- Biomedical Research (2015) Volume 26, Issue 3
Synthesis of some tricyclic indeno [1, 2-d] pyrimidine derivatives as a new class of anti-breast cancer agents.
Mostafa M. Ghorab1, 2*, Mansour S. Alsaid1
1Department of Pharmacognosy, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Kingdom of Saudi Arabia
2Deprtment of Drug Radiation Research, National Center for Radiation Research and Technology, Atomic Energy, Authority, P.O. Box 29, Nasr City, Cairo, Egypt.
- *Corresponding Author:
- Mostafa M. Ghorab
Department of Pharmacognosy
College of Pharmacy King Saud University
P.O. Box 2457, Riyadh 11451 Saudi Arabia
Accepted date March 21 2015
Abstract
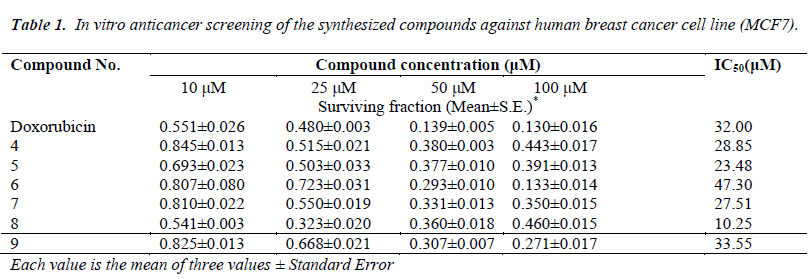
As part of our search for anti-breast cancer agents a new series of tricyclic 4-substituted-2-yl)-2- thioxo-3, 4-dihydro-1H-indeno [1, 2-d] pyrimidin-5(2H)-one 4-9 were obtained in one pot synthesis by a modification of the Biginelli Reaction. The structures of the synthesized compounds were characterized by microanalyses, IR, 1H-NMR, 13C-NMR and mass spectral data. All the synthesized compounds were evaluated for their in vitro anticancer activity against human breast cancer cell line (MCF7). Most of the screened compounds showed interesting cytotoxic activities compared to Doxorubicin as reference drug. Compounds 8, 5, 7 and 4 (IC50 values 10.25, 23.48, 27.51 and 28.85 μM) revealed higher cytotoxic activities than the Doxorubicin as reference drug with IC50 value (32.00 μM). Also, compound 9 is nearly as active as Doxorubicin with IC50 values (33.55 μM). Compound 6 showed moderate activity.
Keywords
Synthesis, indenopyrimidines, anti-breast cancer activity.
Introduction
Most cancer patients are subjected to chemotherapy for the treatment of advanced cancers. However, most metastatic solid tumors eventually remain incurable even by treatment with recent anticancer drugs. Also, Cancer is a disease of striking significance in the world today. It is the second leading cause of death in the world after cardiovascular diseases and it is projected to beginning the primary cause of death there within the coming years [1, 2]. Cancer is a top killer of human beings. Thus, great urgency to develop highly efficacious and minimally toxic treatments for cancer. Although tremendous progress has been achieved in the development of novel cancer treatments, most of the current cancer drugs usually exhibit high toxicity and are severely resisted by tumor cells in the clinic. This dilemma is particularly true for DNAdamaging agents, the mainstay of cancer treatment [3]. Cancer is still continuing to be a major earth problem Worldwide. The development of new anticancer therapeutic agents is one of the fundamental goals in medicinal chemistry as cancer causes about 13% of all the death [4].
Surpassing cardiovascular diseases, it is taking the position number one killer due to various factors [5]. Also the treatment of cancer is associated with various side effects which include bone marrow depression, alopecia, druginduced cancer, hepatotoxicity, and many more. Because of the need and value of anticancer drugs, many laboratories are intensively investigating the chemistry and biology of novel anticancer agents. Also the development of resistance against the existing anticancer drugs and cytotoxicity and genotoxicity of anticancer drugs to the normal cells are othermajor problems in cancer therapy, keeping research window open in search for newer anticancer molecules [6]. But the window passage has become narrower because it is rather hard to search a molecule that can selectively inhibit the proliferation of abnormal cells only with least or no affect on normal cells. Multicomponent condensation reactions (MCRs) have recently been discover to be a powerful method for the synthesis of organic compounds, since the products are formed in a single step and diversity can be achieved by simply varying each component [7-9].
Indenopyrimidine and their derivatives have been studied due to a variety of chemical and biological significance. The importance of indenopyrimidines as biologically active compounds includes their use as antibacterial [10- 12], antiallergic[13], antitumor [11,12] [14, 15] antifolate [16], tyrosine kinase [17], antimicroibial [18], calcium channel antagonists [19], antibacterial [20-23], anti inflammatory, analgesic [24], antihypertensive [25], antileishmanial [26] tuberculostatic [27], anticonvulsants [28], diuretic, potassium sparing [29], and antiaggressive activities [30]. Also, indenopyrimidines were found to possess several pharmacological properties, including anticancer activity [31-35]. On the other hand, the pyrimidines constitute an important class of drug, with several types of pharmacological agents possessing anticancer activity [36-39] among others. A large number of structurally novel pyrimidines have ultimately been reported to show substantial anticancer activity in-vitro and in vivo [40]. Several mechanisms have been reported for anticancer activity of the pyrimidine compounds and the most prominent of these mechanisms was through the inhibition of the carbonic anhydrase [41-44]. The mechanism of tumor inhibition by pyrimidine carbonic anhydrase (CA) inhibitor was suggested by Chegwidden and Spencer [45]. In continuation of our work it seemed of interest to design and synthesize some novel series of indeno [1, 2-d] pyrimidine derivatives to evaluate their antibreast cancer activity.
Experimental
Melting points (oC, uncorrected) were determined in open capillaries on a Gallenkemp melting point apparatus (Sanyo Gallenkemp, Southborough, UK). Recoated silica gel plates (silica gel 0.25 mm, 60 G F 254; Merck, Germany) were used for thin layer chromatography, dichloromethane/ methanol (9.5: 0.5 mL) mixture was used as a developing solvent system and the spots were visualized by ultraviolet light and/or iodine. Infrared spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). 1H-NMR spectra (in DMSO-d6) were recorded on Bruker Ac-300 ultra-shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 300 MHz, using TMS as internal standard. Electron impact Mass Spectra were recorded on a, Shimadzu Gc- Ms- Qp 5000 instrument (Shimadzu, Tokyo, Japan). Elemental analyses were performed on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany). All compounds were within ± 0.4% of the theoretical values.
Results
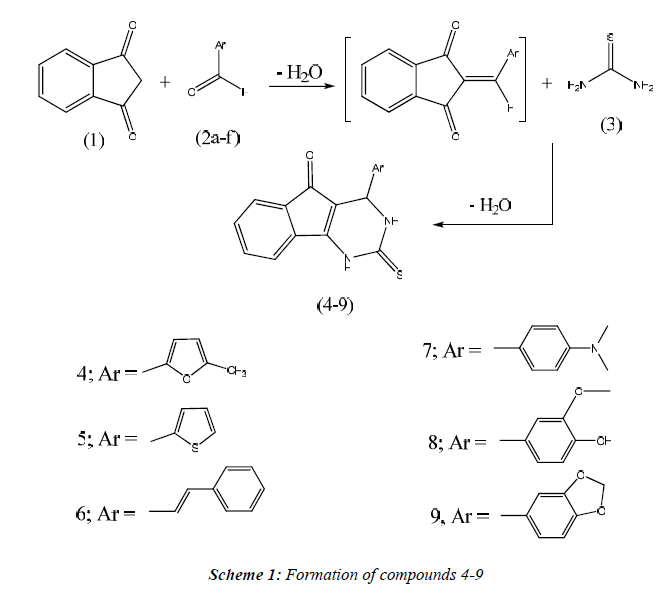
General Procedure for the synthesis of compounds 4-9.
A mixture of thiourea (0.76g, 0.01 mole). Aldehydes (0.01 mole) and 1H-indene-1, 3(2H) - dione (1.46g, 0.01 mole) in absolute ethanol containing 37% HCl (4 drops) was heated under reflux for 3 h. The reaction mixture was allowed to cool, filtered off and recrystallized from dioxane to give compounds 4-9, respectively.
Synthesis of 4-(5-methylfuran-2-yl)-2-thioxo-3, 4- dihydro-1H- indeno [1, 2-d] - pyrimidin-5(2H) - one (4). Yield, 89%; m.p. 192.5 °C; IR (KBr, cm-1): 3468 (NH), 3057 (CH arom.), 2986, 2841 (CH aliph.), 1716 (C=O), 1240 (C=S). 1H NMR (DMSO-d6) δ: 2.4 [s, 3H, CH3], 5.3 [s, 1H, CH-4], 6.1, 6.2 [2d, 2H, 2CH furan, J= 7.7 Hz], 7.1-8.1 [m, 4H, Ar-H], 9.3, 12.8 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C-NMR (DMSO-d6): 12.7, 57.4, 110.3, 110.4, 111.3, 121.6, 124.8, 127.6, 131.9, 133.7, 134.5, 152.6, 152.8, 153.7, 172.1, 191.6. MS m/z (%): 296 [M+] (23.12), 81 (100). Anal. Calcd. for C16H12N2O2S: C, 64.85; H, 4.08; N, 9.45. Found: C, 64.49; H, 4.37; N, 9.74.
Synthesis of 4-(thiophen-2-yl)-2-thioxo-3, 4-dihydro-1Hindeno [1, 2-d] pyrimi-din-5(2H)-one (5). Yield, 79%; m.p. 162.6 oC; IR (KBr, cm-1): 3446 (NH), 3074 (CH arom.), 1722 (C=O), 1271 (C=S). 1H NMR (DMSO-d6) δ: 5.2 [s, 1H, CH-4], 6.7-8.2 [m, 7H, Ar-H], 9.4, 12.8 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C-NMR (DMSO-d6): 57.2, 108.6, 122.4, 123.5, 124.7, 125.9, 126.4, 127.7, 131.8, 133.6, 134.0, 137.3, 154.1, 175.6, 193.0. MS m/z (%): 298 [M+] (9.17), 92 (100). Anal. Calcd. for C15H10N2OS2: C, 60.38; H, 3.38; N, 9.39. Found: C, 60.07; H, 3.76; N, 9.78.
Synthesis of 4-(styryl-2-thioxo-3, 4-dihydro-1H- indeno [1, 2-d] pyrimidin-5(2H)-one (6). Yield, 70%; m.p. >320 oC; IR (KBr, cm-1): 3420 (NH), 3091 (CH arom.), 2922, 2838 (CH aliph.), 1714 (C=O), 1279 (C=S). 1H NMR (DMSO-d6) δ: 4.5 [s, 1H, CH-4], 6.4, 6.6 [2d, 2H, CH=CH, J= 7.3 Hz], 7.1-8.2 [m, 9H, Ar- H], 9.5, 13.0 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C-NMR (DMSO-d6): 58.3, 104.6, 120.7, 120.9, 125.5, 126.7, 127.2, 127.6 (2), 127.9 (2), 128.0, 132.6, 133.7, 134.9, 135.5, 153.0, 171.6, 190.8. MS m/z (%): 318 [M+] (3.78), 102 (100). Anal. Calcd. for C19H14N2OS: C, 71.67; H, 4.43; N, 8.80. Found: C, 71.39; H, 4.15; N, 9.11.
Synthesis of 4-(4(dimethylamino) phenyl) - 2-thioxo-3, 4- dihydro-1H-I ndeno [1, 2 -d] pyrimidin-5(2H) - one (7). Yield, 92%; m.p. 239.8 °C; IR (KBr, cm-1): 3420, 3157 (NH), 3100 (CH arom.), 2955, 2876 (CH aliph.), 1706 (C=O), 1266 (C=S). 1H NMR (DMSO-d6) δ: 3.2 [s, 6H, 2-N (CH3)], 4.9 [s, 1H, CH-4], 6.8, 8.2 [m, 8H, Ar-H], 9.1, 12.6 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C-NMR (DMSO-d6): 42.7 (2), 55.8, 103.4, 110.6 (2), 119.9, 123.8 (2), 124.7, 126.8, 130.6, 132.8, 133.9, 134.1, 151.3, 157.0, 169.8, 189.3. MS m/z (%): 335 [M+] (33.54), 119 (100). Anal. Calcd. for C19H17N3OS: C, 68.03; H, 5.11; N, 12.53. Found: C, 68.32; H, 4.88; N, 12.21.
Synthesis of 4-(4-hydroxy-3-methoxyphenyl) - 2-thioxo-3, 4-dihydro-1H-indeno [1, 2-d] pyrimidin-5(2H)-one (8). Yield, 69%; m.p. 218.9 °C; IR (KBr, cm-1): 3436 (OH), 3391 (NH), 3088 (CH arom.), 2966, 2839 (CH aliph.), 1720 (C=O), 1282 (C=S). 1H NMR (DMSO-d6) δ: 3.7 [s, 3H, OCH3], 4.8 [s, 1H, CH-4], 6.8, 8.3 [m, 8H, Ar-H], 9.4, 12.8 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C NMR (DMSO-d6): 54.3, 56.7, 105.6, 110.1, 114.2, 117.6, 120.8, 124.7, 126.9, 131.7, 133.5, 134.2, 135.6, 143.9, 145.2, 157.3, 172.6, 189.7. MS m/z (%): 338 [M+] (2.78), 122 (100). Anal. Calcd. for C18H14N2O3S: C, 63.89; H, 4.17; N, 8.28. Found: C, 63.57; H, 4.49; N, 8.53.
Synthesis of 4-(benzo[d][1,3]dioxol-5-yl))- 2-thioxo-3,4- dihydro-1H-indeno[1,2-d]pyrimidin-5(2H)-one (9). Yield, 88%; m.p. 214.3 °C; IR (KBr, cm-1): 3438 (NH), 3081 (CH arom.), 2918, 2863 (CH aliph.), 1718 (C=O), 1237 (C=S). 1H NMR (DMSO-d6) δ: 5.1 [s, 1H, CH-4], 6.2 [s, 2H, CH2], 6.7-8.0 [m, 7H, Ar-H], 9.0, 12.3 [2s, 2H, N1-H + N3-H, exchangeable with D2O]. 13C -NMR (DMSO-d6): 55.3, 100.1, 104.3, 111.6, 111.8, 116.6, 121.4, 128.3, 128.4, 132.6, 133.1, 134.5, 134.7, 148.4, 148.7, 156.1, 176.0, 194.8. MS m/z (%): 336 [M+] (4.65), 74 (100). Anal. Calcd. for C18H12N2O3S: C, 64.27; H, 3.60; N, 8.33. Found: C, 64.54; H, 3.26; N, 8.02.
In vitro anticancer activity
The cytotoxic activity was measured in vitro for the newly synthesized compounds using the SulfoRhodamine-B stain (SRB) assay using the method of [45]. The in vitro anticancer screening was done at the Pharmacology Unit, the National Cancer Institute, Cairo University. Cells were plated in 96- multiwall microtiter plate (104- cells/well) for 24h before treatment with the compound(s) to allow attachment of cell to the wall of the plate. Test compounds were dissolved in DMSO and diluted with saline to the appropriate volume. Different concentrations of the compound under test (10, 25, 50 and 100 μM) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the compound(s) for 48h at 37 oC and in atmosphere of 5% CO2. After 48h, cells were fixed, washed, and stained for 30 min. with 0.4% (W/V) with SRB dissolved in 1% acetic acid. Excess unbound dye was removed by four washes with 1% acetic acid and attached stain was recovered with Tris -EDTA buffer. Color intensity was measured in an enzyme- linked immunosrbent assay ELISA reader. The relation between surviving fraction and drug concentration is plotted to get the survival curve for breast tumor cell line after the specified time [45]. The molar concentration required for 50% inhibition of cell viability (IC50) was calculated and the results are given in (Table 1). The relationship between surviving fraction and drug concentration was plotted to obtain the survival curve of breast cancer cell line (MCF7). The response parameter calculated was IC50 value, which corresponds to the concentration required for 50% inhibition of cell viability.
In- vitro anti-breast cancer activity
All the synthesized compounds were evaluated for their in vitro anticancer activity against human breast cancer cell line, MCF7. Doxorubicin, which is one of the most effect tive anticancer agents, was used as the reference drug in this study. The relationship between surviving fraction and drug concentration was plotted to obtain the survival curve of breast cancer cell line (MCF7). The response parameter calculated was the IC50 value, which corresponds to the concentration required for 50% inhibition of cell viability. Table 1 shows the in vitro cytotoxic activity of the synthesized compounds. Most of the tested compounds exhibited significant activity compared to the Doxorubicin as reference drug. From the results of Table 1, it was found that indenopyrimidine containing biologically active 3- methoxy-4-hydroxyphenyl, 2-thioxo 8, 2-thienyl at 4-position with thioxo group at 2-position 5, Ndimethylphenyl at 4-position with thioxo moiety at 2-position 7 and 5-methylfuran at 4-position with thioxo moiety at 2-position 4 with IC50 values (10.25, 23.48, 27.51, and 28.85 μM) exhibitedmore potent anti-breast cancer activity than the reference drug with IC50 value (32.00 μM). Farther, indenopyrimidine bearing the biologically active pipronyl moiety at 4-position with thioxo group at 2-position 9 with IC50 values (33.55 μM) is nearly as active as Doxorubicin as positive control. On the other hand, compounds 6 having styryl moiety at 4-position with thioxo group at 2-position revealed slightly lower activity of Doxorubicin with IC50 value (than that 47.30 μM).
Discussion
The aim of this work was the design and synthesis of some new series of tricyclic indeno [1, 2-d] pyrimidine 4- 9 carrying a miscellanies biologically active moiety at 4- position, thioxo group at 2-position and carbonyl group at 5-position (Scheme 1) and evaluation of their anticancer activity. The structure of compounds 4-9 was elucidated on the basis of microanalyses, IR, 1H-NMR, 13C-NMR, and mass spectral data. The IR spectrum of compound 4 revealed the presence of bands for NH at 3468 cm-1, (C=O) at 1716 cm-1, (C=S) at 1240 cm-1 Also, 1H-NMR spectrum indicated the presence of signals at 2.4 ppm which could be assigned to CH3 group, 5.3 ppm attributed to CH-4 and two doublets at 6.1, 6.2 for furan ring. 13CNMR spectrum of compound 4 in (DMSO-d6) revealed signals at 172.1 ppm assigned to (C=S), 191.6 due to (C=O) group. IR spectrum of compound 5 exhibited the characteristic bands at 3446 cm-1 (NH), 1722 cm-1 (C=O), 1271 cm-1 (C=S). 1H-NMR spectrum of 5 showed signals at 5.2 ppm according to CH-4, 9.4, 12.8 ppm assigned to N1-H and N3-H. 13C-NMR spectrum of compound 5 revealed signals at 175.6 ppm due to (C=S) group and 193.0 ppm attributed to (C=O). IR spectrum of compound 6 showed the characteristic bands at 3420 cm-1 (NH), 1714 cm-1 (C=O), 1279 cm-1 (C=S). 1H-NMR spectrum of 6 exhibited signals at 4.5 ppm assigned to CH-4, two dublets appeared at 6.4, 66 ppm for CH=CH and 9.5, 13.0 ppm assigned to N1-H and N3-H. 13C-NMR spectrum of compound 6 revealed signals at 171.6 ppm due to (C=S) group and 190.8 ppm attributed to (C=O) group. IR spectrum of compound 7 revealed the presence of bands for NH at 3420 cm-1, (C=O) at 1706 cm-1, (C=S) at 1266 cm-1 1H-NMR spectrum of compound 7 indicated the presence of signals at 3.2 ppm which could be assigned to N- (CH3)2 group, 4.9 ppm attributed to CH-4. 13C-NMR spectrum of compound 7 in (DMSO-d6) revealed signals at 169.8 ppm assigned to (C=S), 189.3 ppm due to (C=O) group. IR spectrum of compound 8 revealed the presence of bands for NH at 3436 cm-1 (OH), 3391 cm-1 (NH), 3088 cm-1 (CH arom.), 2966, 2839 cm-1 (CH aliph.), 1720 cm-1 (C=O), 1282 cm-1 (C=S). Also, 1H-NMR spectrum indicated the presence of signals at 3.7 ppm which could be assigned to OCH3 group, 4.8 ppm attrib uted to CH-4. 13C-NMR spectrum of compound 8 in (DMSO-d6) revealed signals at 172.6 ppm assigned to (C=S), 189.7 attributed to (C=O) group. IR spectrum of compound 9 revealed the presence of bands for NH at 3438 cm-1 (NH), 3081 cm-1 (CH arom.), 2918, 2863 cm-1 (CH aliph.), 1718 cm-1 (C=O), 1237 cm-1 (C=S) . 1HNMR spectrum indicated the presence of signals at 6.2 ppm which could be assigned to CH2 group of pipronyl moiety, 5.1 ppm attributed to CH-4 and two signals at 9.0, 12.3 ppm for 2NH groups. 13C-NMR spectrum of compound 9 in (DMSO-d6) revealed signals at 176.0 ppm assigned to (C=S), 194.8 due to (C=O) group.
Conclusion
The objective of the present study was to synthesize and investigate the anticancer activity of some indenopyrimidine derivatives carrying the biologically active thione moiety at 2-position. Compounds 8, 5, 7 and 4 showed promising anti-breast cancer activity higher than that of Doxorubicin as reference drug , while compounds 9 is nearly as active as Doxorubicin. In addition compound 6 revealed a moderate activity compared with the Doxorubicin as positive control.
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project no. RGP- VPP- 302.
References
- Gibbs JB. Mechanism-based target dentification anddrug discovery in cancer research. Science 2000; 287: 1969-1971.
- Unger C. New therapeutic approaches in cancer treatment.Drug Future 1997; 22: 1337-1345.
- Poul EP. Oral cancer prevention and control – The approach of the World Health Organization, Oral Oncology 2009; 45: 454-460.
- Noolvi MN, Patel HM, Bhardwaj V, Chauhan A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: search for anticancer agent. Eur J Med Chem 2011; 46 (6): 2327-2346.
- Aydemir N, Bilalo?glu R.Genotoxicity of two anticancer drugs, gemcitabine and topotecan, in mouse bone marrow in vivo, Mutation Research 2003; 537 (1): 43-51.
- Dandia A, Arya K, Khaturia S. Facile one pot microwave enhanced multistep synthesis of novel biologically important scaffold spiro [indolepyridopyrimidines. Arkivoc 2005; (xiii): 80-88.
- Domling A, Ugi I, Multicomponent reactions with isocyanides. AngewChemInt Ed 2000; 39(18): 3168- 3210.
- James DS, Stephen FM. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds, Chemistry - A Eur J 2009; 15, (6): 1300- 1308.
- Nargund LVG, Reddy YSR, Jose R. Synthesis and antibacterial activity of pyrido [1, 2-a] pyrimidin-4 (1H)-ones. Indian Drugs 1991; 29 (1): 45-46.
- Broom AD, Shim JL, Anderson GL. Pyrido [2, 3-d] pyrimidines. IV. Synthetic studies leading to various oxopyrido [2, 3-d] pyrimidines. J Org Chem 1976; 41 (7): 1095-1099.
- Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA. Synthesis and antitumor activity of 2,4-diamino-6- (2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d] pyrimidine. J Med Chem 1980; 23: 327-329.
- Furukawa K, Hasegawa T. Preparation of pyrido[2, 3- d] pyrimidine-2, 4-di-one derivatives as antiasthmaticsand antiallergics. Chem Abs 1996; 124: 289568c.
- Rosowsky A, Mota CE, Queener SF. Synthesis pyrido[4, 3-d] pyrimidine analogues of trimetrexate and piritrexim. J Hetero Chem 1995; 32 (1): 335-340.
- Thompson AM, Bridges AJ, Fry DW, Kraker AJ, Denny WA. Tyrosine kinase inhibitors.7.7-amino-4- (phenylamino)-and7-amino-4-phenylmethyl) amino] pyrido[ 4, 3-d] pyrimidines: A new class of inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J Med Chem 1995; 38: 3780-3788.
- Donkor IO, Klein CL, Liang L, Zhu N, Bradley E, Clark AM. Synthesis and antimicrobial activity of
- some 6, 7-annulatedpyrido [2, 3-d] pyrimidines. J Pharm Sci 1995; 84(5): 661-664.
- Pastor A, Alajarin R, Vaquero JJ, Alvarez-Builla J, Casa-Juana MFD, Sunkel C, Priego JG, Fonseca I,
- Sanz-Aparicio J. Synthesis and structure of new pyrido[2,3-d] pyrimidine derivatives with calcium channel antagonist activity. Tetrahedron 1994; 50 (27): 8085- 8098.
- Matsumoto J, Minami S. Pyrido [2, 3-d] pyrimidine antibacterial agents. 3. 8-alkyl- and 8-vinyl-5, 8-
- dihydro-5-oxo-2-(1-piperazinyl) pyrido [2, 3- d] pyrimidine- 6-carboxylic acids and their derivatives. J Med Chem 1975; 18: 74-79.
- Suzuki N. Synthesis of antimicrobial agents. V. Synthesis and antimicrobial activities of some heterocyclic condensed- 1, 8-naphthyridine derivatives. ChemPharm Bull 1980; 28 (3): 761-768.
- Oakes V, Rydon HN. Polyazanaphthalenes. Part IV. Further derivatives of 1:3:5- and 1:3:8-triazanaphthalene. J ChemSoc 1956; 4433-4438.
- Degraw JI, Kisliuk RL, Gaumont Y, Baugh CM. Antimicrobial activity of 8-deazafolic acid. J Med Chem1974; 17 (4): 470-471.
- Kolla VE, Deyanov AB, Nazmetdinov FY, KashinaZN, Drovosekova LP. Investigation of the anti-inflammatory and analgesic activity of 2-substituted 1- aryl-6-carboxy-(carbethoxy)-7-methyl-4-oxo-1,4-dihydropyrido [2,3-d] pyrimidines. J. Pharm Chem 1993; 27 (9): 635-636.
- Ellingboe JW, Princeton NJ.Substituted pyridopyrimidinesand antihypertensives.Chem Abs 1996;
- 124: 176134q.
- Agarwal A, Ashutosh R, Goyal N, Chauhan PMS, Gupta S. Dihydropyrido [2, 3-d] pyrimidines as a new class of antileishmanial agents. J Bioorg Med Chem2005; 13 (24): 6678-6684.
- Bystryakova ID, Burova OA, Chelysheva GM, ZhilinkovaSV, Smirnova NM, Safonova TS.Synthesis and biological activity ofpyrido [2, 3-d] pyrimidines. J Pharm Chem 1991; 25 (12): 874-876.
- Deyanov AB, Niyazov RK, Nazmetdivov FY, SyropyatovBY, Kolla VE, Konshin ME. Synthesis and biological activity of amides and nitriles of 2- arylamino- 5-carboxy (carbethoxy)-6-methylnicotinic acids and 1- aryl-6-carbethoxy-7-methyl-4-oxo-1, 4-dihydropyrido [2, 3-d] pyrimidines.J Pharm Chem 1991; 25 (4): 248- 250.
- Monge A, Martinez-Merino Sanmartin VC, Fernandez FJ, Ochoa MC, Berllver C, Artigas P, Fernandez- Alvarez E. 2-Arylamino-4-oxo-3,4-dihydropyrido [2, 3-d] pyrimidines: Synthesis and diuretic activity. Eur J Med Chem 1989; 24 (3): 209-216.
- Saladowska H, Bartoszko-Malik A, Zawisza T. Synthesis and properties of new derivatives of ethyl 7-methyl- 2, 4-dioxo-1, 2, 3, 4-tetrahydropyrido [2, 3-d] pyrimidine- 5-carboxylate.Farmaco 1990; 45 (1): 101-110.
- Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer 2002; 2: 188-200.
- Israel M, Jons LC, Modest EJ. 6H-indeno [1, 2-b] pyrido [3,2-e]pyrazines. A new heterocyclic ring system.J Heterocyclic Chem 1972; 9 (2): 255-262.
- Vigante B, Trizitis G, Tirzite D, Chekavichus B, UldrikisJ, Sobolev A, Duburs G. 4-(10-Methyl 10Hphenothiazin- 3-yl) 1,4-dihydropyridines, 4,5- dihydroindeno[1,2-b]-and5,5-dioxo-4,5 dihydrobenzothieno [ 3,2-b] pyridines. Chem Hetero-cyclic Compounds 2007; 43 (2): 225-232.
- Utsugi T, Aoyagi K, Asao T, Okazaki S, Aoyagi Y, Sano M, Wierzba K, Yamada Y. Antitumor activity of a novel quinoline derivative, TAS-103, with inhibitory effects on topoisomerases I and II. Jpn J Cancer Res 1997; 88 (10): 992-1002.
- Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong YS, Anderson LA, Ortega AJ, Van SS, SteelantWF, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Komienko A. Three-component synthesis and anticancer evaluation of polycyclic indenopyridines lead to the discovery of a novel indenoheterocycle with potent apoptosis inducing properties. Org BiomolChem 2007; 5 (23): 3865-3872.
- Ghahremanzadeh R, Shakibaei GI, Ahadi S, Bazgir AJ. One-pot, pseudo four-component synthesis of a spiro[diindeno[1, 2-b:2′,1′-e]pyridine-11,3′-indoline] trioneLibrary. J Comb Chem 2010; 12 (1): 191-194.
- Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: E7070, a sulfonamide anticancer agent, potently inhibits cytosolic isozymes I and II, and transmembrane, tumor-associated isozyme IX. BioorgMed ChemLett 2004; 14: 217-223.
- Ghorab MM, Noaman E, Ismail MM, Heiba HI, AmmarYA, Sayed MY. Novel antitumor and radioprotectivesulfonamides containing pyrrolo [2, 3-d] pyrimidines.Arzneimittelforschung 2006; 56: 405-413.
- Ismail MM,Ghorab MM, Noaman E, Ammar YA, Heiba HI, Sayed MY. Novel synthesis of pyrrolo [2, 3- d] pyrimidines bearing sulfonamide moieties as potential antitumor and radioprotective agents.Arzneimittelforschung2006; 56: 301-308.
- Rostom SA. Synthesis and in vitro antitumor evaluation of some indeno [1, 2-c] pyrazol (in) es substituted with sulfonamide, sulfonylurea (thiourea) pharmacophores, and some derived thiazole ring systems. Bioorg Med Chem 2006; 14: 6475-6485.
- Supuran CT, Casini A, Mastrolorenzo A, ScozzafavaA. COX-2 selective inhibitors, carbonic anhydrase inhibition and anticancer properties of sulfonamides belonging to this class of pharmacological agents. Mini- Rev Med Chem 2004; 4: 625-632.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential, Exp. OpinTher Patents2000; 10: 575-600.
- Andres AC, Arthur IC. Oxidative stress toxicology, and pharmacology of CYP2E1. Annual Review of Pharmacology and Toxicology 2004; 44: 27-42.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem – ImmunolEndocrMetabolAgents 2001; 1(1): 61-97.
- Kivela AJ, Kivela J,Saamio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours, World J Gastroenterol 2005; 11: 155-163.
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Bioorg Med Chem2001; 9(3): 703–714.
- Ghorab MM, Ismail ZH, Abdalla M. Synthesis and biological activities of some novel triazoloquinazolinesand triaz-inoquinazolines containing benzenesulfonamidemoieties. Arzneimittelforschung 2010; 60 (2): 87-95.
- Skehan P, StorengR, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer- drug screening. J Natl Cancer Inst 1990; 82: 1107-1112.