Research Article - Journal of Clinical and Experimental Toxicology (2017) Volume 1, Issue 1
180-Days repeated dose oral toxicity study of polyherbal unani formulation: Jawarish Shahi
Mohammad Ahmed Khan1, Mohd Urooj1, Thejaswini G1, Syed Shoeb Ahmed1, Munawwar Husain Kazmi2, Gulam Mohammed Husain1*
1Department of Pharmacology, Central Research Institute of Unani Medicine, Hyderabad, India
2Department of Ilmul Advia, Central Research Institute of Unani Medicine, Hyderabad, India
- *Corresponding Author:
- Dr. Gulam Mohammed Husain
Department of Pharmacology, Central Research Institute of Unani Medicine, India
Tel: +918179537035
E-mail: gmhusain@gmail.com
Accepted date: December 14, 2017
Abstract
Background Jawarish Shahi (JS) is a compound Unani Pharmacopoeial formulation indicated for Khafqan (Palpitation), Nafkhe Shikam (Flatulence) and Waswas (Insanity; false perception and hallucinations). However, JS has not been evaluated for its safety. Therefore, present study was conducted to evaluated long term toxicity / safety of this Unani formulation in India during the year 2016-17. Objective The following study was carried out to study the 180 days repeated dose toxicity of JS in SD rats. Methods Study was carried out on SD rats of both sexes. Animals were divided into various groups (n=15). JS was orally administered at a limit dose of 2000 mg/kg bw/day for 180 days. After completion of 180 days blood samples were collected for haematological and biochemical analysis and animals were sacrificed and organs were harvested for weight determination and histopathological evaluation was carried out. Results Animals in groups treated with JS did not show any abnormal behaviour or clinical signs indicative of systemic toxicity based on functional observation parameters observed throughout study duration. There was no toxicologically significant alteration observed in relative organ weights, haematological and biochemical parameters of control and JS treated rats of either sex. The findings were further corroborated by histopathological studies. Conclusion No toxicologically significant changes were observed with respect to clinical signs of toxicity, body weight, feed intake, haematology, biochemistry profile, gross necropsy and histopathology in JS treated rats as compared to control group. It may be concluded based on above observations that No Observed Adverse Effect Level (NOAEL) of JS in SD rats is >2000 mg/kg bw.
Keywords
Toxicity, unani medicine, Jawarish Shahi.
Introduction
Traditional herbal medicine has been recently gaining recognition among population [1]. The characteristic feature of traditional system of medicine is their holistic approach towards therapy with an emphasis on strengthening the healing ability of one’s body [2]. Despite gaining recognition as relatively safer therapy, adverse effects of herbal products have been reported mainly due to issue of quality. Contamination and adulteration have been reported in many herbal products leading to serious toxicities [3,4]. To achieve clinical success, the traditional medicines need to attain high standards of quality, safety and efficacy. Diverse approaches have been tested to evaluate the toxicity potential of chemicals, drugs including traditional medicines [5-7]. Recently, several metabolomic studies have been conducted to prove the efficacy and safety, explore the underlying mechanisms, and identify the potential biomarkers to understand the mechanism of action of traditional medicines with useful results especially on traditional Chinese medicine [8-10]. Evaluation of toxicity potential in animals is still indispensable step before initiating the clinical trial [11-13]. Strategy of the development of alternative methods should be more directed towards the refinement or reduction of in vivo tests [14].
The Unani system of medicine is a comprehensive traditional system of medicine having its own theory of health and disease. Treatment of many diseases with Unani system of medicine has been proven to be effective through their widespread use as well as clinical evaluation. Further, the safety of such Unani formulations needs to be established to bring them into mainstream as a scientifically validated treatment option for the masses [15].
Jawarish Shahi (JS) is one such traditional Unani formulation. It is a compound Unani pharmacopoeial formulation mentioned in the National Formulary of Unani Medicine, PART-I and other classical text of Unani system of medicine. Jawarish Shahi contains herbs like Halela (Termanalia chebula), Amla (Emblica officinalis), Kishneez (Coriaandrum sativum), Elaichi Khurd, (Elettaria cardamomum), and Bed Mushk (Salix caprea). It is recommended for treatment of Khafqan (Palpitation), NafkheShikam (Flatulence) and Waswas (Insanity; false perception and hallucinations). However, safety studies of JS have not been reported in literature. Therefore, the present study was carried out to evaluate 180 repeated dose toxicity of JS in rats.
Materials and Methods
Experimental animals
Sprague Dawley rats (120 ± 20 g) were used for the study. Rats were obtained from National Institute of Nutrition, Hyderabad, India. The selected females were nulliparous and non-pregnant. Rats were housed in polycarbonate cages in the air conditioned room maintained at the temperature of 22°C ± 3°C and relative humidity of 30-70%, with a 12:12 h light/dark illumination cycle [16]. CPCSEA guidelines of laboratory animal care were followed throughout the experiment [17]. Protocol of the study was approved by the Institutional Animals Ethics Committee vide protocol no. CRIUM/IAEC/2014/02/P02. Animals were provided with standard feed pellets (National Institute of Nutrition, Hyderabad) and water ad libitum, unless stated otherwise. Animals were acclimatized to the laboratory conditions for one week before using them for experiment.
Drug/formulation
JS used in present study was prepared as per the standard Pharmacopoeial procedure in the GMP certified pharmacy of CRIUM Hyderabad.
Dose selection
Single dose of JS at 2,000 mg/kg bw (as per the Limit dose of ICH) was used for the study [18] which is approximately two times of therapeutically equivalent dose. Present study was conducted as per the limit test guidance of OECD-452 at a single dose of 2000 mg/kg bw per day, primarily by considering the low toxicity potential (considering the long term clinical use of Jawarish Shahi without any serious adverse effect reported so far) as well as absence of any systemic toxicity in a sub-chronic (90-days) toxicity study conducted in our lab (unpublished data) at two dose levels i.e., 1028 and 2000 mg/kg bw per day.
Drug administration
JS was administered as aqueous suspension in 0.3% CMC (<2 mL/100 gm b.w.). Suspension was freshly prepared every day. The control animals were administered vehicle only. Doses were administered by oral (gavage), once daily for 180 consecutive days at similar time each day to minimize variations.
Duration of therapy
Duration of toxicity study for JS was 06 months (i.e., 180-days).
Experimental design
The 180-day repeated dose oral toxicity study was performed according to the OECD test guideline-452 [16]. Male and female Sprague Dawley rats were divided into two groups with 30 animals (15 males+15 females) in each group (Table 1). All the experimental animals were observed for mortality and morbidity twice a day, throughout the study duration. Detailed clinical observations (i.e., functional observation parameters such as behavioural, stereotypy, motor and autonomic signs like salivation, lacrimation, piloerection etc.) were made periodically to detect signs of toxicity, at the same time (1h after vehicle or drug administration). Body weight of the animals was recorded once a week. Average feed intake for both sexes were recorded at weekly interval by weighing the amounts of feed given to a cage group and leftovers on the next day. At the end of the treatment period, the overnight fasted (water provided ad-libitum) rats were anaesthetized with isofluorane inhalation (EZ Anaesthesia-1339), blood samples were collected by retroorbital puncture in the EDTA vacutainers (for haematological) and serum vacutainers (for biochemical and electrolyte analysis).
| S. No. | Treatment Group | No. of animals (male + female) |
|---|---|---|
| 1. | Vehicle control (0.3% Aq. CMC suspension) | 15+15 |
| 2. | JS (Limit dose of 2,000 mg/kg bw) | 15+15 |
| Total no. of animals | 30+30=60 (30 Male & 30 Female) |
|
JS: Jawarish Shahi (2000 mg/kg)
Table 1: Treatment schedule.
Haemoglobin (Hb), red blood cell count (RBC), white blood cell count (WBC), haematocrit (HCT) and platelet (PLT) were analysed using fully automated haematology analyser (Swelab Autocounter-920EO+). Serum biochemical parameters such as glucose, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total bilirubin, creatinine, blood urea nitrogen (BUN), total cholesterol (TC), triglycerides (TG), total protein (TP) and albumin were analysed using fully automatic analyser (Erba-EM200). Serum electrolytes such as sodium, potassium, chloride and total calcium were estimated in a fully automated electrolyte analyser (Allcare-AC9801).
At the end of study duration, all animals were subjected to gross necropsy. Organs and tissues were examined macroscopically and internal organs/tissues were isolated, trimmed and weighed. Organs/tissues were preserved in the neutral buffer formalin and subjected to histological examination.
Statistical analyses
Data were expressed in mean ± standard error of mean (SEM). Mean difference between the control and treatment groups was analysed by student’s t-test using GraphPad prism (version 5). P value ≤ 0.05 was considered as statistically significant.
Observation and Results
Effect on body weight and feed intake
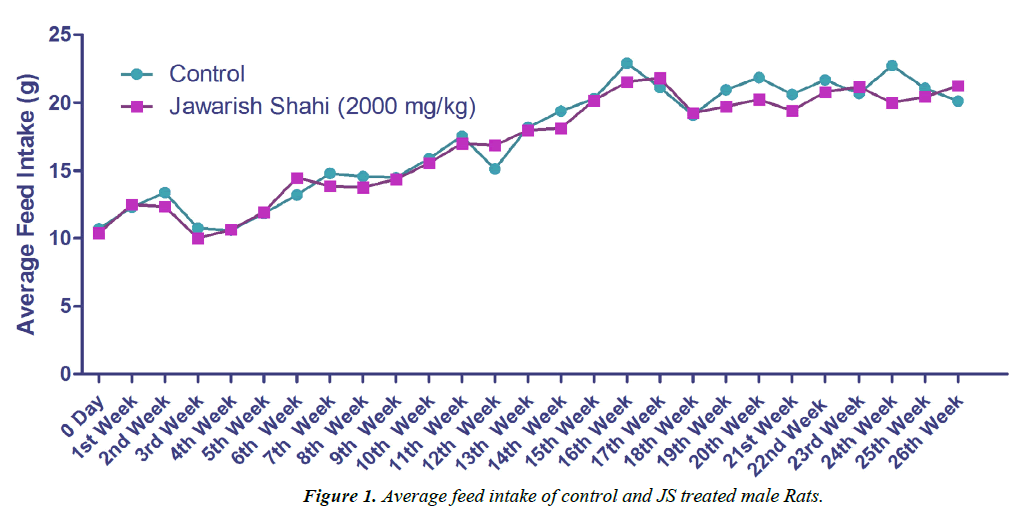
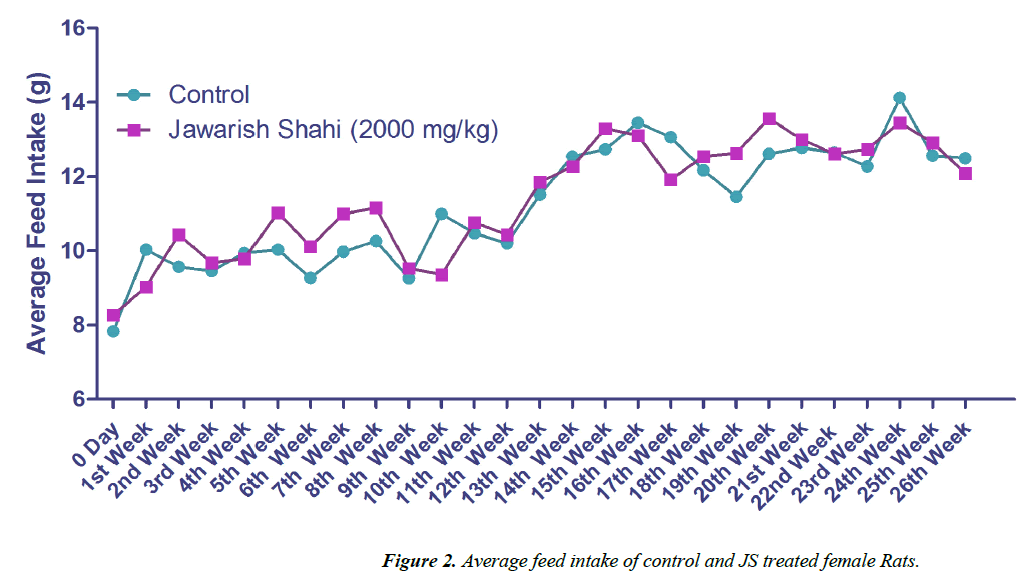
The results of body weight and feed intake of control and JS treated groups are presented in Figures 1 and 2. Body weight gain in control and JS treated groups did not show any significant difference throughout the study (P>0.05). Similarly, feed intake of control and JS groups was also comparable (P>0.05). Rats in control and treatment group showed expected pattern of body weight gain and feed consumption.
Effect on haematological profile
The haematological profile of Control and JS group did not show any significant difference in haematological parameters such as Hb, RBC, WBC count and HCT in rats of either sex (Table 2). Platelet count in Control and JS treated groups were also comparable. However, lymphocyte count was significantly decreased in male rats treated with JS (P<0.05 vs. Control).
| Parameter | Male | Female | ||
|---|---|---|---|---|
| Control | JS | Control | JS | |
| Hb (gm%) | 17.29 ± 0.29 | 17.50 ± 0.25 | 16.65 ± 0.21 | 16.11 ± 0.16 |
| RBC (Million/mm3) | 9.04 ± 0.11 | 9.24 ± 0.07 | 8.28 ± 0.09 | 8.23 ± 0.09 |
| WBC (/mm3) | 7480 ± 252.8 | 8213 ± 351.2 | 5953 ± 255.0 | 5620 ± 424.7 |
| Platelet (lakhs/mm3) | 4.69 ± 0.15 | 5.05 ± 0.14 | 4.39 ± 0.15 | 4.31 ± 0.20 |
| HCT (%) | 45.90 ± 0.50 | 46.69 ± 0.39 | 44.15 ± 0.32 | 43.12 ± 0.44 |
| Neutrophil (%) | 22.07 ± 1.071 | 24.40 ± 1.013 | 24.13 ± 1.287 | 24.07 ± 1.300 |
| Lymphocyte (%) | 69.53 ± 1.06 | 66.47 ± 1.0* | 68.67 ± 1.39 | 69.33 ± 1.39 |
| Eosinophil (%) | 5.07 ± 0.21 | 5.27 ± 0.23 | 4.33 ± 0.23 | 4.0 ± 0.14 |
| Monocyte (%) | 3.33 ± 0.16 | 3.87 ± 0.21 | 2.87 ± 0.19 | 2.60 ± 0.16 |
JS: Jawarish Shahi (2000 mg/kg); Values presented as mean ± SEM; n=15 per sex; Student?s t-test; *P<0.05 vs. Control
Table 2: Effect of JS on Haematological Parameters in Rats.
Effect on biochemical profile
Blood biochemical parameters such as Albumin, Globulin, A/G Ratio, BUN, Creatinine, HDL, LDL, VLDL, Chol/HDL Ratio, HDL/LDL Ratio, in control and drug treated groups (Table 3) did not show treatment related difference. Blood glucose level in male rats was found to be significantly reduced upon treatment with JS (P<0.001 vs. Control).
| Parameters | Male | Female | ||
|---|---|---|---|---|
| Control | JS | Control | JS | |
| Glucose (mg/dL) | 138.5 ± 2.58 | 119.1 ± 5.04** | 122.3 ± 4.42 | 128.5 ± 4.52 |
| ALT (IU/L) | 73.0 ± 2.49 | 89.0 ± 5.11* | 78.9 ± 8.99 | 76.4 ± 3.83 |
| AST (IU/L) | 128.4 ± 5.55 | 150.0 ± 5.82* | 145.5 ± 8.67 | 129.9 ± 3.49 |
| Bilirubin (mg/dL) | 0.153 ± 0.008 | 0.131 ± 0.003* | 0.155 ± 0.007 | 0.137 ± 0.008 |
| ALP (IU/L) | 99.4 ± 5.54 | 120.4 ± 5.93* | 78.5 ± 2.58 | 132.9 ± 9.48*** |
| Total Protein (g/dL) | 6.84 ± 0.050 | 7.09 ± 0.069** | 6.94 ± 0.043 | 7.07 ± 0.122 |
| Albumin (g/dL) | 3.96 ± 0.083 | 4.10 ± 0.048 | 4.11 ± 0.052 | 4.35 ± 0.107 |
| Globulin (g/dL) | 2.88 ± 0.075 | 2.99 ± 0.095 | 2.83 ± 0.052 | 2.73 ± 0.125 |
| A/G Ratio | 1.35 ± 0.062 | 1.33 ± 0.054 | 1.41 ± 0.040 | 1.63 ± 0.142 |
| BUN (mg/dL) | 20.15 ± 0.757 | 19.32 ± 0.402 | 18.85 ± 0.785 | 20.14 ± 0.665 |
| Creatinine (mg/dL) | 0.840 ± 0.025 | 0.813 ± 0.029 | 0.840 ± 0.021 | 0.807 ± 0.018 |
| Cholesterol (mg/dL) | 97.9 ± 3.32 | 106.9 ± 4.06 | 100.3 ± 4.47 | 123.9 ± 6.91** |
| TGs (mg/dL) | 65.60 ± 2.58 | 68.13 ± 3.94 | 79.60 ± 4.08 | 68.00 ± 3.05* |
| HDL (mg/dL) | 43.00 ± 1.05 | 44.60 ± 0.99 | 44.47 ± 0.82 | 45.87 ± 1.05 |
| LDL (mg/dL) | 41.27 ± 2.974 | 48.67 ± 4.267 | 40.00 ± 4.209 | 64.47 ± 7.198 |
| VLDL (mg/dL) | 12.93 ± 0.530 | 13.67 ± 0.797 | 15.80 ± 0.776 | 13.60 ± 0.600 |
| Chol/HDL Ratio | 2.24 ± 0.081 | 2.39 ± 0.112 | 2.21 ± 0.106 | 2.71 ± 0.187 |
| HDL/LDL Ratio | 0.927 ± 0.073 | 1.067 ± 0.108 | 0.872 ± 0.093 | 1.447 ± 0.177 |
JS: Jawarish Shahi (2000 mg/kg); Values presented as mean ± SEM; n=15 per sex; Student?s t-test; ***P<0.001 vs. Control; **P<0.01 vs. Control; *P<0.05 vs. Control
Table 3: Effect of JS on Blood Biochemical Parameters in Rats.
There was significant increase in levels of hepatic enzymes namely AST, ALT and ALP (P<0.05 vs. Control) in male rats treated with JS. In the same group, serum bilirubin level was significantly reduced (P<0.05 vs. Control) and total protein level was significantly increased (P<0.01 vs. Control) upon treatment with JS. ALP level was also increased significantly in JS treated female rats (P<0.01 vs. Control). In addition, serum cholesterol level was increased significantly (P<0.01 vs. Control) whereas serum triglyceride level was significantly decreased (P<0.05 vs. Control) in female rats treated with JS. Serum levels of sodium, potassium, chloride and calcium in JS treated groups did not show any change compared to control groups (Table 4).
| Parameters | Male | Female | ||
|---|---|---|---|---|
| Control | JS | Control | JS | |
| Sodium (mmol/L) | 137.7 ± 0.29 | 136.3 ± 0.71 | 139.3 ± 0.53 | 135.7 ± 0.81 |
| Potassium (mmol/L) | 4.82 ± 0.04 | 4.72 ± 0.056 | 4.34 ± 0.06 | 5.15 ± 0.29 |
| Chloride (mmol/L) | 100.9 ± 0.98 | 103.4 ± 1.24 | 104.9 ± 0.60 | 102.8 ± 1.14 |
| Calcium (mmol/L) | 3.45 ± 0.22 | 3.69 ± 0.48 | 3.05 ± 0.13 | 5.03 ± 0.63 |
JS: Jawarish Shahi (2000 mg/kg); Value
Table 4: Effect of JS on serum electrolytes in rats.
Effect on relative organ weight and histopathology
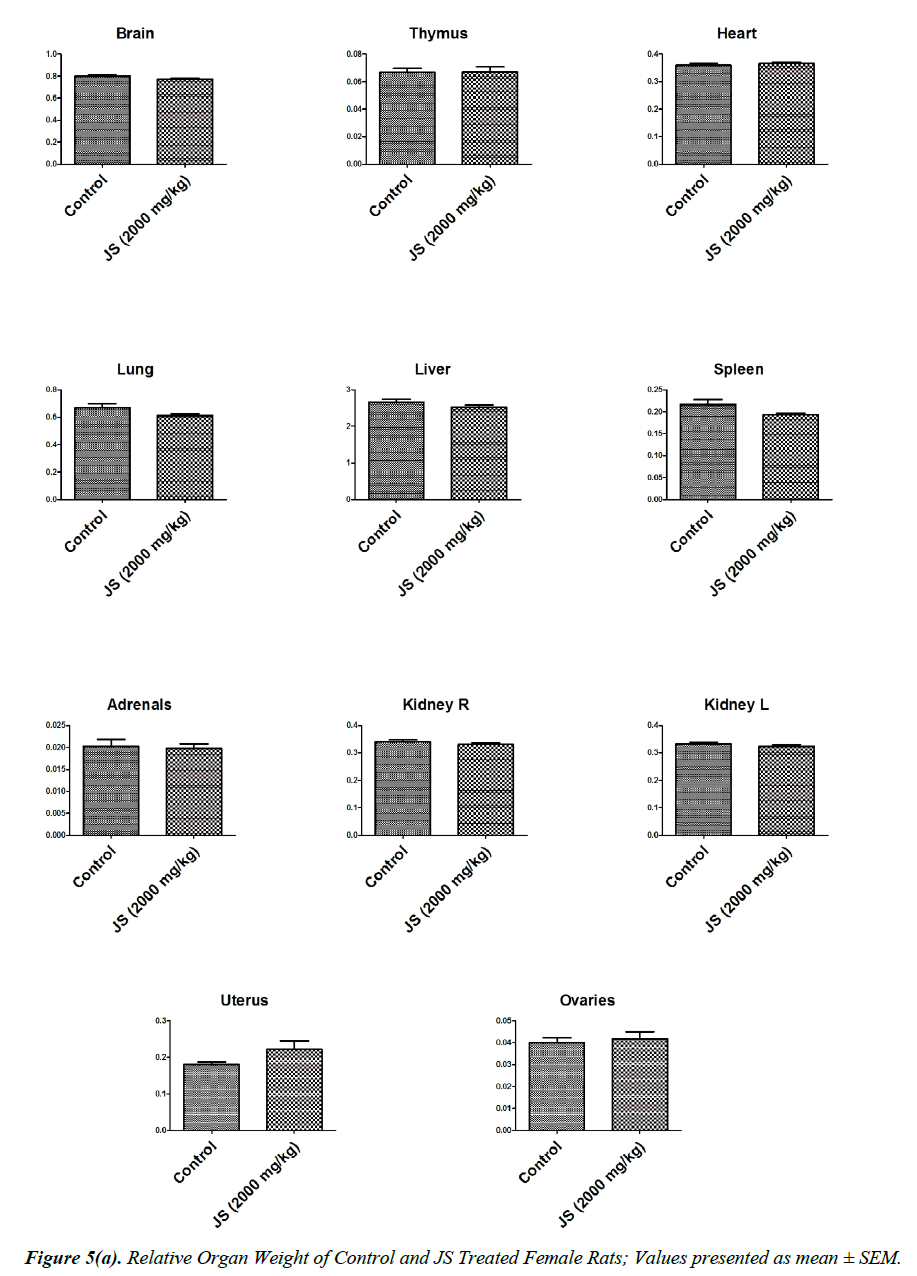
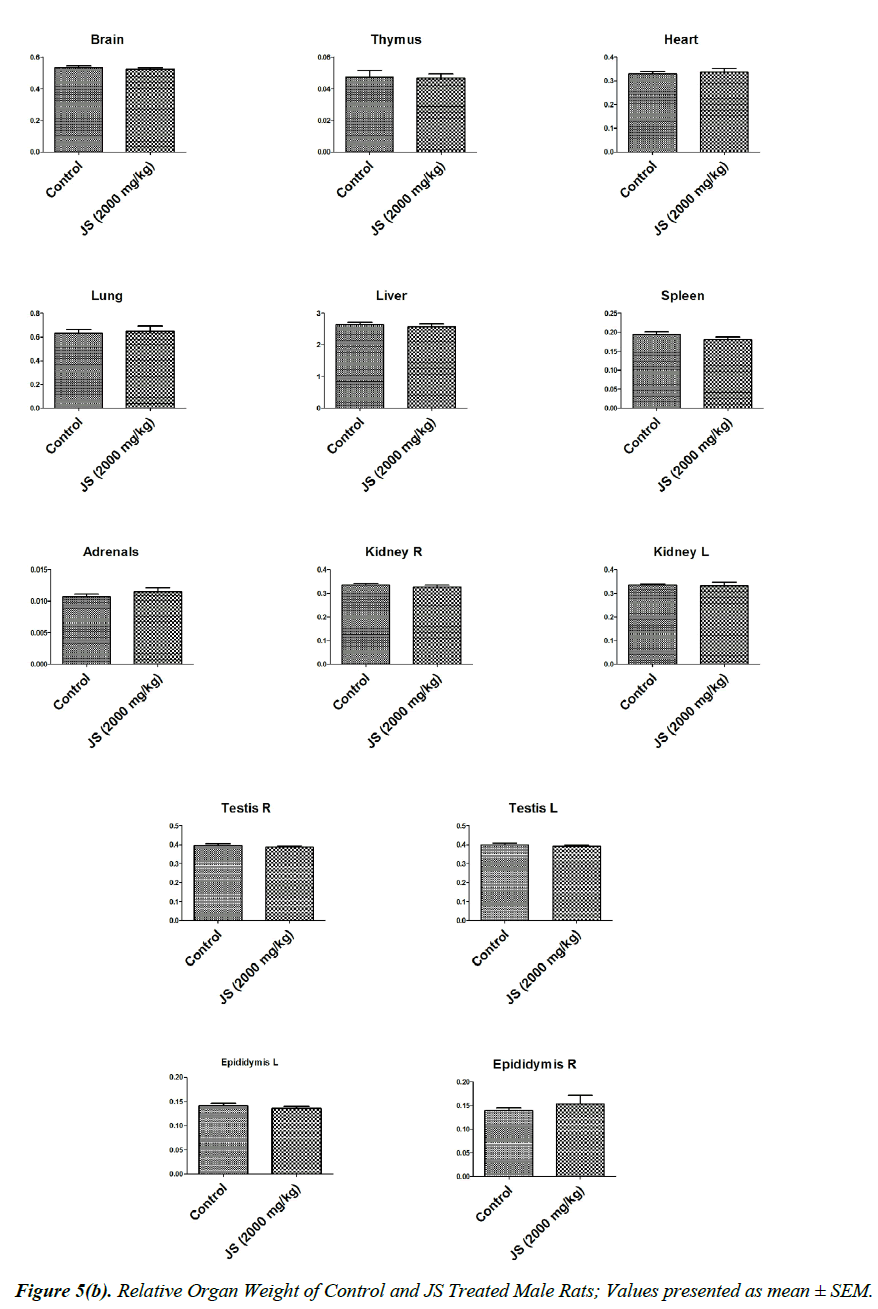
The observations of relative organ weight in control and JS treated groups are presented in Figure 5a and 5b. No significant alteration was observed in relative organ weights of Brain, Thymus, Heart, Lungs, Liver, Spleen, Adrenals, Kidney, Testis, Epididymis, Uterus and Ovaries in JS treated group and it was found comparable to Control (P>0.05).
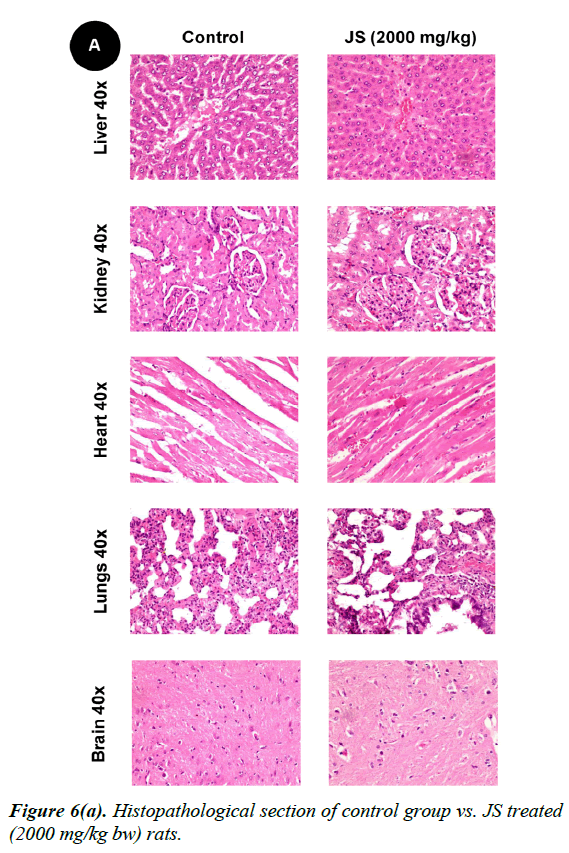
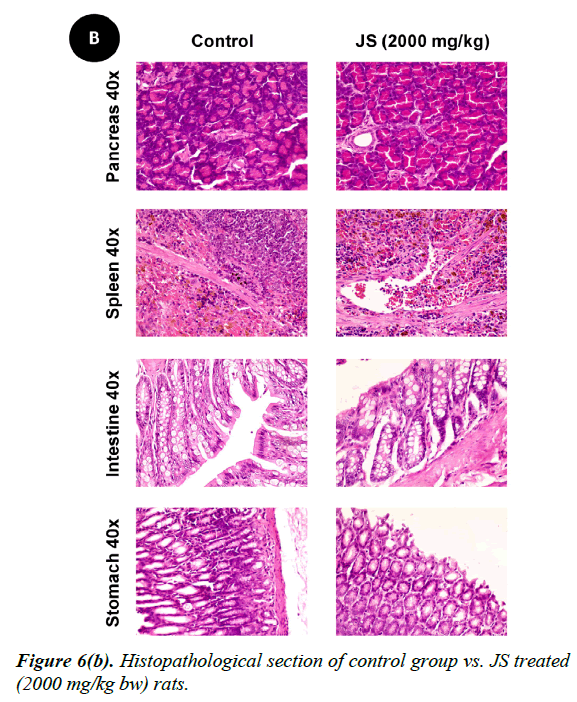
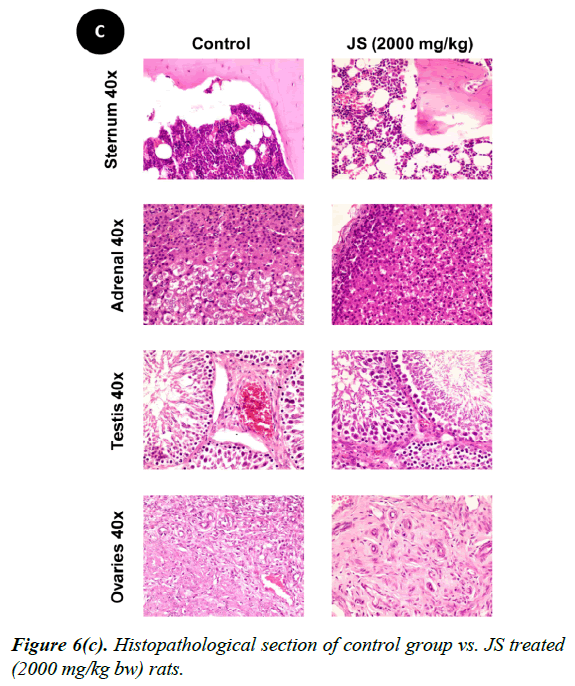
No changes were observed during the gross necropsy of control or JS treated animals. All the collected organs were found histologically normal except few changes which were observed in lungs and livers. In both lungs and livers the histological changes observed in the JS treated group were also observed in the control animals and hence may not be attributed to treatment with JS (Figure 6).
Discussion
The study was carried out to establish safety profile of JS following administration for 180 consecutive days in SD rats. No mortality was observed in any group throughout the study duration of 180 days. Rats in both control and JS treated groups did not show any sign of intoxication during study period.
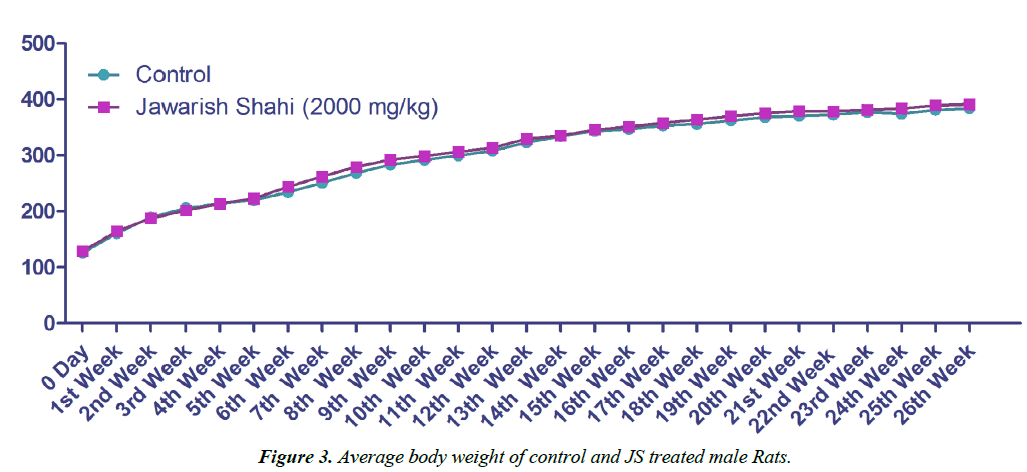
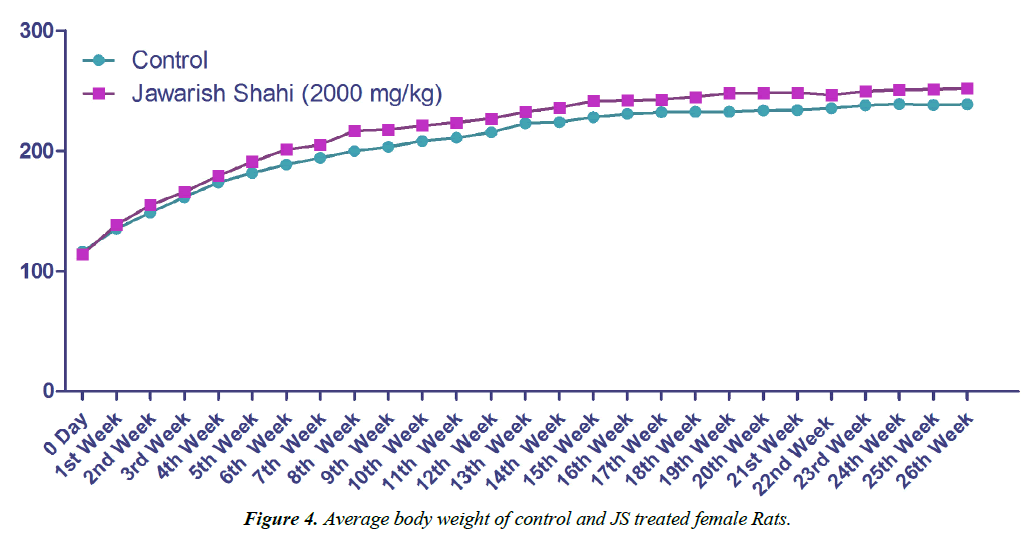
Body weight gain of control and JS treated animal did not show any statistically significant difference (Figures 3 and 4). Body weight gain showed gradual increase indicating no treatment related systemic toxicity [19]. Functional observation also did not reveal any treatment related abnormality in normal behaviour.
Evaluation of haematological parameters is used to determine the extent of the adverse effect on hematopoietic system. This evaluation is important because changes in the haematological parameters have significant correlation to human toxicity upon translation of preclinical data [20]. Observations of hematological parameters did not reveal any treatment-related alterations in the present study (Table 2). Treatment with JS did not cause alteration in hematological profile (i.e., RBC, WBC, Hb, HCT) as well as platelet count indicating no adverse effect on blood cells. Although lymphocyte count was significantly decreased in JS treated male rats the values remained within normal physiological range and hence do not carry toxicological significance [17].
The liver is the main metabolic organ which undertakes metabolism of drugs and detoxification of many carcinogens and toxins in the body. Drug-induced hepatic toxicity can lead to serious consequence in the form of cholestasis, acute hepatitis, or even cirrhosis. Reactive oxygen species generated by metabolic intermediates of Xenobiotics, can generate reactive oxygen species through CYP450 enzyme families. These can also activate inflammatory cells through NADPH oxidases leading to necrosis [21]. Hepatic toxicity can be measured as altered ALT, AST, ALP, and bilirubin levels [22]. Elevation of ALT and/or AST beyond three times of upper value of normal range accompanied by two fold alteration in total bilirubin level signifies liver damage [23,24]. However, in the present study alteration observed in ALT, AST, Bilirubin levels were marginal and hence may not be considered toxicologically relevant (Table 3).
Albumin synthesis is one of the functions of liver, thus, decreased albumin level has a positive correlation with prognosis of liver disease. Bilirubin occurs in both conjugated (glucuronide) as well as unconjugated form. Balance between conjugate and unconjugated form is a measure of reflects balance between balance between production and hepatobiliary excretion. Higher than normal bilirubin level is observed in haemolysis, altered erythropoiesis as well as in muscular injury. Further, conjugated hyperbilirubinaemia signifies liver disease and biliary obstruction [25]. In the present study, all groups exhibited normal values of albumin, total bilirubin and conjugated bilirubin.
Drug-induced nephrotoxicity is a persistent challenge in clinical medicine with its incidence reported to be as high as 60 percent. The potential of drugs to induce toxicity is generally heightened in the kidney microenvironment. The BUN and Creatinine are screening tests of renal function. BUN and Creatinine are handled by glomerular filtration and also essentially reflect glomerular filtration rate [26]. In the present investigation, renal function markers BUN and creatinine levels were found to be well within the normal range for JS treated rats suggesting that JS did not cause any adverse effect on renal function.
Elevated levels of low density lipoprotein and reduced levels of high density lipoprotein along with corresponding high levels of cholesterol is reported to be associated with higher vascular morbidity and mortality. Similarly, elevated TG levels can also lead to higher vascular risk [27]. In the present study, although, value for total cholesterol was significantly increased in female rats treated with JS, the values remained within physiological range. Further, rise in cholesterol level was not associated with any adverse change in HDL, LDL or VLDL level and therefore, may not warrant further investigation. The serum electrolyte levels of JS treated animals were also within normal physiological range, suggesting that JS did not alter ion homeostasis in rats (Table 4).
Organ weight is a sensitive indicator of drug toxicity, since significant alteration in organ weight between control and drug treated groups. Changes in organ weight often precede morphological changes and hence may be observed even in the absence of latter [28]. Relative organ weight data of JS treated male and female rats at the end of 180 days dosing period did not show any significant difference from that of control (Figure 5a and 5b).
The gross necropsy of all animals was carried out at the end of study followed by histopathological evaluation of hematoxylin and eosin-stained tissue sections [29]. The collected organs including vitals upon histopathological examination were found histologically normal except few changes of histological significance were observed in lungs and livers. In both lungs and livers the various histological changes observed in the experimental groups were also present in the control group and hence may not be attributed to treatment with JS (Figure 6a, 6b and 6c).
Conclusions
A chronic toxicity study was conducted on a polyherbal Unani formulation Jawarish Shahi in SD rats. Animals treated with JS did not showed any toxicologically significant changes with respect to behavioural signs of toxicity, body weight gain, feed intake, haematology parameters, biochemistry profile, gross necropsy and histopathology compared to control group. Based on the present findings, No Observed Adverse Effect Level (NOAEL) of JS in SD rats is >2000 mg/kg bw.
Conflicts of Interests
The authors declare no conflict of interest.
Acknowledgement
Support extended by Director General, CCRUM, Ministry of AYUSH, Government of India, New Delhi, in terms of financial resources and infrastructure is greatly acknowledged. Authors are thankful to National Institute of Nutrition for providing technical expertise for histopatholgical evaluation and laboratory services of CRIUM Hyderabad for providing support for biochemistry and haematology of blood samples.
References
- Jafari S, Abdollahi M, Saeidnia S. Personalized medicine: a confluence of traditional and contemporary medicine. Altern Ther Health Med 2014;20:31-40.
- Ikram RRR, Ghani MKA, Abdullah N. An analysis of application of health informatics in Traditional Medicine: A review of four Traditional Medicine Systems. Int J Med Inform 2015;84:988-96.
- Elvin-Lewis M. Should we be concerned about herbal remedies. J Ethnopharmacol 2001;75:141-64.
- Ali A, Sumbul S, Abdin MZ, et al. Development of standard operating procedure and standardization of Habb-e-Banafsha Qawi-A Unani polyherbal formulation. J Pharm Bioallied Sci 2015;7:250-3.
- Yaroshenko IS, Kirsanov DO, Wang P, et al. Determination of the toxicity of herb preparations of the traditional Chinese medicine with a multisensor system. Russian Journal of Applied Chemistry 2015;88:72-81.
- Abdelaziz A, Sushko Y, Novotarskyi S, et al. Using Online Tool (iPrior) for Modeling ToxCast Assays Towards Prioritization of Animal Toxicity Testing. Comb Chem High Throughput Screen 2015;18:420-38.
- Youns M, Hoheisel JD, Efferth T. Toxicogenomics for the prediction of toxicity related to herbs from traditional Chinese medicine. Planta Med 2010;76:2019-25.
- Shi J, Cao B, Wang XW, et al. Metabolomics and its application to the evaluation of the efficacy and toxicity of traditional Chinese herb medicines. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1026:204-16.
- Li CY, Song HT, Wang XX, et al. Urinary metabolomics reveals the therapeutic effect of HuangQi Injections in cisplatin-induced nephrotoxic rats. Sci Rep 2017;7:3619.
- Zhang H, Fu P, Ke B, et al. Metabolomic analysis of biochemical changes in the plasma and urine of collagen-induced arthritis in rats after treatment with Huang-Lian-Jie-Du-Tang. J Ethnopharmacol 2014;154:55-64.
- Iminjan M, Amat N, Li XH, et al. Investigation into the toxicity of traditional Uyghur medicine Quercus infectoria galls water extract. PloS one 2014;9:e90756.
- Chen Y, Chen S, Song C, et al. Acute and subchronic toxicity as well as evaluation of safety pharmacology of traditional Chinese medicine "Huhezi". Int J Clin Exp Med 2015;8:14553-64.
- Ayd?n A, Aktay G, Yesilada E. A guidance manual for the toxicity assessment of traditional herbal medicines. Nat Prod Commun 2016;11:1763-73.
- Lilienblum W, Dekant W, Foth H, et al. Alternative methods to safety studies in experimental animals: role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch Toxicol 2008;82:211-36.
- Husain GM, Ahmed SS, Azhar M, et al. Comparative toxicity study on classical and modified version of Jawarish Jalinoos (a traditional Unani formulation) in rats. Integr Med Res 2017;6:66-78.
- OECD. Guideline 452: Chronic Toxicity Studies 2009.
- CPCSEA. Standard Operating Procedures (SOP) for IAEC 2010;pp:41-2.
- USFDA. Guidance for Industry M3(R2) Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals 2010;pp:1-30.
- Gad SC. Repeat-dose toxicity studies. In: Drug Safety Evaluation. John Wiley & Sons Inc, 2016;pp:159-67.
- Olson H, Betton G, Robinson D, et al. Concordance of the Toxicity of Pharmaceuticals in Humans and in Animals. Regul Toxicol Pharmacol 2000;32:56-67.
- Liu J, Liu Y, Goyer RA, et al. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci 2000;55:460-7.
- Singh A, Bhat TK, Sharma O. Clinical Biochemistry of Hepatotoxicity. J Clin Toxicol 2014;4:1.
- Boone L, Meyer D, Cusick P, et al. Selection and interpretation of clinical pathology indicators of hepatic injury in preclinical studies. Vet Clin Pathol 2005;34:182-8.
- FDA. CDER-PHRMA-AASLD Conference 2000 Clinical White Paper 2000.
- Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J 2003;79:307-12.
- Hosten AO. BUN and Creatinine. Clinical Methods: The History, Physical, and Laboratory Examinations. Butterworths 1990.
- Tziomalos K, Athyros VG, Karagiannis A, et al. Dyslipidemia induced by drugs used for the prevention and treatment of vascular diseases. Open Cardiovasc Med J 2011;5:85-9.
- Piao Y, Liu Y, Xie X. Change trends of organ weight background data in sprague dawley rats at different ages. J Toxicol Pathol 2013;26:29-34.
- Hayes W, Kruger C. Hayes’ Principles and Methods of Toxicology, Sixth Edition. Hayes A, Kruger C, editors. CRC Press 2014;pp:2184.