Review Article - Archives of Digestive Disorders (2017) Archives of Digestive Disorders (Special Issue 2-2017)
A new look of gastric cancer from epidemiology to clinical management.
MX Li1, J Shen1, ZG Xiao1, L Zhang1, X Wu1, CH Cho1,2*1Laboratory of Molecular Pharmacology, Department of Pharmacology, School of Pharmacy, The Southwest Medical University, Sichuan
2School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, China
- *Corresponding Author:
- Prof. CH Cho
School Biomedical Sciences
Faculty of Medicine
The Chinese University of Hong Kong
Shatin, New Territories
Hong Kong
China
Tel: +852-3943 6886
Fax: +852-2603 5139
E-mail: chcho@cuhk.edu.hk
Accepted Date: April 29, 2017
Citation: MX Li, J Shen, ZG Xiao, et al. A new look of gastric cancer from epidemiology to clinical management. Arch Dig Disord. 2017;1(2):4-7.
Abstract
Gastric cancer (GC) is the fourth most common cancer and the third leading cause of cancerrelated death worldwide. Recent advance in diagnosis and treatment has improved longterm survival for patients with early GC, yet the survival rate for advanced cancer patients is still poor. This article reviews the current epidemiology and different risk factors, such as environmental challenges, gene polymorphism and epigenetic profiles. It also introduces the clinical management against these risk factors and repurposes the new clinical application of vitamin D for the prevention and treatment of GC in humans.
Keywords
Gastric cancer, Vitamin D, Helicobacter pylori, Cigarette smoking
Introduction
Epidemiology of gastric cancer
Gastric cancer (GC) is the third leading cause of malignancyrelated death throughout the world. The mortality rate of gastric cancer is 8.9% after lung cancer (19.7%) and breast cancer (12.9%). The incidence of gastric cancer accounts for the fifth place in malignant tumors [1]. There is a marked geographic variation in the gastric cancer incidence rate. It is highest in East Asia including China and Japan, up to nearly 75%, while one of the lowest rates is in the United States [2] The GC cases in China account for nearly half (47%) of all the gastric cancer new cases throughout the world. The incidence also differs remarkably between genders. In the world, the gastric cancer occurs in man versus woman in a ratio up to 2:1 [1].
Cancer risk factors
Epidemiologic studies have indicated that the risk factors of GC are strongly associated with dietary factors, such as high salt or nitrates intake, and low intake of fresh fruits and vegetables [3]. There are remarkable differences in the prevalence of GC in different countries and a large number of epidemiological studies implicate that Asian countries account for nearly 75% of worldwide GC incidences, which might be due to high dietary salt intake or habitual consumption of salted foods, in additional to the major risk factor Helicobacter pylori (H. pylori) infection [4]. A case-control study also showed that higher dietary salt exposure was directly associated with increased GC risk and the association was regardless of H. pylori infection status and virulence, smoking, tumor site or histological types [5]. This evidence is further supported by a number of meta-analysis and case-control studies. They found that salted food intake was directly associated with GC risk in China and Japan [6].
H. pylori infection is one of the main predisposing factors for GC development and accounts for nearly 80% of GC worldwide. Increasing evidences indicated that H. pylori played an important role in gastric pathogenesis through dysregulation of autophagy [7]. On the other hand, it was reported that high salted exposure increased gastric inflammation and exasperated H. pylori infection-induced gastric tumorigenesis [8]. A few studies also suggested a possible causal link between excess salt intake, H. pylori infection, and gastric carcinogenesis, which may explain the higher prevalence of H. pylori infection in Japan than in other industrialized countries.
There are also some other risk factors such as smoking and alcohol drinking. A multiethnic cohort study involved in over 215,000 people revealed an association between smoking and GC risk [9]. Also our previous studies had found that cigarette smoking inhibited gastric ulcer healing and promoted gastric cancer growth, indicating the potential role of smoking in gastric carcinogenesis [10-12]. However, whether the association between alcohol drinking and GC risk is still an open question. A meta-analysis found no significant association between moderate alcohol assumption and GC risk but heavy alcohol drinking was positively correlated with GC risk [13].
Gene polymorphism or epigenetic profiles may also be associated with GC risk. A case-control study analyzed that gene variant rs2016167 of peroxisome proliferator-activated receptor δ (PPAR δ) was strongly associated with GC [14]. In addition, interleukin-1 (IL-1) polymorphism increased the risk of H. pylori-induced hypochlorhydria and might play a determinate role in GC with H. pylori infection [15]. Besides, microRNAs are putatively considered as tumor suppressors or pro-oncogenes and dysregulation of microRNAs might contribute to gastric tumorigenesis [16].
Prevention and Treatment of Gastric Cancer
Regarding prevention of GC, reducing the salt intake or having fresh food and vegetable may reduce the cancer risk. Considering the role of H. pylori in the gastritis-related carcinogenesis, eradication of H. pylori has the potential for the prevention of GC. Proton pump inhibitors (PPIs) are important for H. pylori elimination, because they inhibit gastric acid secretion and maintain a more favorable environment for acid-labile antibiotics. For the guidelines on the management of H. pylori in Europe, triple therapy using a PPI with clarithromycin and amoxicillin or metronidazole given twice daily remains the recommended first choice of drug treatment [17,18]. A population-based H. pylori eradication program which was recently launched in Taiwan demonstrating the reduction of the incidence of GC, was associated with the mass eradication of H. pylori infection [19].
For patients already diagnosed with GC, gastrectomy remains the mainstay of treatment. Endoscopic mucosal resection (EMR) is widely used for treatment with cases of early GC without lymph node metastasis in Japan [20]. Neo-adjuvant and adjuvant chemotherapy could also improve the clinical outcomes. However, drug resistance, tumor recurrence and metastasis are commonly observed in GC patients, thereby reducing the overall survival of these patients. Thus, it is significant and timely to develop therapeutic agents with unique mechanisms of action different from the existing anticancer drugs for GC treatment.
Repurposing vitamin D for gastric cancer
Development of a new class of drugs currently used in humans together with a blank new anti-cancer mechanism different from existing chemotherapeutic agents is the ultimate goal for cancer treatment. Amble studies have indicated that vitamin D3 status in serum might correlate with many types of cancer risk. In addition, patients with low vitamin D levels were diagnosed with poorer survival indicating that vitamin D might protect individuals against the late stages of carcinogenesis. Indeed epidemiological studies on colon cancer provided strong support for inverse association between serum 25-hydroxyvitamin D level and colon cancer risk in both men and women and a dose dependent correlation of calcium supplement with colon cancer incidence in women was also observed [21-23]. In a 197 gastric cancer patients study conducted in the cancer center of Sun Yatsen University demonstrated that vitamin D deficiency might be correlated with the poor prognosis of gastric cancer [24]. However the relationship between vitamin D supplement and gastric cancer risk is still unsubstantial and the prognostic role of serum 25-hydroxyvitamin D concentration is still needed to be further analyzed.
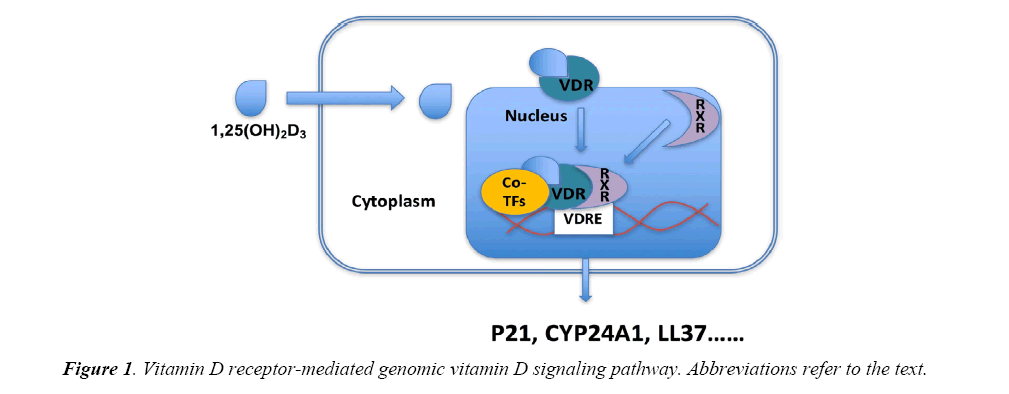
Regarding the biological actions of 1,25(OH)2D3, they are mediated by vitamin D receptor (VDR), via genomic actions and nongenomic actions. Higher VDR expression may be associated with enhanced biological response of 1,25(OH)2D3. The genomic actions mediated by VDR are involved in a number of target genes transcription. VDR when activated by 1,25(OH)2D3 will translocate into nuclear and combine with the retinoid X receptor (RXR) as well as other co-transcriptional factors, thereby activating target genes transcription through interaction with the vitamin D response element (VDRE) located in the promoter of the target genes. A number of genes have been identified as VDR target genes including CYP24A1, p21, cathelicidin (LL37) and involved in different signaling pathways, exerting the action of 1,25(OH)2D3 (Figure 1). 1,25(OH)2D3 can also initiate rapid signaling transduction pathway, such as G proteincoupled receptors, phosphatidylinositol-3-kinase (PI3K), and protein kinase C (PKC), eliciting biological responses through activation of second messengers [25].
Indeed accumulating evidences suggested that vitamin D deficiency was correlated with high cancer risk, implicating the anticancer effect of vitamin D. Consistently, the antitumor activities of 1,25(OH)2D3 have been widely studied in many kinds of cancer. It has antitumor actions through various functional pathways, including those on cell cycle, apoptosis and differentiation and further on angiogenesis in tumors through unique mechanisms of action unlike those of traditional anti-cancer drugs [26]. All these evidences suggest that vitamin D could be an effective alternative for cancer treatment in stomachs and unfold new approaches in developing potential useful therapeutic agent for digestive disorders [27,28].
Discussion and Conclusion
The epidemiology and the different risk factors, such as environmental challenges, gene polymorphism and epigenetic profiles are largely discussed in this review. Although the anticancer effect of vitamin D on cancer cells has been extensively reported, the pharmacological action of vitamin D on cancer stem cells (CSCs) has not been studied. It was only reported that vitamin D analogue EB1809 could induce apoptosis in the subpopulation of gastric cancer cells SGC7901 [29]. In addition to the inhibitory action on gastric cancer cells in vitro [30], there is no doubt that understanding the action of vitamin D on gastric CSCs has even more clinical implications in the development of vitamin D in cancer treatment and perhaps also prevention of the disease. Indeed, a review has been published recently regarding its possible modes of action on gastrointestinal cancers [31]. As vitamin D3 is a clinically used drug for bone disorders, it should have a great potential to be applied as a prophylactic treatment for gastric cancer or other cancers in the gastrointestinal tract.
Acknowledgement
This work was supported by the Health and Medical Research Fund (13120062) from the Food & Health Bureau and the General Research Fund (CUHK463613) from the Research Grant Council of Hong Kong and also the start-up fund from the Southwest Medical University and the National Natural Science Foundation (81473269) from China.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354-362.
- Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10(2):75-83.
- Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109(1):138-143.
- Peleteiro B, Lopes C, Figueiredo C, et al. Salt intake and gastric cancer risk according to Helicobacter pylori infection, smoking, tumour site and histological type. Br J Cancer. 2011;104(1):198-207.
- D'Elia L, Rossi G, Ippolito R, et al. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31(4):489-498.
- Zhang L, Sung JJ, Yu J, et al. Xenophagy in Helicobacter pylori- and Epstein-Barr virus-induced gastric cancer. J Pathol. 2014;233(2):103-112.
- Gaddy JA, Radin JN, Loh JT, et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81(6):2258-2267.
- Nomura AM, Wilkens LR, Henderson BE, et al. The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes Control. 2012;23(1):51-58.
- Ma L, Wang WP, Chow JY, et al. The role of polyamines in gastric mucus synthesis inhibited by cigarette smoke or its extract. Gut. 2000;47(2):170-177.
- Shin VY, Wu WK, Ye YN, et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25(12):2487-2495.
- Shin VY, Wu WKK, Koo Chu KM, et al. Functional role of β-adrenergic receptor in the mitogenic action of nicotine on gastric cancer cells. Tox Sci. 2007;96:21-29.
- Tramacere I, Negri E, Pelucchi C, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28-36.
- Jeon C, Chang SC, Mu L, et al. Genetic variants of peroxisome proliferator-activated receptor delta are associated with gastric cancer. Dig Dis Sci. 2013;58(10):2881-2886.
- El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398-402.
- Shen J, Xiao Z, Wu WK, et al. Epigenetic silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1 to promote Helicobacter pylori-induced gastric carcinogenesis. Cancer Res. 2015;75(4):754-765.
- Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56(6),772-781.
- Malfertheiner P, Megraud F, O'Morain C, et al. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9(1):1-2.
- Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62(5):676-682.
- Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48(2):225-229.
- Garland C, Shekelle RB, Barrett-Connor E, et al. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307-309.
- Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989;2:1176-1178.
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians' Health Study and a meta-analysis of prospective studies. Cancer Prev Res (Phila). 2011;4(5):735-743.
- Ren C, Qiu MZ, Wang DS, et al. Prognostic effects of 25-hydroxyvitamin D levels in gastric cancer. J Transl Med. 2012;10(16).
- Haussler MR, Jurutka PW, Mizwicki M, et al. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25(4):543-559.
- Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr Med Res Opin. 2008;24(1):139-149.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
- Yang WG, Chen CB, Wang ZX, et al. A case-control study on the relationship between salt intake and salty taste and risk of gastric cancer. World J Gastroenterol. 2011;17(15):2049-2053.
- Wang W, Zhao CH, Zhang N, et al. Vitamin D analog EB1089 induces apoptosis in a subpopulation of SGC-7901 gastric cancer cells through a mitochondrial-dependent apoptotic pathway. Nutr Cancer. 2013;65(7):1067-1075.
- Li MX, Li LF, Zhang L, et al. Vitamin D and cancer stem cells in the gastrointestinal tract. Curr Med Chem. 2017;14.
- Li MX, Li LF, Zhang L, et al. 1,25-Dihydroxyvitamin D3 suppresses cancer growth through VDR and mutant p53 in the induction of p21 expression in gastric cancer cells. Life Sci. 2017.