Research Article - Journal of Molecular Oncology Research (2018) Volume 2, Issue 2
The relationship between the calcium-sensing receptor and secreted frizzled-related protein in the breast.
Kelly J Gregory1,2*, Stephanie M Morin3, Brooke Bentley4, Mahmoud Elsayad4, Giovanna M Crisi4 and Sallie S Schneider1,5
1Pioneer Valley Life Sciences Institute, Baystate Medical Center, Springfield, MA, USA
2Biology Department, University of Massachusetts, Amherst, MA, USA
3Molecular and Cellular Biology Program, University of Massachusetts, Amherst, MA, USA
4Department of Pathology, Baystate Medical Center, Springfield, MA, USA
5Veterinary and Animal Sciences, University of Massachusetts, Amherst, MA, USA
- *Corresponding Author:
- Kelly Jane Gregory
Pioneer Valley Life Sciences Institute
Baystate Medical Center, Springfield, MA, USA
E-mail: kelly.gregory@baystatehealth.org
Accepted date: March 29, 2018
Citation: Gregory KJ, Morin SM, Bentley B, et al. The relationship between the calcium-sensing receptor and secreted frizzled related protein in the breast. J Mol Oncol Res. 2018;2(2):27-10.
DOI: 10.35841/molecular-oncology.2.2.27-35
Visit for more related articles at Journal of Molecular Oncology ResearchKeywords
SFRP1, CaSR, Mammary gland, Breast cancer
List of Abbreviations
CASR: Calcium Sensing Receptor; SFRP1: Secreted Frizzled- Related Protein 1; ERK1/2: Extracellular-Signal-Regulated Kinase1/2; PTHrP: Parathyroid Hormone Related Protein; CDKN1B: Cyclin Dependent Kinase 1B; MMP9: Matrix Metalloproteinase 9; IL-6: Interleukin 6.
Introduction
Breast cancer is the most frequently occurring cancer in women and between 12% and 13% of women will develop invasive breast cancer over the course of their lifetime. Secreted frizzled-related protein 1 (SFRP1) is a secreted tumor suppressor gene that is significantly downregulated in estrogen receptor (ER) positive breast tumors and in chemoresistant ER negative tumors [1-4]. Loss of SFRP1 expression is associated with poor overall survival in patients with early breast cancer [3]. When SFRP1 is knocked down in immortalized nonmalignant mammary epithelial cells, the cells exhibit an invasive phenotype which resembles the characteristics observed in metastatic breast cancer stem-like cells [5]. Interestingly, reduced expression of SFRP1 leads to a significant resistance to anchorage independent cell death (anoikis), suggesting that loss of SFRP1 may render cells more capable of surviving while circulating in the bloodstream. Furthermore, reduced SFRP1 results in increased ZEB1 expression and epithelial-mesenchymal transition (EMT). As a result, these cells are capable of migrating and invading through extracellular matrix suggesting that loss of SFRP1 contributes to the aggressiveness and metastatic nature of mammary cells [5].
The calcium sensing receptor (CaSR) is a G-protein coupled membrane receptor expressed in several different organs and is involved in the control of serum calcium concentrations [6]. The activated CaSR is capable of binding to a number of different G-proteins which leads to a range of cellular responses, including the release of extracellular Ca2+ [7]. On a cellular level, CaSR regulates gene expression, cell proliferation, differentiation, and cell death. The CaSR is expressed in mammary cells where it regulates a variety of functions. During lactation, Ca2+ concentrations are controlled by way of CaSR-dependent adjustment of the parathyroid hormone related protein (PTHrP) and milk production [8]. In addition to being expressed in healthy cells, the expression of the CaSR is upregulated in human breast cancer cell lines as well as primary breast tumors [9,10]. Similarly, chemicallyinduced rat mammary tumors express elevated Casr mRNA levels when compared with normal mammary tissue [11]. Immunohistochemical analysis of primary tumors from 65 patients with metastatic breast cancer showed that the levels of CaSR expression were variable among tumors and that the variability correlated with tumor activity. Specifically, primary tumors with high levels of CaSR expression were associated with bone metastases while those with lower levels of CaSR expression were not [12]. These data are consistent with the finding that CaSR expression levels are elevated in highly metastatic breast cancer cells [13].
Considering that loss of SFRP1 results in the acquisition of cancer cell characteristics regulated by CaSR and that the expression of the CaSR is upregulated in breast cancer patients and cell lines, we sought to investigate the relationship between SFRP1 and CaSR expression as well as their activity. Our data reveal that SFRP1 is inversely related to CaSR expression in normal murine mammary glands, non-malignant and malignant mammary epithelial cell lines, and human explant breast tissue. Additionally, we provide evidence that inhibition of CaSR activity promotes cell death and reduces cellular proliferation, migration and invasion in mammary epithelial cells deficient in SFRP1. Finally, we reveal that the effects of CaSR inhibition are due in part to effects on the MAPK pathway as well as cytokine expression.
Methods
Animals
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Baystate Medical Center Institutional Animal Care and Use Committee (Permit Number: 132681). Female control and Sfrp1-/- 129/C57Blk6 mice [14,15] (n=6/genotype) were individually housed in plastic cages with food and water provided continuously, and maintained on a 12:12 light cycle. Animals were euthanized and mammary tissue was procured at the following time points: puberty (virgin week 5 (V5)), pregnancy day 15 (P15), lactation day 6 (L6), and involution day 3 (I3).
Immunohistochemistry
Formalin-fixed and paraffin embedded tissue blocks were sectioned at 4 μm on positively charged slides, deparaffinized in xylene, rehydrated in graded ethanols, and rinsed in phosphate-buffered saline (PBS). Immunohistochemistry (IHC) was performed on a DakoCytomation autostainer using the Envision HRP Detection system (Dako, Carpinteria, CA). Heat induced antigen retrieval was performed in a microwave at 98ºC in 0.01 M citrate buffer for 10 min. After cooling for 20 min, sections were rinsed in TBST and were incubated with the primary rabbit polyclonal anti-CaSR antibody (ThermoFisher PAI-37213) for 30 min. The secondary anti- Rabbit HRP polymer was applied for 30 min and visualized by incubation with chromogen diaminobenzidine (DAB) for 5 min. Tissue sections were counterstained with hematoxylin, dehydrated through graded ethanols and xylene, and cover-slipped. Images were captured with an Olympus BX41 light microscope using SPOT Software 5.1 (SPOT™ Imaging Solutions, Detroit, MI).
Human cell and explant culture
The 76N TERT cell line (obtained from Dr. Vimla Band) was stably transfected with either pSUPER.retro (TERT-pSUPER) or siSFRP1-PSUPER.retro (TERT-siSFRP1) plasmids and MCF7 cells were purchased from ATCC (ATTC#HTB-22) and transfected with either pCDNA3.1 or SFRP1-pCDNA3.1 plasmids as previously described [5,16,17]. Cells were grown to 70% confluence in 6-well plates for RNA isolation. For some experiments, cells were treated with DMSO or 5 μM NPS 2143 (SML0362; Sigma, St. Louis, MO) 24 h prior to RNA isolation. Fresh human breast tissue was obtained from women enrolled in the Rays of Hope Breast Research Patient Registry. All subjects were consented to provide excess tissue not needed for diagnostic purposes. The tissue was dissected to isolate fibroglandular tissue enriched with epithelium from the surrounding adipose tissue. The dissected tissue was placed on Surgifoam gelatin sponges (Ferrosan, Sueborg, Denmark) in 60 mm tissue culture dishes containing 3 mL of medium ((phenol red free DMEM/F12 buffered with Hepes and NaHCO3 from Gibco (Invitrogen, Carlsbad, CA)), 5 μg/mL human insulin, 1X antibiotic/antimycotic (100 U/mL penicillin/streptomycin and 0.250 μg/mL amphotericin B), and 10 μg/mL gentamycin (Sigma). The media was supplemented with either 0.1% BSA, DMSO, 1 μg/ml rSFRP1 (SRP3154, Sigma) or 50 μM WAY 316606 (4767, Tocris Bioscience) for 24 h; the tissue was subsequently flash frozen and stored at -80°C until being processed for RNA isolation.
RNA isolation and real-time PCR analysis
Total RNA was extracted from mouse mammary glands (n=6) as well as cells and tissues (n=3/treatment) using an acid-phenol extraction procedure [18], according to the manufacturer’s instructions (Trizol, Invitrogen, Carlsbad, CA).
Relative expression levels of mRNA were determined by quantitative real-time PCR using the Mx3005P® real-time PCR system (Agilent, Santa Clara, CA) and all values were normalized to the amplification of β-actin (ACTB). The PCR primer sequences for mouse Actb and human ACTB have been published [5,14]. Additionally, primer sequences were designed to cross exon junctions using GenScript Real-time PCR Primer Design and are described in Table 1. The assays were performed using the 1-Step Brilliant® SYBRIII® Green QRT-PCR Master Mix Kit (Agilent) containing 200 nM forward primer, 200 nM reverse primer, and 10 ng total RNA. The conditions for cDNA synthesis and target mRNA amplification were performed as follows: 1 cycle of 50°C for 30 min;1 cycle of 95°C for 10 min; and 35 cycles each of 95°C for 30 s, 55°C for 1 min, and 72°C for 30 s. Non-template controls were included to control for primer dimers and reverse-transcriptase controls were included to control for genomic DNA amplification.
| Amplicon | Forward Primer | Reverse Primer |
|---|---|---|
| mouse Casr | 5'-GGTGAGACAGATGCGAGT-3' | 5'- AAAGAAGAAGCAGATGGCAG-3' |
| human CASR | 5'-CCTCCAGCAGACTCCTCAGC-3' | 5'-ATTGTGCCCACCCAGTTCCA-3' |
| human PTHRH | 5'-TGATCGACGACACACGCACT-3' | 5'-AGTCTCCGCTGCATCGTCTC-3' |
| human MMP9 | 5'-GAACCAATCTCACCGACAGG-3' | 5'-GCCACCCGAGTGTAACCATA-3' |
| human IL6 | 5'-TCAATGAGGAGACTTGCCTG-3' | 5'-GATGAGTTGTCATGTCCTGC-3' |
Table 1: Primers utilized for real-time PCR analysis.
Migration and invasion assays
TERT-siSFRP1 cells were plated to confluence in 6 well plates and the cell monolayer was scraped in a straight line to create a “scratch” with a p200 pipet tip as previously described [19]. The cell debris was removed with a gentle PBS wash and the media was replaced with 2 mL of control or treatment media. The cells were cultured for 24 h and images were captured with a Nikon Eclipse TE-2000C light microscope using NIS Elements Basic Research software (version 4.51). TERT-siSFRP1 cells were seeded in serum free media in either BD BioCoat Matrigel™ invasion chambers (BD Biosciences) above media containing 10% FBS. After a 22 h incubation in control or treatment media, chambers were removed and cells were stained with 10% Crystal Violet. Images were captured with an Olympus BX41 light microscope using SPOT Software 5.1.
Western blot analysis
Treated TERT-siSFRP1 cells were washed twice with cold PBS and 100 μL of cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 100 mM NaF, 10 mM MgCl2, 0.5% NP40, protease inhibitor cocktail, and phosphatase inhibitor I and II (Sigma)) added directly to the plate. The cells were incubated for 30 min at 4°C on a shaker and then harvested using a rubber policeman. The lysates were passed 4 times through a 26 gauge syringe, kept on ice for 30 min, and then centrifuged for 20 min at 12,000 rpms at 4°C. The supernatant was transferred to a new tube and the protein was quantified utilizing the BCA™ Protein Assay Kit (Pierce, Rockford, IL). A total of 30 μg of protein was run on a 10% SDS-Page gel and transferred to a PVDF membrane. The membrane was blocked for 45 min with 5% milk in Tris-buffered saline containing 0.05% Tween-20 (TBS-T). The primary antibodies used in this study were Rabbit mAb Phospho-p44-42 MAPK (ERK1/2)(Thr202/ Tyr204) #4377 (1:1000) (Cell Signaling Technologies, Danvers, MA) and Rabbit pAb anti β-actin ab8227 (1:1000) (Abcam, Cambridge, MA), incubated overnight at 4°C. The secondary antibody Goat anti-rabbit IgG-HRP linked antibody #7074 (1:1000)(Cell Signaling Technologies) was applied and incubated for 45 min at room temperature. The blot was washed and developed using a Western Blot Luminol Reagent (Denville Scientific, Holliston, MA). The integrated band densities were measured using ImageJ software.
Cellular proliferation and death assays
To assay for cell proliferation, 5,000 TERT-siSFRP1 cells were plated per well in 96 well plates and the following day, the media was changed to treatment media for 24 h. Cells were next treated with CellTiter 96® AQueous One solution (Promega) according to the manufacturer’s instructions. After a 4 h incubation, the plate was immediately read using the EnSpire™ Multimode Plate Reader (Perkin Elmer, Hopkinton, MA). To assay for cell death, TERT-siSFRP1 cells were incubated with CellTox™ Green Cytotoxicity Assay reagent in suspension with treatment media and plated at a density of 5,000 cells/well in an opaque walled 96 well plate according to the manufacturer’s instructions (Promega). After a 24 h incubation, the plate was read at regular intervals for 72 h using the EnSpire™ Multimode Plate Reader (Perkin Elmer). Additionally, TERT-siSFRP1 cells were grown to 70% confluencey and treated with LIVE/DEAD® Viability/ Cytotoxicity assay reagent (ThermoFisher Scientific) according to manufacturer’s instructions. Images were captured with captured Nikon Eclipse TE-2000C fluorescent microscope using NIS Elements Basic Research software (version 4.51).
Enzyme linked immunosorbent assay
Supernatant was collected from explant cultures treated with either 0.1% BSA or 1 μg/ml rSFRP1 for 24 h for analysis of IL-6 secretion using a human IL-6 ELISA kit (Thermo Scientific, Grand Island, NY) according to the manufacturer’s instructions. Briefly, all reagents were gradually equilibrated to room temperature before use. The lyophilized standard was reconstituted and prepared in serial dilutions ranging from 400 pg/ml to 0 pg/ml. All of the supernatant samples were diluted 1:100 using explant organ culture media as diluent. 50 μL of Biotinylated Antibody Reagent was added to each well except for the blank, then 50 μL of the reconstituted standards and diluted samples were pipetted into corresponding wells. For wells that do not contain standards or samples except for the blank, 50 μl of standard diluent was added. The plate was covered and incubated for 2 h at room temperature. The plate was then washed and Streptavidin-HRP was prepared and 100 μl was added to each well except for the blank, and incubated for 30 min at room temperature. The plate was again washed and 100 μl of TMB substrate was added to all wells including the blank and the color was allowed to develop in the dark for 30 min at room temperature. Finally, 100 μl of Stop solution was added to each well, including the blank, and the plate was immediately read using the EnSpire™ Multimode Plate Reader (Perkin Elmer, Hopkinton, MA).
Statistical analysis
Group means were compared using Student’s t-tests (Graphpad Prism) and results with P<0.05 were considered significant.
Results
The expression of SFRP1 controls the expression of CaSR in normal and malignant breast cells
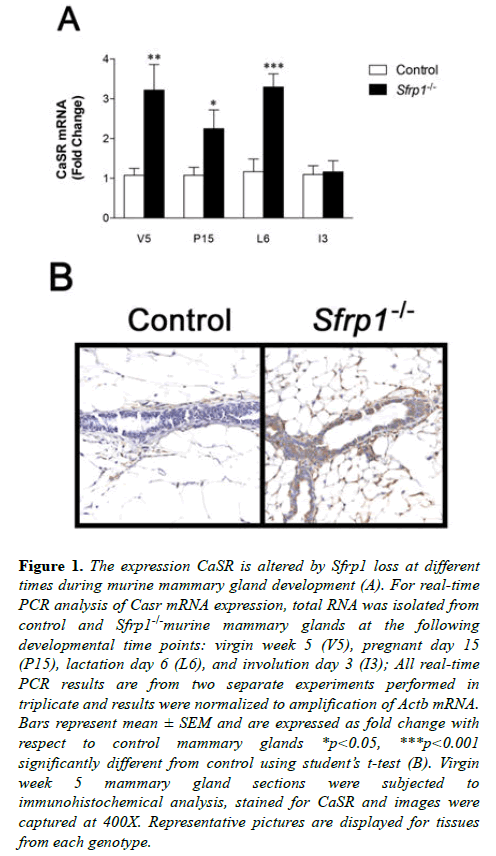
There are several interesting parallels between developmental, bone and cancer signaling pathways when SFRP1 is reduced/ lost or Casr activity is gained. Furthermore, the two proteins appear to be inversely expressed in breast cancer cells. In this study we tested the hypothesis that SFRP1 may in part control the expression level of Casr. Since the expression of Casr is known to increase normally during pregnancy resulting in the highest expression during lactation [8], we chose to examine key developmental time points between puberty and involution to determine whether a targeted deletion of Sfrp1 had any effect on Casr mRNA expression. Total RNA was harvested from mammary glands derived from control and Sfrp-/- mice at the following developmental time points: puberty (virgin week 5 (V5)), pregnancy day 15 (P15), lactation day 6 (L6), and involution day 3 (I3). Real-time PCR analysis revealed that mammary glands from pubescent, pregnant, and lactating Sfrp1-/- mice express significantly higher mRNA levels of Casr mRNA when compared with control wild-type mice (Figure 1A). However, following lactation the presence or absence of SFRP1 did not have an effect on Casr expression. We confirmed the RNA results at the puberty stage by evaluating membrane bound CaSR protein expression. Immunohistochemistry was employed on formalin fixed mammary glands from control and Sfrp1-/- mice. Representative images clearly demonstrate that the membrane of mammary epithelial cells from Sfrp1-/- mice express more CaSR protein (Figure 1B).
Figure 1: The expression CaSR is altered by Sfrp1 loss at different times during murine mammary gland development (A). For real-time PCR analysis of Casr mRNA expression, total RNA was isolated from control and Sfrp1-/-murine mammary glands at the following developmental time points: virgin week 5 (V5), pregnant day 15 (P15), lactation day 6 (L6), and involution day 3 (I3); All real-time PCR results are from two separate experiments performed in triplicate and results were normalized to amplification of Actb mRNA. Bars represent mean ± SEM and are expressed as fold change with respect to control mammary glands *p<0.05, ***p<0.001 significantly different from control using student?s t-test (B). Virgin week 5 mammary gland sections were subjected to immunohistochemical analysis, stained for CaSR and images were captured at 400X. Representative pictures are displayed for tissues from each genotype.
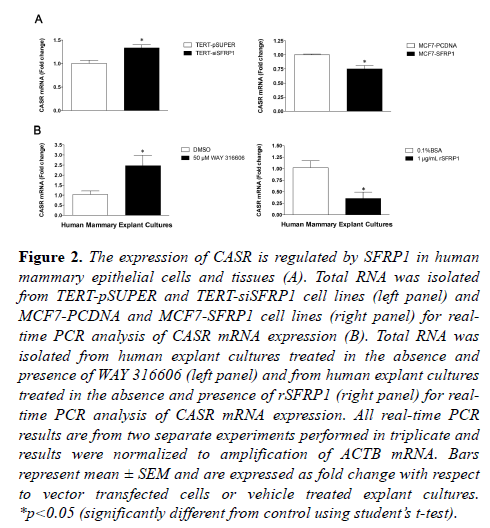
To extend our mouse model findings to human mammary epithelial cells, we examined CASR RNA expression in a variety of breast cell lines in which we modulated SFRP1 via siRNA, gene overexpression or pharmacologic inhibition [5,16,17]. We used an SFRP1 knockdown non-malignant immortalized mammary epithelial cell line (76nTert) to study how loss of SFRP1 affects CASR expression. RNA was isolated from TERT-pSUPER (control) and TERT-siSFRP1 mammary epithelial cells for real-time PCR analysis of CASR mRNA expression. The mRNA levels of CASR were significantly upregulated in response to SFRP1 reduction (Figure 2A, left panel). In order to assess whether CASR expression could be inhibited by SFRP1, we used a breast cancer cell line (MCF7), which does not express SFRP1 normally. RNA analysis of MCF7 cells with re-expression of SFRP1 (MCF7-SFRP1) demonstrated that there is a significant reduction in the expression of CASR and it’s downstream target PTHrP when compared with vector transfected cells (MCF7- pCDNA) (Figure 2B, left panel; Figure S1).
Figure 2: The expression of CASR is regulated by SFRP1 in human mammary epithelial cells and tissues (A). Total RNA was isolated from TERT-pSUPER and TERT-siSFRP1 cell lines (left panel) and MCF7-PCDNA and MCF7-SFRP1 cell lines (right panel) for realtime PCR analysis of CASR mRNA expression (B). Total RNA was isolated from human explant cultures treated in the absence and presence of WAY 316606 (left panel) and from human explant cultures treated in the absence and presence of rSFRP1 (right panel) for realtime PCR analysis of CASR mRNA expression. All real-time PCR results are from two separate experiments performed in triplicate and results were normalized to amplification of ACTB mRNA. Bars represent mean ± SEM and are expressed as fold change with respect to vector transfected cells or vehicle treated explant cultures. *p<0.05 (significantly different from control using student?s t-test).
Additionally, as cell lines can acquire many genetic changes that could alter responses, we examined the effect of SFRP1 inhibition or addition to benign human breast tissue. We employed our explant tissue culture system utilizing breast surgical specimens [17] and cultivated them in the presence and absence of a SFRP1 inhibitor (WAY 316606) or rSFRP1 protein. Consistent with our in vivo and in vitro findings, we show that CASR mRNA expression is significantly upregulated in response to treatment with WAY 316606 (Figure 2B, left panel) and CASR expression is clearly downregulated in explant cultures treated with rSFRP1 protein (Figure 2B, right panel).
Inhibition of CaSR activity reverses the effect of SFRP1 loss on proliferation, survival and migration
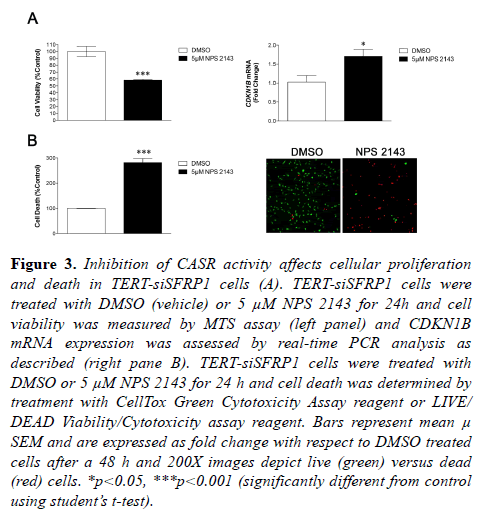
We have previously shown that reducing expression of SFRP1 in immortalized mammary epithelial cells caused them to exhibit characteristics analogous to cancer cells, including increased cellular proliferation, decreased death response, and increased cellular migration and invasion [5]. The CaSR has been previously shown to play a role in these aforementioned cellular processes in breast cancer cells [11,16,17]. Given our finding that CASR is upregulated in TERT-siSFRP1 cells, we sought to determine whether increased CaSR activity may partially explain the more aggressive characteristics observed in the TERT-siSFRP1 cells. Indeed, when TERT-siSFRP1 cells are treated with a CaSR 145 inhibitor (NPS 2143), cell viability is significantly reduced (Figure 3A, left panel). The CaSR has been 146 shown to increase cell proliferation by inhibiting the cyclin dependent kinase, CDKN1B (11). Here we show that inhibition of CaSR activity significantly increases the expression of CDKN1B in these cells (Figure 3A, right panel). Further, we clearly demonstrate that TERT-siSFRP1 cells treated with NPS 2143 exhibit a significant increase in cell death (Figure 3B).
Figure 3: Inhibition of CASR activity affects cellular proliferation and death in TERT-siSFRP1 cells (A). TERT-siSFRP1 cells were treated with DMSO (vehicle) or 5 µM NPS 2143 for 24h and cell viability was measured by MTS assay (left panel) and CDKN1B mRNA expression was assessed by real-time PCR analysis as described (right pane B). TERT-siSFRP1 cells were treated with DMSO or 5 µM NPS 2143 for 24 h and cell death was determined by treatment with CellTox Green Cytotoxicity Assay reagent or LIVE/ DEAD Viability/Cytotoxicity assay reagent. Bars represent mean µ SEM and are expressed as fold change with respect to DMSO treated cells after a 48 h and 200X images depict live (green) versus dead (red) cells. *p<0.05, ***p<0.001 (significantly different from control using student?s t-test).
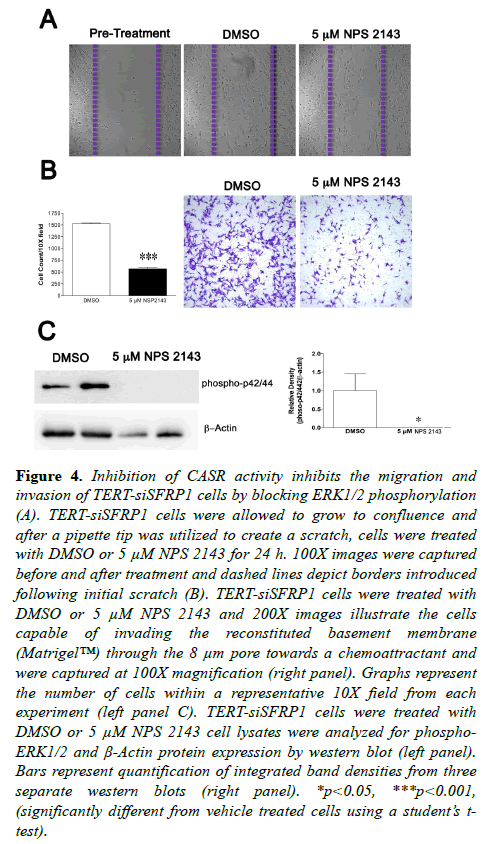
We next measured the migratory and invasive properties of TERT-siSFRP1 cells in the absence and presence of NPS 2143. A scratch wound assay suggested that after a 24 h TERTsiSFRP1 cells were less motile when CaSR activity was inhibited (Figure 4A). To confirm these results and extend them to invasion, TERT-siSFRP1 cells treated with vehicle or NPS 2143 were plated in BD BioCoatTM MatrigelTM Invasion Chambers. The cells that were able to invade the reconstituted basement membrane (MatrigelTM) through the 8 μm pore towards a chemoattractant were stained with 10% crystal violet (Figure 4B). Quantification revealed that TERT-siSFRP1 cells were significantly less invasive when treated with the CaSR inhibitor. The CaSR has been shown to regulate cellular migration through regulation of MAPK/ERK signalling [21].We have demonstrated that phosphorylation of ERK1/2 is elevated TERT-siSFRP1 cells when compared to control transfected cells. Therefore, we sought to establish whether the decrease in cellular migration and invasion in response to CaSR inhibition might be due to a decrease in ERK1/2 phosphorylation. Western blot analysis revealed that when TERT-siSFRP1 cells were treated with NPS 2143, ERK1/2 phosphorylation was completely abolished (Figure 4C).
Figure 4: Inhibition of CASR activity inhibits the migration and invasion of TERT-siSFRP1 cells by blocking ERK1/2 phosphorylation (A). TERT-siSFRP1 cells were allowed to grow to confluence and after a pipette tip was utilized to create a scratch, cells were treated with DMSO or 5 µM NPS 2143 for 24 h. 100X images were captured before and after treatment and dashed lines depict borders introduced following initial scratch (B). TERT-siSFRP1 cells were treated with DMSO or 5 µM NPS 2143 and 200X images illustrate the cells capable of invading the reconstituted basement membrane (Matrigel?) through the 8 µm pore towards a chemoattractant and were captured at 100X magnification (right panel). Graphs represent the number of cells within a representative 10X field from each experiment (left panel C). TERT-siSFRP1 cells were treated with DMSO or 5 µM NPS 2143 cell lysates were analyzed for phospho- ERK1/2 and Ã-Actin protein expression by western blot (left panel). Bars represent quantification of integrated band densities from three separate western blots (right panel). *p<0.05, ***p<0.001, (significantly different from vehicle treated cells using a student?s ttest).
We have demonstrated that phosphorylation of ERK1/2 is elevated TERT-siSFRP1 cells when compared to control transfected cells. Therefore, we sought to establish whether the decrease in cellular migration and invasion in response to CaSR inhibition might be due to a decrease in ERK1/2 phosphorylation. Western blot analysis revealed that when TERT-siSFRP1 cells were treated with NPS 2143, ERK1/2 phosphorylation was completely abolished (Figure 4C).
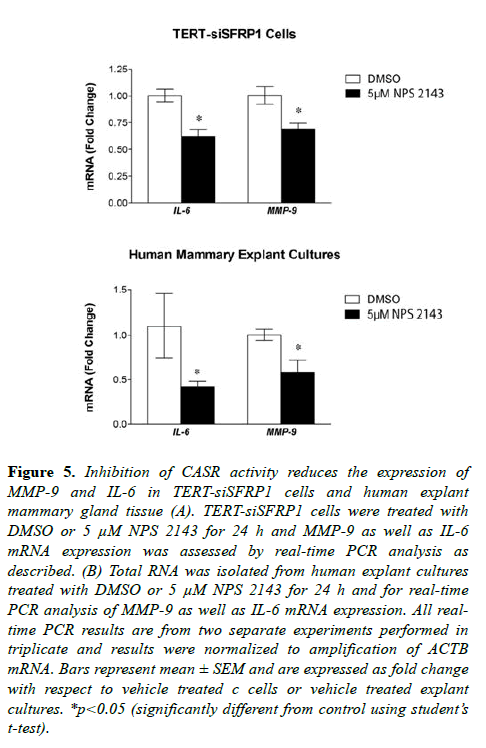
Studies have suggested that secretion of SFRP1 also controls inflammatory cytokine production [22-24], and our published findings reveal that Sfrp1-/- mice exhibit increased cytokine expression in response to diet-induced obesity in murine mammary glands and fat depots [25]. Upregulation of the CaSR has been implicated in metastatic breast cancer by affecting secretory factors including IL-6 as well as protease MMP-9 [20,26]. Considering that TERT-siSFRP1 cells express significantly higher levels of IL-6 and MMP-9 when compared with TERT-pSUPER cells (Figure S2), we sought to determine whether blocking CaSR activity could reverse the expression of this cytokine and protease. We clearly demonstrate that TERT-siSFRP1 cells treated with NPS 2143 exhibit a significant reduction in MMP-9 and IL-6 expression (Figure 5A). Moreover, we found that when CaSR activity is inhibited in normal human breast explants, MMP-9 and IL-6 expression levels are also diminished (Figure 5B).
Figure 5: Inhibition of CASR activity reduces the expression of MMP-9 and IL-6 in TERT-siSFRP1 cells and human explant mammary gland tissue (A). TERT-siSFRP1 cells were treated with DMSO or 5 µM NPS 2143 for 24 h and MMP-9 as well as IL-6 mRNA expression was assessed by real-time PCR analysis as described. (B) Total RNA was isolated from human explant cultures treated with DMSO or 5 µM NPS 2143 for 24 h and for real-time PCR analysis of MMP-9 as well as IL-6 mRNA expression. All realtime PCR results are from two separate experiments performed in triplicate and results were normalized to amplification of ACTB mRNA. Bars represent mean ± SEM and are expressed as fold change with respect to vehicle treated c cells or vehicle treated explant cultures. *p<0.05 (significantly different from control using student?s t-test).
Discussion
The present study reveals that mice with a targeted deletion of Sfrp1 express higher levels of the CaSR in the mammary epithelium during puberty, pregnancy, and lactation. Consistent with these findings, when SFRP1 is downregulated or blocked in human mammary epithelial cells and tissues, CaSR expression is also upregulated. Furthermore, SFRP1 overexpression or treatment diminishes the expression of the CASR mRNA in human breast cancer cells and explant mammary tissues. To investigate the role CaSR signaling plays in the cellular behavior of mammary epithelial cells deficient in SFRP1, TERT-siSFRP1 cells were treated with a CaSR antagonist, NPS 2143, and the consequences on cellular proliferation, death, migration, and invasion were investigated.
We clearly demonstrate that inhibition of CaSR signaling diminishes proliferation and enhances cell death. There is also a reduction in cellular migration and invasion as well as decreased ERK1/2 phosphorphylation in response to CaSR inhibition. Finally, when the effect of CaSR activity on IL-6 and MMP-9 expression was evaluated in TERT-siSFRP1 cells and mammary gland explant cultures, we found that NPS 2143 significantly reduced the expression of MMP-9 and IL-6.
Previously published data from our laboratory has taken advantage of mice with a targeted deletion of SFRP1 [27]. These mice are noted for the increased density of their trabecular bones due to a reduction in the ability of osteoclasts and osteoblasts to undergo apoptosis [28]. Our mammary gland developmental studies in these mice have uncovered an intriguing phenotype of precocious tertiary branching in virgin pre- and post-pubescent mice. Interestingly, the CaSR plays a novel role in lung ontogeny in which extracellular calcium exerts an effect on branching morphogenesis and alveolar expansion via the CaSR [29].
Thus, it is reasonable to speculate that the branching phenotype observed in Sfrp1-/- mice could be due, at least in part, to altered expression of the CaSR. The data presented here clearly demonstrate that when precocious mammary gland branching is first observed in Sfrp-/- mice (V5) [14], the expression of CaSR is significantly upregulated in the mammary epithelial cells when compared with control mice. Normally, Casr mRNA levels in the mouse mammary gland are low during ductal development [8] which supports the notion that SFRP1 regulates the levels of Casr mRNA during this time point and that the atypical mammary gland development may be due in part to aberrant CaSR expression. We also observed differences in CaSR expression between control and Sfrp1-/- mice during pregnancy and lactation which is a time when CaSR levels are typically on the rise, early to mid-pregnancy and subsequently at a peak during lactation [8]. There are no morphological differences between control and Sfrp1-/- mice after the cession of lactation and we also do not see a difference in Casr mRNA levels at this time when the expression of the CaSR is ordinarily is rapidly reduced [8]. This was unexpected because involution is when the levels of SFRP1 are at their highest and we thought we might see a significant decrease in Casr expression. These results suggest that the mechanism of regulation is lost at this point and another transcriptional regulator takes control, it is the loss of SFRP1 that affects CASR and not levels elevated beyond a certain point or the levels of CASR are as low as they go at that point in time.
When we extended our studies to investigate the role SFRP1 expression plays in CASR mRNA regulation in human mammary epithelial cells, we first found that knockdown of SFRP1 (TERT-siSFRP1) increased CaSR expression. Published findings have shown that fully transformed breast cancer cell lines have higher CaSR levels then nonmalignant breast cell lines [13,30]. TERT-siSFRP1 cells exhibit several characteristics consistent with cancer cells [5,31] and this most recent finding certainly adds to this list of attributes. Chemical inhibition of SFRP1 in explant human mammary gland cultures also upregulated CaSR mRNA levels strengthening the inverse relationship between SFRP1 and CaSR. Therefore, we sought to determine whether SFRP1 expression or addition of recombinant SFRP1 protein could diminish the expression of the CaSR. Not only does re-expression of SFRP1 decrease the expression of CaSR in MCF-7 breast cancer cells, but rSFRP addition to human breast explant cultures from women undergoing elective breast surgery confirms this result. The add back experiments suggest that addition of exogenous SFRP1 at some level can temper CASR expression. Additionally, overexpression of SFRP1 in MCF-7 cells also reduces the expression of PTHrP which is linked to the oncogenic role of the CaSR [32]. PTHrP is a peptide growth factor that contributes to the pathogenesis of cancers by stimulating osteolytic bone destruction and releasing bone derived growth factors. Activation of osteoclasts is achieved by increasing the expression of receptor activator of nuclear factor kappa B ligand (RANKL), which is upregulated in Sfrp1-/- mice [14]. Furthermore, SFRP1 has been shown to bind to and inhibit RANKL mediated action [33]. Taken together, these data suggest a novel pathway by which SFRP1 loss may affect tumorigenesis.
Cancer results from cellular mutations that enhance proliferation, decrease anti-proliferative signals, and decrease programmed cell death; and from cellular alterations that enhance metastasis [34]. We hypothesized that the upregulation of the CaSR in TERT-siSFRP1 may play a role in the cancer cell characteristics observed in TERT-siSFRP1 cells [5]. First we found that inhibition of CaSR activity reduced the proliferation of TERT-siSFRP1 cells and promoted cell death. These findings are fully consistent with the data described by Kim et al. showing that down regulation of the CaSR in breast cancer cells inhibited proliferation and stimulated cell death [11]. Additionally, these studies revealed that the CaSR promoted proliferation by reducing the levels of the cyclin dependent kinase inhibitor 1B (CDKN1B). Accordingly, here we show that inhibition of CaSR activity results in an increase in CDNK1B mRNA expression. The CaSR has also been shown to play a role in cellular proliferation by El Hiani et al. in part by increasing ERK1/2 phosphorylation [20,35]. Our data support these findings by showing that inhibition of CaSR reduces ERK1/2 phosphorylation in TERT-siSFRP1 cells.Tumor cell metastasis occurs when primary epithelial cells exit their site of origin and colonize at a distant site. In order for this process to occur, the cells must invade the extracellular matrix, extravasate into blood vessels, survive during loss of attachment and extravasate in secondary organs. Our previous research has shown that TERT-siSFRP1 cells are significantly more migratory and invasive then TERT-pSUPER cells which is due in part to constitutively phosphorylated ERK1/2 expression [5,31]. The data presented here clearly demonstrate that blocking CaSR activity resulted in a reduction in the migratory and invasive behavior of TERT-siSFRP1 cells. Supporting our findings, Saidak et al. showed that CaSR stimulation enhanced the migratory activity of breast cancer cells in part by activating the ERK1/2 pathway [21]. Although activation of the CaSR has been shown affect migration in other cell types including medullary thyroid carcinoma cells and keratinocytes [36,37], the role CaSR plays in cellular invasion presented here is novel. Hiani et al. described a pathway by which stimulation of the CaSR activated membrane metalloproteinases (MMPs) leads to stimulation of ERK1/2 signaling. The expression of MMP-9 is upregulated in TERT-siSFRP1 cells and we therefore hypothesized that the decrease in cellular invasion in response to CaSR inhibition may be partially explained by an effect on MMP-9 expression. We clearly demonstrate that when CaSR activity is blocked in TERT-siSFRP1 and human explant mammary tissue, MMP-9 levels are reduced. Taken together, we speculate that the upregulation of the CaSR in TERT-siSFRP1 cells contributes MMP-9 expression and ERK1/2 mediated cellular invasion which supports the notion that SFRP1 loss could be implicated in cellular metastasis.
Activation of the CaSR has been shown to induce the secretion of a series of pro-inflammatory cytokines in breast cancer cells [26] and its expression may also in turn be regulated by cytokines [38]. SFRP1 is important for tempering Wnt5a and inflammation in macrophages [24,39]. A loss of Sfrp1 has been linked to obesity induced inflammation in the mammary gland as evidenced by increased IL-6 expression and macrophage accumulation [25]. The regulation of IL-6 by SFRP1 is also observed in TERT-siSFRP1 cells and its expression and secretion are decreased when human explant cultures are treated with rSFRP1 (Figure S2A, Figure S3). Here we show that when CaSR activity is blocked in TERT-siSFRP1 cells and human explant mammary gland cultures, IL-6 expression is repressed. These results are fully consistent with the findings presented by Hernandez-Bedolla et al. showing that the same chemical inhibitor utilized in our studies, NPS 2143, decreased the expression and secretion of IL-6 in breast cancer cells [26].
Taken together, these observations provide insight into the role loss of SFRP1 plays in the acquisition of cancer cell characteristics regulated by CaSR. Specifically, when CaSR activity is blocked in epithelial cells deficient in SFRP1, cell death is enhanced and there is a striking reduction in cellular proliferation, migration and invasion which is due in part to effects on the MAPK pathway as well as cytokine expression. Our studies suggest that targeting the CaSR in SFRP1 negative breast cancers may be a powerful therapeutic strategy aimed at preventing breast cancer metastasis or treating established bone metastases.
Acknowledgements
The design of this study, collection, analysis, interpretation, and writing of the manuscript were made possible because of the funding support by the Rays of Hope Foundation.
References
- Zhou Z, Wang J, Han X. Up-regulation of human secreted frizzled homolog in apoptosis and its down-regulation in breast tumors. Int J Cancer. 1998;78(1):95-9.
- Wong SC, Lo SF, Lee KC, et al. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196(2):145-53.
- Klopocki E, Kristiansen G, Wild PJ, et al. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol. 2004;25(3):641-9.
- Bernemann C, Hulsewig C, Ruckert C, et al. Influence of secreted frizzled receptor protein 1 (SFRP1) on neoadjuvant chemotherapy in triple negative breast cancer does not rely on WNT signaling. Mol Cancer. 2014;13:174.
- Gauger KJ, Hugh JM, Troester MA, et al. Down-regulation of sfrp1 in a mammary epithelial cell line promotes the development of a cd44high/cd24low population which is invasive and resistant to anoikis. Cancer Cell Int. 2009;9:11.
- Brown EM. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab. 2013;27(3):333-43.
- Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575-80.
- VanHouten J, Dann P, McGeoch G, et al. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest. 2004;113(4):598-608.
- Cheng I, Klingensmith ME, Chattopadhyay N, et al. Identification and localization of the extracellular calcium-sensing receptor in human breast. J Clin Endocrinol Metab. 1998;83(2):703-7.
- Sanders JL, Chattopadhyay N, Kifor O, et al. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141(12):4357-64.
- Kim W, Takyar FM, Swan K, et al. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein. Cancer Res. 2016;76(18):5348-60.
- Mihai R, Stevens J, McKinney C, et al. Expression of the calcium receptor in human breast cancer--a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol. 2006;32(5):511-5.
- Huang C, Hydo LM, Liu S, et al. Activation of choline kinase by extracellular Ca2+ is Ca(2+)-sensing receptor, Galpha12 and Rho-dependent in breast cancer cells. Cell Signal. 2009;21(12):1894-900.
- Gauger KJ, Shimono A, Crisi GM, et al. Loss of SFRP1 promotes ductal branching in the murine mammary gland. BMC Dev Biol. 2012;12:25.
- Satoh W, Matsuyama M, Takemura H, et al. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis. 2008;46(2):92-103.
- Gregory KJ, Schneider SS. Estrogen-mediated signaling is differentially affected by the expression levels of Sfrp1 in mammary epithelial cells. Cell Biol Int. 2015.
- Gregory KJ, Morin SM, Schneider SS. Regulation of early growth response 2 expression by secreted frizzled related protein 1. BMC Cancer. 2017;17(1):473.
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987;162(1):156-9.
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for 547 analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329-33.
- El Hiani Y, Ahidouch A, Lehen'kyi V, et al. Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sensing receptor-stimulated MCF-7 breast cancer cell proliferation. Cell Physiol Biochem. 2009;23(4-6):335-46.
- Saidak Z, Boudot C, Abdoune R, et al. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp Cell Res. 2009;315(12):2072-80.
- Ehrlund A, Mejhert N, Lorente-Cebrian S, et al. Characterization of the Wnt inhibitors secreted frizzledrelated proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98(3):E503-8. 18.
- Barandon L, Casassus F, Leroux L, et al. Secreted frizzledrelated protein-1 improves postinfarction scar formation through a modulation of inflammatory response. Arterioscler Thromb Vasc Biol. 2011;31(11):e80-7. 19.
- Pereira C, Schaer DJ, Bachli EB, et al. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28(3):504-10.
- Gauger KJ, Bassa, L.M, Henchey, et al. Mice deficient in Sfrp1 exhibit increased adiposity, dysregulated glucose metabolim, and enhanced macrophage infiltration. PLoS One. 2013;8(12):e78320.
- Hernandez-Bedolla MA, Carretero-Ortega J, ValadezSanchez M, et al. Chemotactic and proangiogenic role of calcium sensing receptor is linked to secretion of multiple cytokines and growth factors in breast cancer MDAMB-231 cells. Biochim Biophys Acta. 2015;1853(1): 166-82.
- Satoh W, Gotoh T, Tsunematsu Y, et al. Sfrp1 and Sfrp2 regulate anteroposterior axis elongation and somite segmentation during mouse embryogenesis. Development. 2006;133(6):989-99.
- Bodine PV, Billiard J, Moran RA, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem. 2005;96(6):1212-30.
- Finney BA, Moral PM, Wilkinson WJ, et al. Regulation of mouse lung development by the extracellular calciumsensing receptor, CaR. J Physiol. 2008;586(Pt 24): 6007-19.
- Vanhouten JN, Wysolmerski JJ. The calcium-sensing receptor in the breast. Best Pract Res Clin Endocrinol Metab. 2013;27(3):403-14 27.
- Gauger KJ, Chenausky KL, Murray ME, et al. SFRP1 reduction results in an increased sensitivity to TGF-beta signaling. BMC Cancer. 2011;11:59.
- Chakravarti B, Dwivedi SK, Mithal A, et al. Calciumsensing receptor in cancer: good cop or bad cop? Endocrine. 2009;35(3):271-84.
- Hausler KD, Horwood NJ, Chuman Y, et al. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res. 2004;19(11): 1873-81.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57-70.
- El Hiani Y, Lehen'kyi V, Ouadid-Ahidouch H, et al. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys. 2009;486(1): 58-63.
- Tharmalingam S, Daulat AM, Antflick JE, et al. Calciumsensing receptor modulates cell adhesion and migration via integrins. J Biol Chem. 2011;286(47):40922-33. 33.
- Tu CL, You M. Obligatory roles of filamin A in Ecadherin-mediated cell-cell adhesion in epidermal keratinocytes. J Dermatol Sci. 2014;73(2):142-51.
- Cifuentes M, Fuentes C, Mattar P, et al. Obesity-associated proinflammatory cytokines increase calcium sensing receptor (CaSR) protein expression in primary human adipocytes and LS14 human adipose cell line. Arch Biochem Biophys. 2010;500(2):151-6. 35.
- Pereira CP, Bachli EB, Schoedon G. The wnt pathway: a macrophage effector molecule that triggers inflammation. Curr Atheroscler Rep. 2009;11(3):236-42.