Research Paper - Journal of Chemical Technology and Applications (2018) Volume 2, Issue 1
A compact, algebraic formulation of disproportionation and symproportionation in Bromine systems.
- *Corresponding Author:
- Tadeusz Micha?owski
Department of Oncology The University Hospital in Cracow 31-501 Cracow, Poland
Tel: +212667845404
E-mail: michalot@o2.pl
Accepted date: July 03, 2018
Citation: Michalowska-Kaczmarczyk AM, Michalowski T. A compact, algebraic formulation of disproportionation and symproportionation in Bromine systems. J Chem Tech App. 2018;2(2):1-14.
Abstract
Numerous examples of disproportionation and symproportionation of bromine in dynamic redox systems are resolved according to GATES/GEB principles and represented graphically by the functions E = E(Φ) and pH = pH(Φ) of the fraction titrated Φ, and completed by dynamic speciation diagrams log zi ( ) i i log ??X ?? = ? Φ , for different species zi i X . The results of calculations can be considered from the viewpoint of relative efficiency of the competing reactions, on different steps of the titration procedure. The idea of linear combination of the balances related to electrolytic systems (aqueous media) is presented in context of GEB formulation GEB according to Approach II. Oxidation number, oxidant, reductant, are perceived as derivative (not primary) concepts within GATES/GEB. The equivalency of Approaches I and II to GEB is also proved.
Keywords
Thermodynamics of electrolytic redox systems, GEB, GATES/GEB, disproportionation, symproportionation, Potentiometric titration.
Notations: GATES: Generalized Approach to Electrolytic Systems; GEB: Generalized Electron Balance; ON: Oxidation Number
Introduction
Disproportionation [1] and symproportionation [2] are two mutually opposite phenomena classified as redox reactions. We will focus here our interest on aqueous solutions as electrolytic redox systems, of which physicochemical knowledge is relatively extensive.
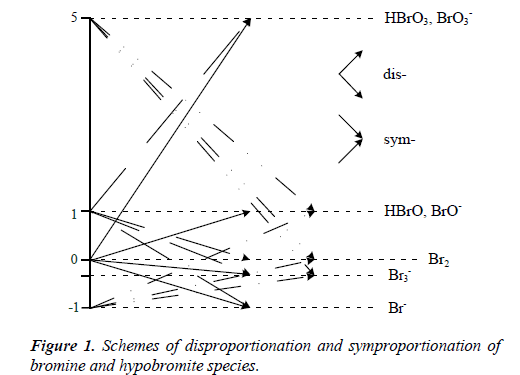
Disproportionation is a special type of redox reaction, where an element on an intermediate oxidation number (ON) in a species is transformed – simultaneously – to the species with lower and higher ONs of this element. It means that this element must be able to form the species with at least three different oxidation numbers (ONs). For example, bromine forms the species with five ONs (–1, –1/3, 0, 1, 5) (Figure 1). In Br2 and BrO-1, bromine has intermediate ONs: 0 and 1, resp. In particular, the disproportionation of Br2, affected by OH-1 ions, can be written as follows [3]:
3Br2 + 6OH-1 = BrO3-1 + 5Br-1 + 3H2O (1a)
Br2 + 2OH-1 = BrO-1 + Br-1 + H2O (1b)
etc. In the symproportionation, two reactants containing the same element, here: Br with different ONs, react with formation of the species on intermediate ONs of this element (Figure 1). For example, symproportionation of BrO3-1 and Br-1, affected by H+1 ions, can be written as follows:
BrO3-1 + 5Br-1 + 6H+1 = 3Br2 + 3H2O (2a)
BrO3-1 + 8Br-1 + 6H+1 = 3Br3-1 + 3H2O 2(b)
etc.
A remark. The disproportionation reactions in biological systems are termed as dismutation, when associated with superoxide dismutases (SODs) – the enzymes catalysing a dismutation of toxic superoxide (O2−1) radical [4]. In French, the term dismutation refers also to non-biological systems [5]. Comproportionation [6] and synproportionation [7]), as the synonyms of symproportionation, are also found in literature.
The disproportionation may be affected by an action of the solvent, e.g. dilution with water, to which the bromine compound at an intermediate oxidation state, e.g. HBrO, has been introduced. The disproportionation effect can be greatly enhanced by the action of an acid or base. In some instances, it can also be stated that the disproportionating agent acts also as an oxidant or reductant [3]. In a particular case, namely in reaction Br2 + Br-1 = Br3-1, the symproportionation is indistinguishable from the complexation effect.
The redox systems are formulated, from thermodynamic viewpoint, according to Generalized Approach to Electrolytic Systems (GATES) [8,9] principles, formulated by Michałowski. For this purpose, the set of K algebraic equations, f0,f12,f3,…,fK, is formulated. It is composed of: charge balance (ChB, f0), the linear combination f12 = 2∙f2 – f1, of elemental balances: f1 = f(H) for H and f2 = f(O) for O, and K–2 elemental/core balances f(Yk) (k=3,…,K) for elements/cores Yk (≠ H, O). The f12 is the primary form of the Generalized Electron Balance (GEB), discovered by Michałowski, and formulated as the Approach II to GEB [3,8-24]. The GATES related to redox systems will be denoted as GATES/GEB. The GATES is related to redox and non-redox systems, and then GATES/GEB ⊂ GATES.
Another option is the Approach I to GEB [25-28], discovered by Michałowski, and considered later as the ‘short’ version of GEB. The Approach I to GEB is based on a ‘card game’ principle, with electron-active elements as ‘players’, electronnon- active elements as ‘fans’, and electrons as ‘money’ [22]. The equivalency of Approaches I and II to GEB will be proved, and then the balances for GEB be formulated for different systems according to the Approach I.
All attainable physicochemical knowledge can be involved in further, numerical calculations, realized with use of an iterative computer program. The results of calculations are presented graphically and discussed. The GATES/GEB is perceived as the best tool for thermodynamic resolution of electrolytic redox systems, according to algebraic principles.
Thermodynamic modelling of redox systems
Modelling of electrolytic redox systems according to GATES/
GEB principles is based on general laws of elements and charge
preservation, related to closed systems composed of condensed
phases, separated from the environment by diathermal walls. In
further discussion, we refer to redox systems formed in aqueous
media, where the species are perceived in their natural form,
as hydrates ![]() , where zi (zi = 0, ±1, ±2,…) is the external charge of
, where zi (zi = 0, ±1, ±2,…) is the external charge of  expressed in elementary charge unit e = F/NA (F –
Faraday constant, NA – Avogadro’s constant), and niW (≥ 0) is the
mean number of water (W = H2O) molecules attached to
expressed in elementary charge unit e = F/NA (F –
Faraday constant, NA – Avogadro’s constant), and niW (≥ 0) is the
mean number of water (W = H2O) molecules attached to 
Components and species: The terms: components and species
are distinguished. Components form a system, the species are
present in the system thus formed. A static system is obtained
after disposable mixing the components: H2O as solvent, and
solute(s). A dynamic D+T system is a result of addition of
titrant T into titrand D, in consecutive portions. The D and T
are composed separately before the titration, where the D+T
mixture is formed; the D and T are subsystems of the D+T
system. A volume V mL of T is added into V0 mL of D, up to a
given point of the titration, and V0+V mL of D+T mixture is thus
obtained, if the assumption of the volumes additivity is valid/
tolerable. In the notation applied here, N0j (j=1,2,…,J) is the
number of molecules of the component of j-th kind, including
water, forming D and T in dynamic D+T system. The D+T
system thus obtained involves N1 molecules of H2O ( 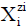 = H2O,
z1=0) and Ni species of i-th kind,
= H2O,
z1=0) and Ni species of i-th kind, ![]() (i=2,3,…,I), denoted
briefly as
(i=2,3,…,I), denoted
briefly as 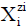 (Ni ,ni) where ni≡niW≡niH2O.
(Ni ,ni) where ni≡niW≡niH2O.
The known chemical formulas of 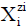 and their respective charges
provide the information necessary/sufficient to formulate the
respective balances, for elements or cores. A core is a cluster
of elements with defined composition, structure and external
charge that remains unchanged in a system considered.
and their respective charges
provide the information necessary/sufficient to formulate the
respective balances, for elements or cores. A core is a cluster
of elements with defined composition, structure and external
charge that remains unchanged in a system considered.
Formulation of balances: general remarks: It is advisable
to start the balancing from the interrelations between numbers
of particular entities: N0j for components represented by
molecules composing D and T, and Ni – for the species (ions
and molecules) of i-th kind (i = 1,…,I), where I is the number of
kinds of the species ![]() in D+T. The mono- or two-phase
electrolytic D+T system thus obtained involve N1 molecules of
H2O and Ni species of i-th kind,
in D+T. The mono- or two-phase
electrolytic D+T system thus obtained involve N1 molecules of
H2O and Ni species of i-th kind, ![]() (i=2,3,…,I), specified
briefly as
(i=2,3,…,I), specified
briefly as 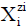 (Ni ,ni) ,where ni ≡niW ≡niH2O is the mean number
of hydrating water molecules (W=H2O) attached to
(Ni ,ni) ,where ni ≡niW ≡niH2O is the mean number
of hydrating water molecules (W=H2O) attached to 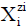 .The net charge of
.The net charge of ![]() equals to the charge of
equals to the charge of 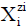 , i.e., zi + niW∙0 = zi.For ordering purposes, we write the sequence: H+1 (N2, n2), OH-1 (N3, n3), … , i.e., z2 = 1, z3 = –1, … . The
, i.e., zi + niW∙0 = zi.For ordering purposes, we write the sequence: H+1 (N2, n2), OH-1 (N3, n3), … , i.e., z2 = 1, z3 = –1, … . The 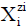 's, with different
numbers of H2O molecules involved in
's, with different
numbers of H2O molecules involved in ![]() , e.g. {H+1, H3O+1,H9O4+1}, {H4IO6-1, IO4-1} are considered equivalently, i.e., as the
same species in this medium. The charge of a species
, e.g. {H+1, H3O+1,H9O4+1}, {H4IO6-1, IO4-1} are considered equivalently, i.e., as the
same species in this medium. The charge of a species ![]() , expressed in elementary charge units, results from the numbers
of protons in nuclei, and orbital electrons in atoms composing
the species.
, expressed in elementary charge units, results from the numbers
of protons in nuclei, and orbital electrons in atoms composing
the species.
Presentation of the species in natural forms in aqueous media,
i.e., as ![]() , has several advantages. This way, after linear
combinations of the related balances, one can discover some
regularities hidden earlier by notation of the species in the
form
, has several advantages. This way, after linear
combinations of the related balances, one can discover some
regularities hidden earlier by notation of the species in the
form 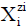 This notation can be extended on electrolytic systems
in mixed-solvent As (s=1,…,S) media, where mixed solvates
This notation can be extended on electrolytic systems
in mixed-solvent As (s=1,…,S) media, where mixed solvates 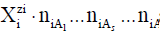 are assumed, and
are assumed, and 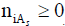 is the mean numbers
of As (s=1,…,S) molecules attached to
is the mean numbers
of As (s=1,…,S) molecules attached to 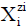 [15,21,29]. In other
instances (reaction notation), the common/simpler notation
[15,21,29]. In other
instances (reaction notation), the common/simpler notation 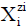 of the species, e.g. HSO4-1∙ n4H2O as HSO4-1, will be practiced. Molar concentrations [mol/L] of the species be
denoted as
of the species, e.g. HSO4-1∙ n4H2O as HSO4-1, will be practiced. Molar concentrations [mol/L] of the species be
denoted as 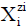 , for brevity.
, for brevity.
The notation ![]() of the species is useful on the step of
formulation of the related balances: charge balance (f0 = ChB) and elemental balances, fk = f(Yk), in the system where K
elements Yk (k=1,…,K) are involved. The ChB expresses the
electroneutrality of the electrolytic system, whereas the f(Yk),
(k=1,…,K) express the conservation of all the elements in the
closed system, chosen for modelling purposes. For simplicity/
uniformity of notation, we assume the sequence: f1 = f(H), f2 = f(O),… ,fk= f(Yk).
of the species is useful on the step of
formulation of the related balances: charge balance (f0 = ChB) and elemental balances, fk = f(Yk), in the system where K
elements Yk (k=1,…,K) are involved. The ChB expresses the
electroneutrality of the electrolytic system, whereas the f(Yk),
(k=1,…,K) express the conservation of all the elements in the
closed system, chosen for modelling purposes. For simplicity/
uniformity of notation, we assume the sequence: f1 = f(H), f2 = f(O),… ,fk= f(Yk).
The charged/ionic species ![]() of the system, i.e., the species
with zi ≠ 0 (zi > 0 for cations, zi < 0 for anions), are involved in
the charge balance
of the system, i.e., the species
with zi ≠ 0 (zi > 0 for cations, zi < 0 for anions), are involved in
the charge balance
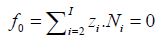 (3)
(3)
Free water particles, and water bound in the hydrates ![]() are included in the balances: f1 = f(H), f2 = f(O):
are included in the balances: f1 = f(H), f2 = f(O):
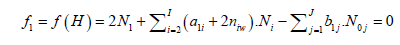 (4)
(4)
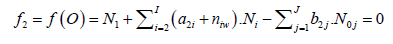 (5)
(5)
Then the balance
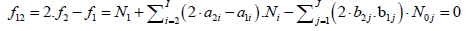 (6)
(6)
is formulated.
The elemental balances: f3,...,fK , interrelating the numbers of atoms Yk ≠ H, O in components and species, are as follows
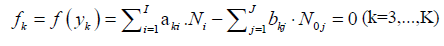 (7)
(7)
where aki and bkj in equations 4, 5 and 7 are the numbers of
element Yk (k=1,...,K) in ![]() , and in the j-th component of
the system, resp.
, and in the j-th component of
the system, resp.
The linear combination
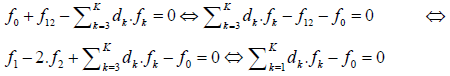 (8)
(8)
involves K balances: f0, f12, f3 ,…,fK. In particular, d1 = +1, d2 = –2. As will be indicated below, when the multipliers dk are equal to (or involved with) the oxidation numbers (ON’s) of the corresponding elements Ek (k=1,…,K) in a redox system, we get the simplest (most desired) form of the related linear combination (eq. 8), as will be explained on the example presented in section 3.5.
In eq. 6 and then in eq. 8, the terms involved with water, i.e., N1, N0j (for j related to H2O as the component), and all ni = niW are not involved. The necessity of prior knowledge of niW values in the balancing is thus avoided. The ni = niW = niH2O values are virtually unknown – even for X2z2 = H+1 [30] in aqueous media, and depend on ionic strength (I) of the solution.
For a redox system, (f0, f12, f3 ,…,fK) is the set of K independent, algebraic equations. The f12 is the primary form of Generalized Electron Balance (GEB), f12 = pr-GEB [12,17]. All the balances thus obtained are expressed in terms of concentrations, see Table 1 in context of Table 2. The charge balance has there the form
| 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| System | D (V0) | T (V) | GEB | Charge balance | Concentration balances |
| S1 | Br2 (C0) | NaOH (C) | P1Br = 2ZBr?β0 | α ? P2Br + β = 0 | P3Br = 2?β0 |
| S2 | HBrO (C0) | NaOH (C) | P1Br = (ZBr?1)?β0 | α ? P2Br + β = 0 | P3Br = β0 |
| S3 | NaBr (C0) | KBrO3 (C) | P1Br = (ZBr+1)?β0 + (ZBr?5)?β | α ? P2Br + β0 + β = 0 | P3Br = β0 + β |
| S4 | NaBr (C0) + H2SO4 (C01) | KBrO3 (C) | P1Br = (ZBr+1)?β0 + (ZBr?5)?β | α ? P2Br ? P2S + β0 + β = 0 | P3Br = β0 + β, P3S = β01 |
| S5 | NaBr (C0) | NaBrO (C) | P1Br = (ZBr+1)?β0 + (ZBr?1)?β | α ? P2Br + β0 + β = 0 | P3Br = β0 + β |
| S6 | NaBr (C0) +H2SO4 (C01) | NaBrO (C) | P1Br = (ZBr+1)?β0 + (ZBr?1)?β | α ? P2Br ? P2S + β0 + β = 0 | P3Br = β0 + β, P3S = β01 |
| S7 | NaBr (C0) | Br2 (C) | P1Br = (ZBr+1)?β0 + 2ZBr?β | α ? P2Br + β0 = 0 | P3Br = β0 + 2?β |
| S8 | NaBr (C0) +H2SO4 (C01) | Br2 (C) | P1Br = (ZBr+1)?β0 + 2ZBr?β | α ? P2Br ? P2S + β0 = 0 | P3Br = β0 + 2?β, P3S = β01 |
| S9 | NaBr (C0) | KBrO3 (C) | P1Br = (ZBr+1)?β0 + (ZBr?5)?β | α ? P2Br + β0 + β = 0 | P3Br = β0 + β |
| S10 | NaBr (C0) + Br2 (C01) | KBrO3 (C) | P1Br = (ZBr+1)?β0 + 2ZBr?β01 + (ZBr?5)?β | α ? P2Br + β0 + β = 0 | P3Br = β0 + 2?β01 + β |
| S11 | NaBr (C0) + Br2 (C01) + H2SO4 (C02) | KBrO3 (C) | P1Br = (ZBr+1)?β0 + 2ZBr?β01 + (ZBr?5)?β | α ? P2Br ? P2S + β0 + β = 0 | P3Br = β0 + 2?β01 + β, P3S = β02 |
Table 1: Composition of titrand D and titrant T in the systems S1,?,S11.
| System S1 | System S2 | ||||
|---|---|---|---|---|---|
| NaOH → Br2 | NaOH → HBrO | ||||
| ? | pH | E | ? | pH | E |
| 1,995 | 6,666 | 1,0491 | 0,995 | 6,347 | 1,0720 |
| 1,996 | 6,728 | 1,0455 | 0,996 | 6,411 | 1,0681 |
| 1,997 | 6,811 | 1,0406 | 0,997 | 6,498 | 1,0630 |
| 1,998 | 6,933 | 1,0334 | 0,998 | 6,625 | 1,0555 |
| 1,999 | 7,161 | 1,0199 | 0,999 | 6,866 | 1,0412 |
| 2,000 | 8,143 | 0,9619 | 1,000 | 8,102 | 0,9682 |
| 2,001 | 8,966 | 0,9132 | 1,001 | 9,002 | 0,9150 |
| 2,002 | 9,244 | 0,8968 | 1,002 | 9,281 | 0,8985 |
| 2,003 | 9,413 | 0,8868 | 1,003 | 9,450 | 0,8885 |
| 2,004 | 9,534 | 0,8797 | 1,004 | 9,571 | 0,8814 |
| 2,005 | 9,628 | 0,8741 | 1,005 | 9,666 | 0,8758 |
Table 2: (S1,S2). The sets of (?, ph, E) values taken from the vicinity of the equivalence points, at (C0,V0,C) = (0.01,100,0.1).
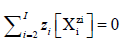 (3a)
(3a)
Note that [ Xm] = 0 for a species m mw Xm.nmw with zero charge (zm=0), e.g., 0∙[H2O] = 0 (z1=0).
The term charge balance (ChB) is used for both forms of this relation, e.g., for ChB expressed by equations: 3 (in terms of Ni, N0j), and 3a (in terms of concentrations); it is done in accordance with the ‘Ockham razor’ principle. This conceptual ‘abuse’ should not lead to ambiguities, in the right context. In addition, the term GEB will be applied both to f12, and to the linear combinations, expressed by eq. 8 (in terms of Ni, N0j), and to the related balances written in terms of concentrations. The elemental/core balances expressed in terms of concentrations are named as concentration balances, for Yk ≠ H, O (k=3,…,K). The balances expressed in terms of concentrations are compatible with expressions for equilibrium constants, specified in Table 3. Consequently, the system of balances related to a redox system consists of three types of balances, expressed in terms of concentrations: GEB, ChB, and K–2 concentration balances for the related elements/cores Yk ≠ H, O; k= 3,…,K. For modelling purposes, the balances are related to the closed system, separated from the environment by diathermal walls [22].
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|
| System: | ? | pBrO3 | pBrO | pBr | Δ1 = pBrO ? pBrO3 | Δ2 = pBr ? pBrO3 |
| S1 | 1.5 | 2.669 | 6.857 | 1.976 | 4.188 | 0.693 |
| 2.5 | 2.574 | 6.758 | 1.875 | 4.184 | 0.699 | |
| S2 | 1.5 | 2.538 | 6.988 | 2.237 | 4.450 | 0.301 |
| 2.5 | 2.574 | 7.024 | 2.273 | 4.450 | 0.301 |
Table 3: Supplementary computational data for the systems S1 and S2.
As indicated elsewhere [31], for a non-redox system, the
equation 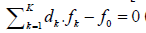 (eq. 8) is transformed into identity,
0 = 0, and then f12 is not an independent equation. Consequently,
in non-redox systems, f12 is the equation dependent on f0,f3,…,fK, and
(eq. 8) is transformed into identity,
0 = 0, and then f12 is not an independent equation. Consequently,
in non-redox systems, f12 is the equation dependent on f0,f3,…,fK, and 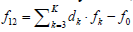 . In other words, f0,f3,…,fK is the set of K –
1 independent equations for a non-redox system. For non-redox
systems, f0,f3,…,fK is transformed into charge balance (eq. 3a)
and K – 2 concentration balances for Yk ≠ H, O. In other words, f1, f2 and f12 are not formulated for non-redox systems. The
dependency or independency of f12 from the balances f0,f3,…,fK is the general criterion distinguishing between non-redox and
redox systems [11].
. In other words, f0,f3,…,fK is the set of K –
1 independent equations for a non-redox system. For non-redox
systems, f0,f3,…,fK is transformed into charge balance (eq. 3a)
and K – 2 concentration balances for Yk ≠ H, O. In other words, f1, f2 and f12 are not formulated for non-redox systems. The
dependency or independency of f12 from the balances f0,f3,…,fK is the general criterion distinguishing between non-redox and
redox systems [11].
Formulation of the proper (i.e., with dk equal to ON’s) linear combinations is applicable to check the linear dependency or independency of the balances. This way is realized a very useful/ effective manner for checking/stating the linear dependence of the balances: f0, f12, f3 ,…,fK related to non-redox systems, named as the transformation of the linear combination (6) to the identity, 0 = 0 [12]. For this purpose, in all instances, we try to obtain the simplest form of the linear combination (6).
Let us repeat: For a redox system, the proper linear combination (6), with dk equal to ON’s, is the way towards the simplest/ shortest form of GEB; for a non-redox system, it is the way towards identity, 0 = 0.
To avoid possible/simple mistakes in the realization of the linear combination procedure, we apply the equivalent relations:
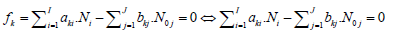 (9)
(9)
for elements with negative oxidation numbers, or
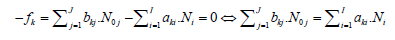 (10)
(10)
for elements with positive oxidation numbers, k ∈ 3,…,K. In this notation, fk will be essentially treated not as the algebraic expression on the left side of the equation fk = 0, but as an equation that can be expressed in alternative forms presented above.
Note, for example, that 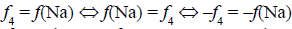 . The change of places of numbers Ni for components and N0j for species (see equations 9, 10) facilitates the purposeful
linear combination of the balances, and enables to avoid simple
mistakes in this operation.
. The change of places of numbers Ni for components and N0j for species (see equations 9, 10) facilitates the purposeful
linear combination of the balances, and enables to avoid simple
mistakes in this operation.
Concentrations of solutes in D and T of D+T redox systems S1 – S11 considered in this paper are specified in Table 1 (columns 2, 3). The set of balances for a particular redox system, specified in rows of Table 1, consists of: generalized electron balance (GEB, column 4), charge balance (ChB, column 5) and concentration balance(s) (column 6). The symbols used in columns 4,5,6 of Table 1 and further notations are as follows:
C0 – concentration of analyte (A) in D, C01 – concentration of H2SO4 in D, C – concentration of reagent (B) in T;
u = V/(V0+V); u0 = V0/(V0+V);
β = C∙u, β0= C0∙u0, β01 = C01∙u0 ;
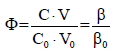 – fraction titrated ;
– fraction titrated ;
Atomic number ZBr=35 for Br;
Molar concentration 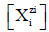 of the species
of the species ![]() in the D+T
mixture is involved in the relation
in the D+T
mixture is involved in the relation
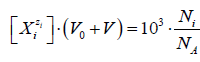 ;
;
α = [H+1] – [OH-1] = 10-pH – 10pH-14 ;
ϑ0 = RT/F∙lN10 – Nernstian slope; ϑ0 = 0.05916 V at T = 298 K;A = 1/0.05916 = 16.9;
P1Br = (ZBr–5)([HBrO3]+[BrO3-1]) + (ZBr–1)([HBrO]+[BrO-1]) + 2ZBr[Br2] + (3ZBr+1)[Br3-1] + (ZBr+1)[Br-1];
P2Br = [BrO3-1] + [BrO-1] + [Br3-1] + [Br-1];
P3Br = [HBrO3] + [BrO3-1] + [HBrO] + [BrO-1] + 2[Br2] + 3[Br3-1]+ [Br-1];
P2S = [HSO4-1] + 2[SO4-2] ; P3S = [HSO4-1] + [SO4-2].
All concentrations of components and species involved with notation applied in Table 1 are expressed in mol/L, and all volumes – in mL. In this notation, common segments of the related balances are distinguished; it allows to simplify the extensive formulation, and may be helpful in construction of the appropriate algorithms. Numerical values of equilibrium constants needed/used in the calculations are involved in the relations:
[H+1] = 10-pH ; [OH-1] = 10pH-14 ; [HSO4-1] = 101.8-pH[SO4-2]; n b
[Br2] = 102A(E-1.087)-2pBr ; [Br3-1] = 102A(E-1.05)-2pBr ; [BrO-1] = 102A(E-0.76)-pBr+2pH-28 ;
[HBrO] = 108.6-pH∙[BrO-1] ; [BrO3-1] = 106A(E-1.45)-pBr+6pH ; [HBrO3] = 100.7-pH∙[BrO3-1];
As stated above, formulation of GEB can be realized according to Approaches I or II to GEB; both Approaches are equivalent, i.e.,
Approach I to GEB ![]() Approach II to GEB (11)
Approach II to GEB (11)
A remark: The GEB concept is quite different from the ‘electron balancing’ procedure applied in laying the redox reactions according to stoichiometric ‘rules’, criticized extensively in [8,18,19,32-34]; it is also different from the term ‘electron balance’ applied for description of: microbial metabolism [35], electrons in light-emitting diodes [36], etc., and… from ‘electronic balance’, as the device for mass measurement [37].
Formulation of the system S1
Preliminary data: Formulation of GEB according to both Approaches (I and II) to GEB will be exemplified first by the system S1 in Table 1, formed from D and T, considered as subsystems of the D+T system. V0 mL of D is composed of Br2 (N01 molecules) + H2O (N02molecules), and V mL of T is composed of NaOH (N03 molecules) + H2O (N04 molecules). In the D+T system we have I = 11 species:
H2O (N1), H+1 (N2,n2), OH-1 (N3,n3), HBrO3 (N4,n4), BrO3-1 (N5,n5), HBrO (N6,n6), BrO-1 (N7,n7), Br2 (N8,n8),
Br3-1 (N9,n9), Br-1 (N10,n10), Na+1 (N11,n11) (12)
Note, for example, that N4 molecules of HBrO3∙n4H2O (z4=0) involve: N4(1+2n4) atoms of H, N4(3+n4 ) atoms of O, and N4 atoms of Br.
The species formed from Br2 and H2O in D, and then present in D+T, resulted from hydrolytic disproportionation: 3Br2 + 3H2O = BrO3-1 + 5Br-1 + 6H+1; 3Br2 + 3H2O = HBrO3 + 5Br-1 + 5H+1; Br2 + H2O = HBrO + Br-1 + H+1; Br2 + H2O = BrO-1 + Br-1 + 2H+1. The symproportionation reaction Br2 + Br-1 = Br3 -1 can be also perceived as complexation. The ONs for H, O and Na are not changed, i.e., these elements are not oxidized or reduced.
Approach I to GEB in S1: The Approach I to GEB needs prior knowledge of ON’s for all elements in components and species of the system in question. In S1, one element (Br) is considered a priori as the only one electron-active element (player); K*=1 is here the number of players.
In the system S1, bromine (as Br2) is the carrier/distributor of its own, bromine electrons. One atom of Br has ZBr bromine electrons, and then one molecule of Br2 has 2ZBr bromine electrons, i.e., N01 molecules of Br2 involve 2ZBr∙N01 bromine electrons. The oxidation degree x of an atom in a simple species, such as ones formed here by bromine, is calculated on the basis of known oxidation degrees: +1 for H, and –2 for O, and external charge of this species. We have, by turns, the relations: 1∙1 + 1∙x + 3∙(–2) = 0 → x= 5 for HBrO3; 1∙x + 3∙(–2) = –1 → x = 5 for BrO3-1; 1∙1 + 1∙x + 1∙(–2) = 0 → x = 1 for HBrO;…; 3∙x = –1 → x = –1/3; 1∙x = –1 → x = –1 for Br-1.
The oxidation degree is the net charge resulting from the presence of charge carriers, inherently involved in an atom: protons in nuclei and orbital electrons, expressed in elementary charge units as: +1 for protons, and –1 for electrons. The number y of bromine electrons in one molecule of HBrO3 is calculated from the formula: ZBr∙(+1) + y∙(–1) = 5, i.e., bromine involves y = Z Br–5 bromine electrons, etc. On this basis, we state that [22,38]:
N4 species HBrO3∙N4H2O involve (ZBr–5)∙N4 bromine electrons;
N5 species BrO3-1∙N5H2O involve (ZBr–5)∙N5 bromine electrons;
N6 species HBrO∙N6H2O involve (ZBr–1)∙N6 bromine electrons;
N7 species BrO-1∙N7H2O involve (ZBr–1)∙N7 bromine electrons;
N8 species Br2∙N8H2O involve 2ZBr∙N8 bromine electrons;
N9 species Br3 -1∙N9H2O involve (3ZBr+1)∙N9 bromine electrons;
N10 species Br-1∙N10H2O involve (ZBr+1)∙N10 bromine electrons;
The N01 molecules of Br2 involved 2ZBr∙N01 bromine electrons. These (bromine) electrons were dissipated between different bromine species, indicated above. The balance for the bromine electrons is then as follows:
2ZBr∙N01 = (ZBr–5)∙N4 + (ZBr–5)∙N5 + (ZBr–1)∙N6 + (ZBr–1)∙N7 + 2ZBr∙N8 + (3ZBr+1)∙N9 + (ZBr+1)∙N10 (13)
Applying in (13) the relations:
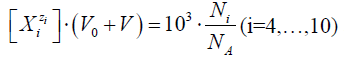 and
and
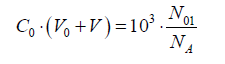 (14)
(14)
we get
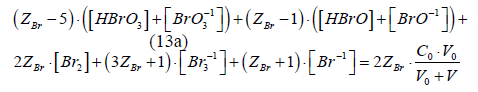
Eq. 13a is the GEB for S1, obtained according to the Approach I to GEB. Applying the notation from Table 2, from eq. 13a we get the balance P1Br = 2ZBr∙β0, identical with the one specified in column 4 for S1.
Approach II to GEB in S1: The balances related to the D+T mixture are as follows:
f0 = ChB
N2 – N3 – N5 – N7 – N9 – N10 + N11 = 0 (15)
f1 = f(H)
2N1 + N2(1+2n2) + N3(1+2n3) + N4(1+2n4) + 2N5n5 + N6(1+2n6) + 2N7n7 + 2N8n8 + 2N9n9 +
2N10n10 + 2N11n11 = 2N02 + N03 + 2N04 (16)
f2 = f(O)
N1 + N2n2 + N3(1+n3) + N4(3+n4) + N5(3+n5) + N6(1+n6) + N7(1+n7) + N8n8 + N9n9 + N10n10 + N11n11
= N02 + N03 + N04 (17)
– f3 = – f(Na)
N03 = N11 (18)
f4 = f(Br)
N4 + N5 + N6 + N7 + 2N8 + 3N9 + N10 = 2N01 (19)
From Equations 16 and 17 we have
f12 = 2∙f2– f1 :
– N2+ N3 + 5N4 + 6N5 + N6 + 2N7 = N03 (20)
From equations 15, 18 and 20 we have
f12 + f0 – f3 :
5N4 + 5N5 + N6 + N7 – N9 – N10 = 0 (21)
Applying the atomic number ZBr for Br, from Equations 19 and 21 we obtain the equation
ZBr∙f4 – (f12 + f0 – f3) :
(ZBr– 5)(N4+N5) + (ZBr– 1)(N6+N7) + 2ZBrN8 + (3ZBr+1)N9 + (ZBr+1)N10 = 2ZBrN01
identical with eq. 13. This way, the equivalency of the Approaches I and II (eq. 11) is proved for the system S1.
In the balance f12 = 2∙f2 – f1 = 2∙f(O) – f(H) (eq. 20), the numbers of water molecules: N1, niW, and those N0j related to H2O as component/solvent (here: N02 and N04) used for preparation of D and T are cancelled. In other systems, also hydrating water molecules introduced by some components (e.g. CuSO4∙5H2O in [20]), are also cancelled within f12.
Charge and concentration balances for S1: From equations 14, 15, 18, 19 and Tables 1 and 2 we have
[H+1] – [OH-1] – [BrO3-1] – [BrO-1] – [Br3-1] – [Br-1] + [Na+1] = 0
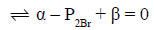 (15a)
(15a)
 (18a)
(18a)
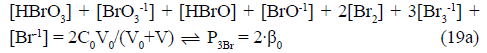
Eq. 19a is the concentration balance for Br, see column 6 in Table 1 for S1. Note that α, P2Br and β are considered as segments of eq. 15a, applied also in balances related to other systems specified in Table 1.
Other/equivalent forms of GEB in S1: Note that the GEB for the system S1, obtained from f12 (eq. 20), has the form
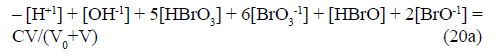
From eq. 21 we have
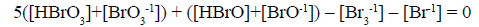 (21a)
(21a)
Other combinations of f12 (eq. 20) with f0, f3 and f4 (equations 15,18,19) have also full properties of GEB for the system S1. Among others, we have
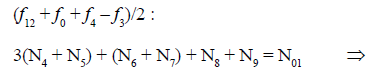 (22)
(22)
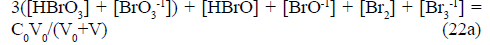
Equations 21a and 22a can be perceived as the steps towards the shortest (involving the smallest number of terms) form of GEB in S1. Summarizing, the equations 13a, 20a, 21a and 22a are the equivalent forms of GEB in the system S1.
Computer program for the system S1: Some of the balances specified above are involved in the set of independent balances applied in the computer program.
function F = NaOH_Br2(x)
global V C0 V0 C yy
E = x(1);
pH = x(2);
pBr = x(3);
H = 10^(-pH);
Kw = 10^-14;
pKw = 14;
OH = Kw/H;
A = 16.9;
Br = 10^-pBr;
ZBr = 35;
Br2=Br^2*10^(2*A*(E-1.087));
Br3=Br^3*10^(2*A*(E-1.05));
BrO=Br*10^(2*A*(E-0.76)+2*pH-2*pKw);
BrO3=Br*10^(6*A*(E-1.45)+6*pH);
HBrO = 10^8.6*H*BrO;
HBrO3=10^0.7*H*BrO3;
Na=C*V/(V0+V);
F = [%Charge balance
(H-OH -Br-Br3-BrO-BrO3+Na);
%Concentration balance for Br
(Br+3*Br3+2*Br2+HBrO+BrO+HBrO3+BrO3-2*C0*V0/ (V0+V));
%Electron balance
( ( Z B r + 1 ) * B r + ( 3 * Z B r + 1 ) * B r 3 + 2 * Z B r * B r 2 + ( Z B r - 1)*(HBrO+BrO)...
+(ZBr-5)*(HBrO3+BrO3)-2*ZBr*C0*V0/(V0+V))];
yy(1)=log10(Br);
yy(2)=log10(Br3);
yy(3)=log10(Br2);
yy(4)=log10(HBrO);
yy(5)=log10(BrO);
yy(6)=log10(HBrO3);
yy(7)=log10(BrO3);
yy(8)=log10(Na);
end
The calculation procedure is realized according to an iterative computer program, here: MATLAB [8]. The volume V of the titrant (T) added is the parameter. In this program, the set of 3 independent variables, forming a (transposed) vector is considered. The number of the (independent, ‘homogeneous’) variables is equal to the number of equations; this ensures a unique solution of the equations related to the system S1, at the pre-set C0, C and V0 values, and the V-value at which the calculations are realized, at defined step of the calculation procedure. The set of equations {15a, 19a, 13a}, involving GEB, obtained according to Approach I to GEB, was applied there. The set of independent equations: {15a, 19a, 20a}, {15a, 19a, 21a} or {15a, 19a, 22a} can be chosen, optionally, for this purpose. The complete set of interrelations between concentrations of the species in the balances, taken from Table 3, is applied.
xT = (x(1), x(2), x(3)) = (E, pH, pBr) (23)
The ‘homogeneity’ of the variables in (23) results from the fact that all them are found in the exponents of the power for 10 in: [e-1] = 10-A∙E, [H+1] = 10-pH, [Br-1] = 10-pBr, where A = 16.9 at T = 298 K (Table 2).
In the computer program, two measurable variables: potential E and pH are involved in the set (23). The E values are related here to the normal hydrogen electrode (NHE) scale. In all cases presented in this paper, the curves E = E(Φ), pH = pH(Φ) and speciation diagrams with the curves zi log [Xizi] vs. Φ are plotted.
Equations and equalities in S1: Among the concentration balances for the systems specified in Table 1, one can distinguish equations and equalities. In the system S1, we have an equality, represented by the balance 18a, which involves only one species (here: Na+1). In the equality 18a, the value for [Na+1] is a number (not variable) for the pre-assumed C and V0 values, at given V-value; as such, it can enter immediately the related ChB, column 5 for S1 in Table 1. Other balances, here: 13a, 15a and 19a, involve more species, and then are classified as equations. Then (18a) is not considered as equation, if the number of equations be compared with the number of independent variables, here: 3 = 3.
Oxidation number, oxidant and reductant as the redundant terms in S1: The GEB related to the system S1, and expressed by eq. 21a, obtained according to Approach II to GEB, can be rewritten as follows:
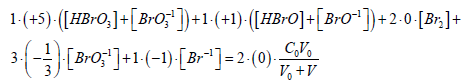 (21b)
(21b)
As we see, the balance (21b), obtained from f12 + f0 – f3, i.e., from
the linear combination of f0 and balances for ‘fans’ (H, O, Na),
has the oxidation numbers (ON) of Br in its species equal to (or
involved with) the coefficient/multiplier at the concentration of
the Br-species, and at concentration of the Br-component (here:
Br2). If the species or component involves more Br-atoms, e.g.,
Br3-1, then we have 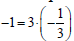 , i.e. the product of the number of
Br-atoms, and the ON for Br in Br3-1.
, i.e. the product of the number of
Br-atoms, and the ON for Br in Br3-1.
This regularity can be extended on other redox systems. Concluding, the formulation of GEB according to Approach II to GEB
• needs none prior knowledge of ON’s for elements participating the redox system; it means that ON is the derivative concept within GATES/GEB;
• the terms: oxidant and reductant (as distinguisher, attribute, differentia specifica) are not assigned a priori to individual components and species of the redox system; there is simply no need for this, i.e., full ‘democracy’ in this respect is assumed.
In this context, the linear combination f12 + f0 – f3 and the resulting eq. 21b exemplify the ‘purposeful’ linear combination.
Completeness/redundancy/compatibility of equilibrium constants: The preparatory step in the formulation of redox systems according to GATES/GEB principles involves gathering of the corresponding equilibrium data, i.e., the standard potentials E0’s, and other equilibrium constants (Table 3). The set of equilibrium constants should be complete, as far as possible. The point is that these sets of data, often presented in the corresponding tables of equilibrium constants, are usually incomplete and/or refer to different equations of the related reactions. The related equilibrium constants can be obtained from other equilibrium constants, as were shown in [12,28], where the problem of redundancy and compatibility was considered in context with the system S1. The problem of redundancy is involved with seemingly excessive number of physicochemical data, collected from various thematic studies, or different works.
Disproportionation in dynamic bromine systems
In algorithms applied for all dynamic systems S1 – S6, specified in Table 1 and presented below, it is assumed that V0 = 100, C0 = 0.01, C = 0.1. All the systems will be illustrated graphically, on the graphs (a) E = E(Φ), (b) pH = pH(Φ) and (c) log [Xizi] =ϕ (Φ) plotted as the functions of the fraction titrated
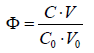 (24)
(24)
It provides a kind of normalization in the related graphs, i.e., independency on V0 value. In principle, C0 is related to an analyte (A), and C – to a reagent B for this analyte (Tables 1 and 2). Some dynamic systems are presented in extended, graphical forms.
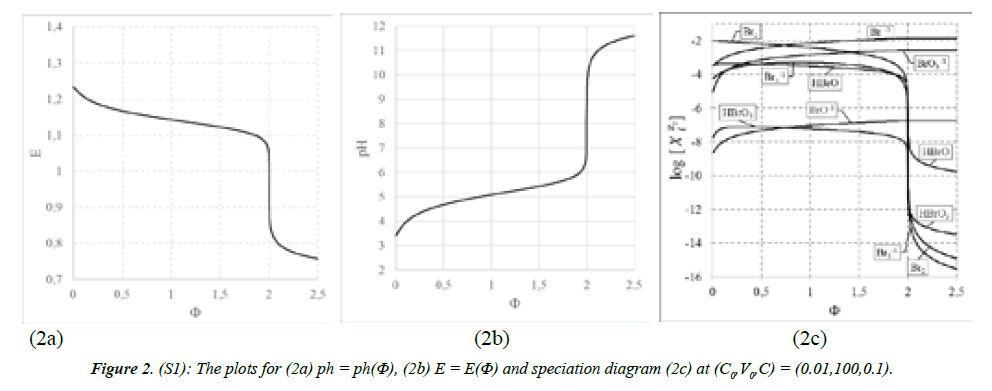
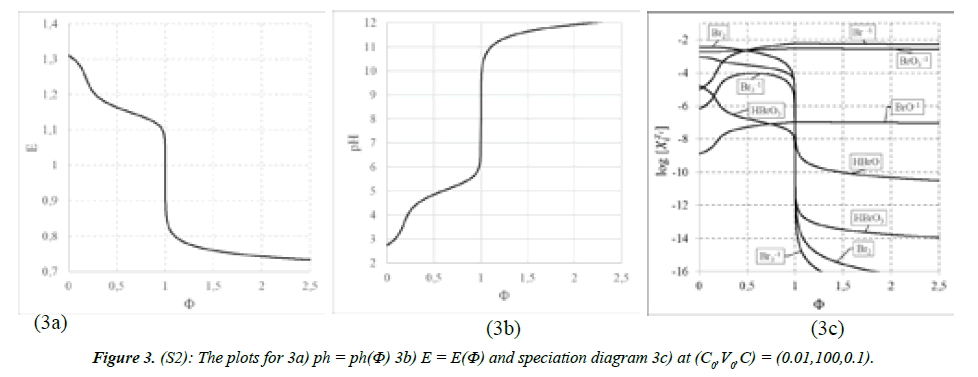
Systems S1 : NaOH ⇒ Br2 and S2 : NaOH ⇒ HBrO: The curves are presented in (Figures 2A-C) for S1, and 3a,b,c for S2. The points (Φ,E,pH) from the vicinity of the related equivalence points on the titration curves: E = E(Φ) and pH = pH(Φ) are collected in Table 2. Moreover, from the result files we have the set of (Φ,pBrO3,pBrO,pBr) values collected in Table 3, where:
pBrO3 = –log[BrO3-1], pBrO = –log[BrO-1], pBr = –log[Br-1]
On this basis, we can compare two main competing reactions: (1a) and (1b) in the system S1, and:
3HBrO + 3OH-1= BrO3-1 + 2Br-1 + 3H2O and (25)
HBrO + OH-1 = BrO-1 + H2O (26)
in the system S2. Note that BrO-1 is the main competing product relative to BrO3-1, both in S1 and S2 (Figures 2C,3C), at a due excess of NaOH. On this basis, we find the relative efficiencies equal to 1041 (column 6) : 104.188 at Φ = 1.5 and 104.184 at Φ = 2.5 for reactions (1a), (1b); and 104.450 at Φ =1.5 and 2.5 for reactions (25) and (26). Note that (26) is the dissociation (not disproportionation) reaction. Stoichiometries of reactions (1a) and (1b) are the same for the competing pairs of reactions: 3:6 = 1:2 for reactions (1a), (1b), and 3:3 = 1:1 for reactions (25) and (26). Moreover, we find the ratio [BrO3-1]/[Br-1] equal to 1042 = 100.699 = 5 = 5:1 for S1 at Φ = 2.5 (i.e. at the excess of NaOH), and close to 5 at the point where 1.5/2 = 75% of Br2 is already consumed. The stoichiometry of products of reaction (25) is confirmed by the ratio [Br-1]/[BrO3-1] = 100.301 = 2 = 2 : 1 (Table 3). This confirms the reaction 1a, and testifies against the reaction 1b (of the same stoichiometry!), commonly met (‘given to believe’) in literature and elsewhere, e.g. [38].
Symproportionation in dynamic bromine systems
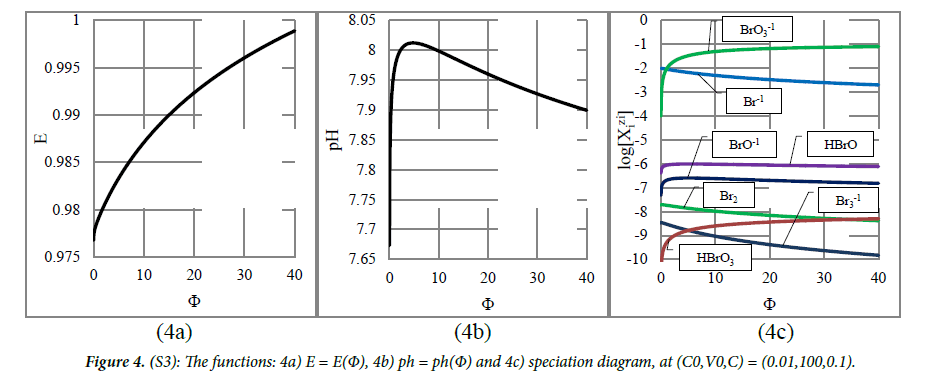
System 3 : kbro3 ⇒ nabr: In this case, symproportionation practically does not occur (Figure 4C); concentration of HBrO, as the major product formed in the symproportionation reaction
BrO3-1 + 2Br-1 + 3H+1 = 3HBrO (27)
is ca. 10-6 mol/L. The potential E increases monotonically (Figure 4A), whereas pH first increases, passes through maximum and then decreases (Figure 4B). The relevant pH and E changes are small. Binding the H+1 ions in reaction (27) causes a weakly alkaline reaction (Figure 4B).
Table 2 (S1,S2). The sets of (Φ, pH, E) values taken from the vicinity of the equivalence points, at (C0,V0,C) = (0.01,100,0.1).
System S4 : KBrO3 ⇒ NaBr + H2SO4: The stoichiometry 1 : 5, i.e., Φeq = 0.2, stated for C01 values indicated at the curves plotted in Figure 5 (column 5a), results from reaction (2a). At Φ > 0.2, an increase of efficiency of the competing reaction (27) is noted. A growth of C01 value causes a small extension of the potential range in the jump region, on the side of higher E-values (Figure 5, column 5a). With an increase of the C01 value, the graphs of pH vs. Φ resemble two almost straight line segments intersecting at Φeq = 0.2 (Figure 5, column 5b).
However, the pH-ranges covered by the titration curves are gradually narrowed (Figure 5, column 5b).
System S5 : NaBrO ⇒ NaBr: The basic reaction in this system (Figure 6B) results from the relation
[HBrO3]+[HBrO]+2[Br2]+ 2[Br3-1] = [OH-1] – [H+1] > 0