Keywords
|
| Zingiberaceae, Light microscope, Lipid profile, Diabetes mellitus. |
Introduction
|
| Pancreas is a vital endocrine-exocrine organ that produces several hormones and enzymes. Its enzymes help in the digestion of carbohydrates, fats, and proteins whereas its hormones such as insulin regulate carbohydrate metabolism in the body and maintains passage of glucose across the cell membrane [1]. Therefore, any change in the function of the organ may directly affect the physiological function of the body [2,3]. Diabetes mellitus is a chronic endocrine disease widely present in all parts of the world affecting nearly 10% of the population all over the world and considered as one of the leading cause of death in humans [4-6]. The World Health Organization (WHO) estimates that over 300 million people worldwide will have diabetes mellitus by the year 2025 [7]. Diabetes is characterized by multiple defects in its pathophysiology [8] and abnormalities in protein, fat and carbohydrate metabolism [9,10]. It is evident that this disease leads to hyperglycemia, and to many other complications such as hyperlipidemia, hypertension, atherosclerosis, retinopathy, neuropathy and nephropathy [5]. |
| Zingiber officinal roscoe (Z. officinalee) is one of the Zingiberaceae family [11]. Z. officinalee is commonly called ginger. Ginger rhizome is widely used worldwide as a spice. It has been used as a medicine in Asian, Indian and Arabic herbal traditions [12]. Major active components of Z. officinale are thought to be volatile oils, and phenol compounds such as gingerols, Zingbrene and shogaols [13,14]. A lot of researches were done to clarify ginger's influence on lipid profile; HDLC, LDL-C, TC and Triglycerides (TG) [15]. Ginger's effect on glucose and lipid metabolism in both liver and blood was investigated [13,16]. It was reported that high dose (>6 g) intake of ginger might cause gastric reflux in human [17] and IgE-mediated allergy may be produced, due to ginger dust inhalation [18]. Thus, when ginger is consumed at a higher dosages and for long time, it could be toxic [19]. The present study aims to investigate the biochemical and histological changes in the pancreas of rats exposed to aqueous extract of ginger and to observe the hypoglycemic activity of ginger in alloxan induced diabetic female rats. |
Materials and Methods
|
| The fresh rhizomes of ginger were obtained from a local market in Amman, Jordan. |
| Preparation of plant extracts |
| The raw extract was prepared according to the method used by Elshater et al., [20]. Plant parts were separated and washed with distilled water, dried and then grind using blender. Each 30 g powdered plant material was extracted by refluxing with 100 ml of hot water for two weeks at room temperature with shaking at 150 rpm. The extract was filtered through White canvas and filter paper. The mixture was filtered, then the filtrate was centrifuged and the clear supernatant fraction was separated. The concentration was considered to have 1g/ml based on the weight of the starting material. The extracts was purified by filtration through 0.22 μm filter units and kept at -20°C until use. |
| Animals |
| The study was performed on 20 healthy adult female albino rats, with a weight ranging from 190-210 g. The rats were obtained from the animal house of the Faculty of Medicine, University of Jordan, and kept under controlled laboratory conditions under a 12 h dark and 12 h light cycle, at 25°C, and were provided with a diet and water ad libitum. The study was approved by the Institutional Review Board of Al-Balqa Applied University and the University of Jordan, Amman. All procedures related to use of animals was conducted in compliance with ethical guidelines guaranteed by the intuition ethical committee. |
| Induction of Diabetes |
| Alloxan is known as a useful drug to induce diabetes in experimental animals [21]. After overnight fasting diabetes was induced in rats by intraperitoneal injection of alloxan monohydrate (Sigma, St. Louis, MO, USA) dissolved in 1ml distilled water at a dose of 65mg/kg BW [22]. After the injection, animals had free access of food and water, also after 6 hours of injection rats were given solution of 5% D-glucose solution for the next 24 h. Rats were allowed to stabilize for 3 days and blood samples were drawn from the eye vein on the 3rd day to determine the blood glucose concentrations to confirm the occurrence of diabetes, the rats which have blood glucose more than 150 mg/dl were considered diabetic and were used for further experiments [5,23,24]. The treatment with ginger extract was started after induction of diabetes by alloxan and considered as the first day of treatment. The treatment continued for 21 days. |
| Experimental design |
| The rats were randomly divided into 4 groups: I, II, III and IV, 5 animals in each group. Groups III and IV consisted of diabetic rats receiving daily an oral single dose of ginger plant extract through a stomach tube. All animals were fasted before the treatment. Group I (control group; did not receive alloxan), which included rats that were allowed water ad libitum and were fed a standard diet. Group II: Untreated diabetic rats (injected with 65 mg/kg BW of alloxan); received distilled water. Group III: Diabetic rats; received 500 mg/kg BW /day of the Ginger extract Group IV: Diabetic rats; received 1000 mg/kg BW /day of the Ginger extract. All rats were weighed before the start of the experiment and at the end of the experiment. |
| At the end of the (21) days, the rats were sacrificed, relative pancreas weight was computed by expressing the absolute weight of the organ to the animal’s body weight as described below [25]. Relative organ weight=organ weight (g)/body weight (g) × 100. The pancreas was obtained and fixed in 10% Formalin solution for histological examination. It was trimmed, processed, embedded in paraffin wax, sectioned, placed on glass microscope slides, and stained with hematoxylin and eosin (H.E.). |
| Collection of blood samples |
| Blood samples were collected in clean plain tubes from eye vein of all animals three times and the serum was analyzed in laboratory to determine the serum levels of blood glucose and other biochemical parameters. The blood was collected firstly at the beginning of the experiment before injection of alloxan, then on the 3rd day after injection of alloxan to determine the blood glucose concentrations to confirm the development of diabetes mellitus and finally at the end of the experiment (three weeks after injection of alloxan). The collected blood was left to clot then centrifuged at 3500 rpm for 5 min. The serum was separated and stored at - 80°C for later analyses. |
| Biochemical study |
| Laboratory analyses were performed at Jabal Al-Hussein Laboratory, Amman, Jordan. The serum levels of blood glucose were measured using enzymatic colorimetric (GODPAP) method, according to the manufacturer’s instructions (BioSystem, Spain). Insulin was determined using the access ultrasensitive insulin assay (Beckman Coulter, Inc, California). Lipid assays (TG), (TC), (LDL-C) and (HDL-C) were performed using ACCENT-200 methods (CORMAY S.A., Poland). All tests were analyzed according to the manufacturer’s instructions. |
| Statistical analyses |
| Data for all groups were expressed as mean ± standard deviation. Statistical analyses were performed using one-way analysis of variance (ANOVA) and student t-test. Differences were considered to be statistically significant when P value was <0.05, <0.01 or <0.001. All statistical analyses were performed with SPSS software, (version 20 Inc. Chicago, Illinois, IL). |
Results
|
| The final body weight and the percentage weight gain of the rats were not significantly (P>0.05) different from those of the control at the two doses of the extract investigated (Table 1). This study also showed that aqueous extract of ginger had no significant effect on body weight of both the diabetic and nondiabetic rats. Table 2 shows the effect of ginger on serum glucose concentration and insulin level in alloxan-diabetic rats. Administration of ginger to diabetic rats showed remarkably reduction in glucose concentration, there were very highly significant (P<0.001) in rats have 500 mg ginger/kg BW as well as 1000 mg ginger/kg BW when compared with group II (diabetic group). In diabetic rats there were a very highly significant (P<0.001) reduction in insulin level as compared with group I (control group). Concerning the rats treated with the two doses of ginger, there were elevation in insulin level but it was not significant (P>0.05) when compared with group II. Table 3 shows that alloxan produced significant increase in the levels of serum cholesterol (P<0.01), LDL-C (P<0.05), and TG (P<0.01), and a significant decrease in the levels of serum HDL-C (P<0.05), when compared with control group. Moreover, treatment with both doses of ginger extract to diabetic rats produced significant reduction in the levels of serum TC, when compared with diabetic group, while there was a significant (P<0.05) reduction in TG levels in rats treated with 1000 mg ginger/kg BW, when compared with diabetic group. |
| The extract did not significantly (P>0.05) alter the level of HDL-C and LDL-C at both dose investigated. The computed pancreas body weight ratio of the diabetic rats and those treated with 500 mg ginger/kg BW, were not significantly (P>0.05) different from those of their control. |
| However the computed pancreas body weight ratio significantly (P<0.05) increase in rats treated with 1000 mg ginger/kg BW, when compared with diabetic group (Table 4). |
| Histopathological investigation |
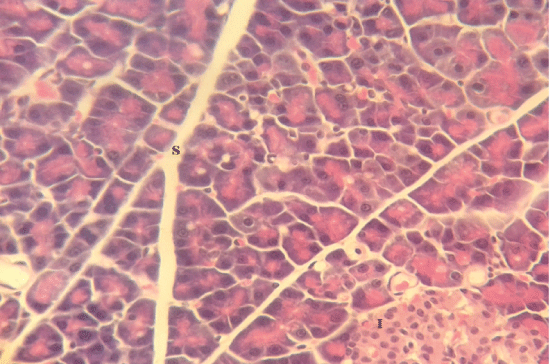
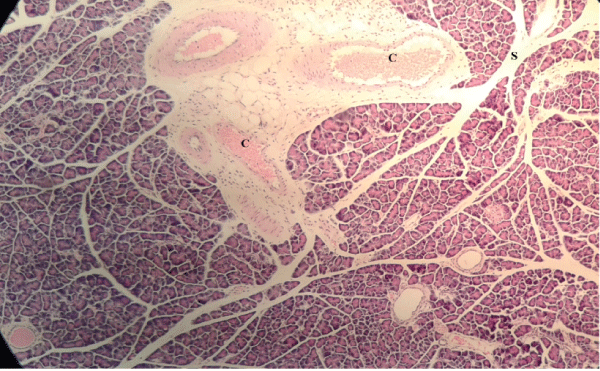
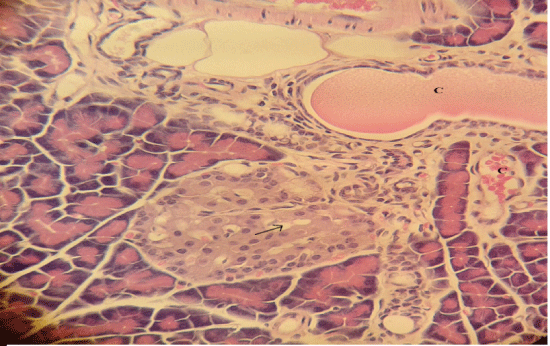
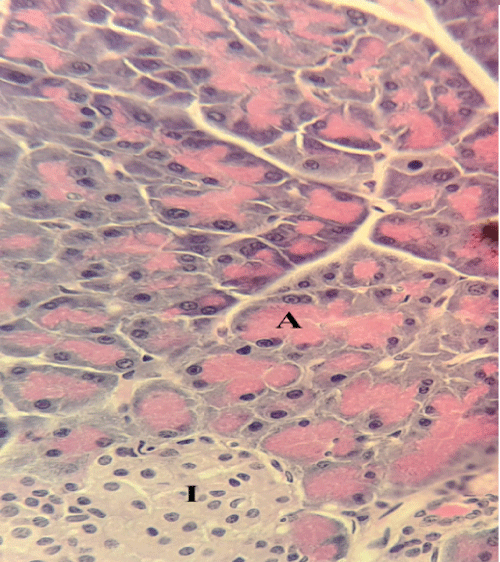
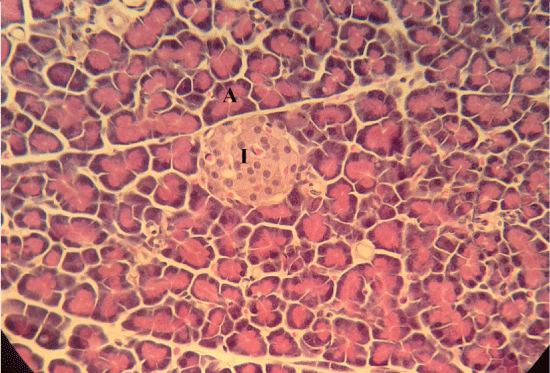
| Microscopically, pancreas from control rat group showed the normal histological structure, the histological appearance of the pancreatic islet cells showed no histopathological changes (Figure 1). In diabetic control rats, abnormalities in the pancreas were recorded. The congestion of RBCs in blood vessels (Figures 2 and 3) as well as thickening of the septa (Figure 2) were obviously seen. In addition, the islets of Langerhans have been prominently changed (Figures 3 and 4) and a slight vaculation of its cells was observed as shown in Figure 3. |
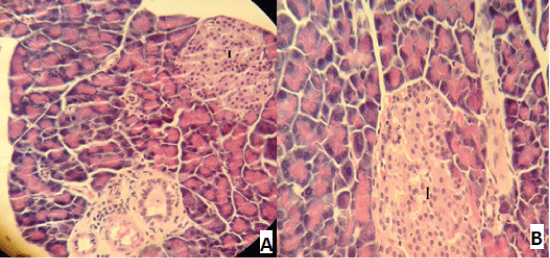
| Compared with the diabetic untreated group, approximately normal structure of acinar cells and islets of Langerhans were seen in animals given oral 500 mg/Kg BW of ginger (Figure 5). Moreover, Microscopic examination of the pancreatic sections of rats treated with 1000mg /Kg BW ginger revealed numerous small sized normal islets of Langerhans (Figure 6). |
Discussion
|
| Diabetes is one of many leading causes of morbidity and mortality in all countries of the world. There are reports that several medicinal plant such as ginger rhizome have hypoglycemic and hypolipidemic effects [24,26]. The results of this study indicated that administration of ginger extract at 500 and 1000 mg/kg of diabetic female rats for 21 days decreased blood glucose level, TC and TG and increased serum insulin and HDL-C levels. These findings were in agreement with other previous studies [27,28]. Khattab et al., [26] found that diabetic rats exhibited significant improvement in biological evaluation, glucose, insulin and lipid profile, when compared with untreated diabetic group. |
| The mechanism of hypolipedemic action of medicinal plant might be due to the inhibition of dietary lipid absorption in the intestine or its production by liver or stimulation of the bilary secretion of cholesterol and cholesterol excretion in the feces [29-31]. In this study, treatment of diabetic rats with aqueous ginger extract was significantly reduced serum glucose level in comparison with diabetic group. Although the reduction was significantly higher in comparison to normal control group, it was not enough to achieve normal values in control rats as illustrated in Table 2. These results are in agreement with previous studies [32]. |
| In the current study the decreasing levels of serum triglycerides after the treatment at a dose of 1000 mg/kg BW with ginger extract may be due to the stimulating effect of ginger extract on insulin. A similar result was reported by Akhani et al., [33] who reported that treatment of diabetic rats with ginger produced a significant increase in insulin levels and a decrease in fasting glucose levels in diabetic rats. Treatment with ginger was also found to decrease in serum cholesterol, serum triglyceride and blood pressure in diabetic rats. Ozougwu and Eyo, [34] found that the increasing dosages (200, 250 and 300mg/kg BW) of Z. officinalee aqueous extracts produced dose-dependent significant (P<0.05) reductions in the blood glucose levels of diabetic rats. |
| It has been reported that ginger interfere with the activities of some digestive enzymes in rats [19] such as pancreatic amylase and lipase [35]. Besides, in rabbit it acted on the liver to reduce cholesterol biosynthesis and may stimulate cholesterol’s conversion to bile acids and increase its fecal excretion [36]. In the current study, the results showed that, microscopic examination of the pancreatic sections of diabetic untreated group revealed necrosis and atrophy of β-cells of islets of Langerhans, and congestion of pancreatic blood vessels. This results are in accordance with the findings of other studies [26,37]. The number of normal islets of diabetic pancreas was reduced as recorded by Qinna and Badwan, [38]. As well as, the endochylema of islets reported to have serious necrosis and granular degeneration. |
| The examined sections of pancreas for diabetic rats treated with both doses of ginger showed no histopathological changes. On the other hand, the sections for diabetic untreated rats revealed slight vacuolation of sporadic β-cells of islets of Langerhans. This result agree with other reports [39,40]. Ginger is a good source of antioxidant and therefore may be capable of preventing tissue damage, it can protect the pancreas tissues from lipid peroxidation on diabetic rats [41,42]. |
| According to the literature, Streptozotocin and alloxan are considered as the most used diabetogenic compound for inducing diabetes in experimental animal models [38,43]. There is little risk of toxicity when used as a spice. Acute toxicity tests in mice found no mortality or adverse effect when ginger extract was given at doses up to 2.5 g/kg over a 7 day period. Increasing the dose to 3-3.5 g/kg leading to 10-35% mortality [15]. On the other hand, other researchers found that, daily doses administration of ginger 500, 1000 and 2000 mg/kg BW of female and male rats for 35 days, was not associated with any abnormalities and mortalities [19]. |
Conclusion
|
| The levels of blood glucose, total serum lipids and total serum cholesterol, which were raised in alloxan induced diabetic rats can be lowered by ginger aqueous extracts. Histopathological investigation of pancreatic sections of diabetic rats treated with ginger at either doses, revealed approximately normal structure of acinar cells and islets of Langerhans, compared with the diabetic untreated group. Overall, it may be concluded that aqueous extract of ginger possesses hypoglycemic and hypolipidemic potential in alloxan induced diabetic rats. |
Acknowledgment
|
| We are grateful to the University of Jordan, Amman for providing the research facilities. |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
References
|
- Tiwari AK, Rao JM (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects. CurrSci 83: 30-38.
- Hall JE. Secretory function of the alimentary tract. Guyton & Hall’s textbook of medical physiology. 11th ed. 2006; Philadelphia, PA: Saunders. pp: 791-807.
- Abd El-Haleem MR, Mohamed DA. The effects of experimental aflatoxicosis on the pancreas of adult male albino rats and the role of ginger supplementation: a histological and biochemical study. The Egyptian Journal of Histology 2011; 34: 423-435.
- Burke JP,Williams K, Narayan KMV, Leibson C, Haffner SM, Stern MP. A population perspective on diabetes prevention: Whom should we target for preventing weight gain? Diabetes Care.2003; 26: 1999-2004.
- Anfenan MLK. Evaluation of Nutritional and Antidiabetic Activity of Different Forms of Ginger in Rats. Middle-East Journal of Scientific Research 2014; 21:56-62.
- Jafri SA, Rehman K, Qasim M, Kalsoom. Effect of MurrayaKoenigii, CatharanthusRoseus and PsidiumGuajava Leaves Extract on Blood Glucose in Alloxan Induced Diabetic Rats. AmJBiol Life Sci 2014; 2: 1-5.
- Park MK, Jung U, Roh C. Fucoidan from marine brown algae inhibits lipid accumulation. Drugs 2011;9:1359-1367.
- Dheer R, Bhatnagar P. A study of the antidiabetic activity of Barleriaprionitis Linn. Indian J Pharmacol 2010;42:70-73.
- Mohommadi J, Saadipour K, Delaviz H, Mohommadi B. Antidiabetic effects of an alcoholic extract of Juglansregia in an animal model. Turk J Med Sci 2011; 41: 685-91.
- Anoja PA, Kamani APW, Lakmini KBM. Study of antihyperglycaemic activity of medicinal plant extracts in alloxan induced diabetic rats. AncSci Life 2013; 32:193-198.
- Zachariah TJ. Ginger. In: Parthasarathy VA, Champakam B, Zachariah TG. editors. Chemistry of Spices 2008; CABI, pp: 70-100.
- Shinashal RZ, AL-Sultan RK, Abdalmajeed SA. The Effect of Ginger on The Histopatholoical Lesions of Salmonella Typhimurium in Mice Liver in Comparison with Cephalexin. J Kirkuk UnivSci Stud2012; 7: 14-23.
- Ali B, Tanira G, Nemmar A. Some phytochemical, pharmacological and toxicological properties of Ginger (Zingiberofficinale Roscoe), a review of recent research. Food chemToxicol 2008; 46: 409-420.
- Shen CL, Hong KJ, Kim SW. Effects of ginger (ZingiberofficinaleRosc.) on decreasing the production of inflammatory mediators in sow osteoarthritic cartilage explants. J Med Food 2003; 6: 323-328.
- Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacologic investigation of ginger (Zingiberofficinale). J Ethnopharmacol 1989; 27: 129-140.
- Al-Noory AS, Amreen AN, Hymoor S. Antihyperlipidemic effects of ginger extracts in alloxan-induced diabetes and propylthiouracil-induced hypothyroidism in (rats). Phcog Res 2013; 5: 157-161.
- Desai HG, Kalro RH, Choksi AP. Effect of ginger & garlic on DNA content of gastric aspirate. Indian J Med Res 1990; 92:139-141.
- Van Toorenenbergen AW, Dieges PH. Immunoglobulin E antibodies against coriander and other spices. J Allergy ClinImmunol 1985;76: 477-481.
- Xiang R, Peng G, Suzuki T,Yang Q, Yamahara J, Li Y. 35-day gavage safety assessment of ginger in rats. RegToxicolPharmacol 2009; 54: 118-123.
- Elshater AA, Salman MMA, Moussa MMA. Effect of Ginger extract consumption on levels of blood glucose, lipid profile and kidney functions in alloxan-induced diabetic rats. Egypt Acad J BiolSci 2009; 2: 153-162.
- Vinuthan MK, Girish KV, Ravindra JP, Jayaprakash,Narayana K. Effect of extracts of Murrayakoengii leaves on levels of blood glucose and plasma insulin in alloxan-induced diabetic rats. Indian J PhysiolPharmacol 2004; 48: 348-352.
- Al-Logmani A, Zari T. Long-term effects of Nigella sativa L. oil on some physiological parameters in normal and streptozotocin-induced diabetic rats. J DiabMellit 2011;1: 46-53.
- Ledoux SP, Woodley SE, Patton NJ, Willson LG. Mechanism of nitrosourea-induced beta cell damage. Alt DNA Diabetes 1986; 35: 866-872.
- Al-Haj Baddar NW, Aburjai TA, Taha MO, Disi AM. Thujone corrects cholesterol and triglyceride profiles in diabetic rat model. Nat Prod Rese 2011;25: 1180-1184.
- Bashir L, Shittu OK, Busari MB, Sani S, Aisha MI. Safety Evaluation of Giant African land Snails (Archachatinamarginata) Haemolymph on Hematological and Biochemical Parameters of Albino Rats. J Adva Med PharmSci 2015; 3:122-130.
- Khattab HAH, Al-Amoudi NS, Al-Faleh AA. Effect of Ginger, Curcumin and Their Mixture on Blood Glucose and Lipids in Diabetic Rats. J Life Sci 2013;10:428-442.
- Ugwuja EI, Nwibo AN, Ugwu NC, Aloke C. Effects of Aqueous Extract of Spices Mixture Containing Curry, Garlic and Ginger on Plasma Glucose and Lipids in Alloxan-induced Diabetic Rats. Pak J Nutri 2010;9: 1131-1135.
- Prasad SS, Kumar S, Vajpeyee SK, Bhavsar VH. To Establish the Effect of Ginger-Juice ZingiberOfficinale (Zingiberaceae) on Important Parameters of Lipid Profile. Int J PharmaSci Res 2012; 3: 352- 356.
- Garjani A, Fathiazad F, Zakheri A, Akbari NA, Azarmie Y. The effect of total extract of Securigerasecuridaca L. seeds on serum lipid profiles, antioxidant status, and vascular function in hypercholesterolemic rats. J Ethnopharmacol 2009; 126: 525-532.
- Jayasooriya AP, Sakono M, Yukizaki C, Kawano M, Yamamoto K, Fukuda N. Effects of Momordicacharantia powder on serum glucose levels and various lipid parameters in rats fed with cholesterol-free and cholesterol-enriched diets. J Ethnopharmacol 2000;72: 331-336.
- Ahmadvanda H, Tavafic M, Khalatbary AR. Hepatoprotective and Hypolipidemic Effects of SaturejaKhuzestanica Essential Oil in Alloxan-induced Type 1 Diabetic Rats. Iran J Pharma Res 2012;11: 1219-1226.
- Abd-Elraheem AE, Salman MMA, Mahrous MA, Moussa MMA. Effect of Ginger Extract Consumption on levels of blood Glucose, Lipid Profile and Kidney Functions in Alloxan Induced-Diabetic Rats. Egypt Acad J biologSci 2009; 2: 153-162.
- Akhani SP, Vishwarkarma SL, Goval RK. Anti-diabetic activity of Zingiberofficinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol 2004; 56:101-105.
- Ozougwu JC, Eyo JE. Evaluation of the Activity of Zingiberofficinale (Ginger) Aqueous Extraction on Alloxan-Induced Diabetic Rats. Pharmacologyonline 2011; 1: 258-269.
- Ramakrishna RR, Platel K, Srinivasan K. In vitro influence of spices and spice-active principles on digestive enzymes of rat pancreas and small intestine. Nahrung 2003;47:408-412.
- Verma SK, Singh M, Jain P, Bordia A. Protective effect of ginger, ZingiberofficinaleRosc on experimental atherosclerosis in rabbits. Indian J ExpBiol 2004;42: 736-738.
- Qadori YT. Histological studies on pancreatic tissue in diabetic rats by using wild cherry. The Iraqi Postgra Med J 2011;10: 421- 425.
- Qinna NA, Badwan AA. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Design Devel Therapy 2015; 9: 2515-2525.
- Aggarwal BB. Targeting inflammation induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutri 2010; 30:173-199.
- Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda- Bukhsh AR.[6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicolo Letters 2012; 210: 34-43.
- Usha K, Saroja S. Antitubercular potential of selected plant materials. J Med Arom Plant Sci 2000; 22: 182-184.
- Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinal on dyslipidemia in diabetic. J EthnopharMacol 2005; 97:227-230.
- Etuk EU. Animals models for studying diabetes mellitus. AgriBiol J North Am 2010;1: 130-134.
|





