for identification of hundreds of components that are present in natural and biological system (Oleszek & Marston, 2000; Philipson, 2007; Daffre et al., 2008).
Conclusion
The overall results of the study prove that aqueous leaf extract of Citrullus colocynthis has imbibed predominant anticarcinogenic activity. The plant extract proves to have promising anticancer potential by inhibiting the MCF-7 breast cancer cell line profound antibacterial activity on the multidrug resistant strain Staphylococcus aureus, Escherichia coli, and Pseudomonas salmonella Bacillus subtilis. The spectrum analysis of the aqueous leaf extract of Citrullus colocynthis showed the presence of many compounds which are responsible for the anticarcinogenic activity. However, the search for new lead compounds from natural sources with more effectivity and less toxicity would constitute an interesting alternative for the development of anticancer drugs in breast cancer treatment.
References
- NACCL-27 Proceedings V.1, Tao et al. 2015, November 2015Publisher: UCLA and Ohio State University
- Neil-Sztramko SE, Winters-Stone KM, Bland KA, et al. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br J Sports Med. 2019;53(8):504-12.
- Cabrales P, Zanini GM, Meays D, et al. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis. 2011;203(10):1454-63.
- Hussein MA, El-Gizawy HA, Gobba NA, et al. Synthesis of cinnamyl and caffeoyl derivatives of Cucurbitacin-Eglycoside Isolated from Citrullus colocynthis fruits and their structures antioxidant and anti-inflammatory activities relationship. Curr Pharm Biotechnol. 2017;18(8):677-93.
- Al-Snafi AE. Chemical constituents and pharmacological effects of Citrullus colocynthis-A review. IOSR J Pharmacy. 2016;6(3):57-67.
- Alhawiti NM. Antiplatelets and profibrinolytic activity of Citrullus colocynthis in control and high-fat diet-induced obese rats: mechanisms of action. Arch Physiol Biochem. 2018;124(2):156-66.
- Dhakad PK, Sharma PK, Kumar S. A review on phytochemical studies and biological potential of Citrullus colocynthis (L.) Schrad (Cucurbitaceae). J Bioeng Biosci. 2017;5(4):55-64.
- Chen Y, Sa Y, Wang G, et al. The protective effects of citrullus colocynthis on inhibiting oxidative damage and autophagy-associated cell death in Parkinson's disease. Journal of the Taiwan Institute of Chemical Engineers. 2019;100:18-25.
- Abdulridha MK, Al-Marzoqi AH, Ghasemian A. The anticancer efficiency of Citrullus colocynthis toward the colorectal cancer therapy. J Gastrointest Cancer. 2020;51(2):439-44.
- Chawech R, Njeh F, Hamed N, et al. A study of the molluscicidal and larvicidal activities of Citrullus colocynthis (L.) leaf extract and its main cucurbitacins against the mollusc Galba truncatula, intermediate host of Fasciola hepatica. Pest management science. 2017;73(7):1473-7.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref