- Biomedical Research (2013) Volume 24, Issue 4
Study of oxidative stress in pre- and post-hemodialysis in chronic renal failure patients.
N. S. Nagane1, J.V. Ganu2, P.E. Jagtap11Department of Biochemistry, Bharati Vidyapeeth Deemed University Medical College And Hospital, Sangli-416416 (Maharashtra), India
2Department of Biochemistry, Govt. Medical College Miraj, Miraj 416410 (Maharashtra), India
- Corresponding Author:
- N.S. Nagane
Department of Biochemistry
Bharati Vidyapeeth Deemed University Medical College and Hospital
Sangli 416416 (Maharashtra), India
Accepted Date: August 16 2013
Citation: Nagane NS, Ganu JV, Jagtap PE. Study of oxidative stress in pre- and post-hemodialysis in chronic renal failure patients. Biomedical Research 2013; 24 (4): 498-502.
Abstract
Chronic renal failure is a progressive loss of renal function over a period of months or year and Hemodialysis is a suitable treatment which is preferably given to the chronic renal failure ernment RF) . Who are not to undergo renal transplantation therapy. This study was carried out to observe the immediate effect of oxidative stress and inflammation during hemodialysis . In the present study 30 CRF patients undergoing hemodialysis and 50 healthy controls matching in age and sex were included. In the present study prehemodialytic samples showed significant rise (P<0.001) in serum lipid peroxidation (LPO), serum homocysteine and high sensitivity C-reactive Protein (hs-CRP) as compared to controls. Mean values of serum superoxide dismutase (SOD) and serum nitric oxide (NO? )were found to be significantly reduced (P<0.001)in prehemodialytic samples as compared to controls. In post hemodialytic samples mean values of serum superoxide dismutase, serum nitric oxide and serum homocysteine were significantly reduced (P<0.001)when compared to prehemodialytic samples while mean values of serum lipid peroxidation and hs-CRP were significantly increased (P<0.001)in post hemodialytic samples as compared to prehemodialytic samples. The result of our study have been shown there is extensive oxidative stress and inflammation in patients with CRF, which is further augmented by hemodialysis, as supported by altered levels of LPO, SOD, NO?, homocysteine and hs-CRP. It may cause increased risk of cardiovascular disorder (CVD) in patients on chronic hemodialysis.
Keywords
Chronic renal failure (CRF), Hemodialysis (HD), high sensitivity C-reactive protein (hs-CRP), Oxidative stress
Introduction
Chronic renal failure is associated with increased inflammation and oxidative stress which play an important role in the in the development of cardiovascular disorder and it represent leading cause of death in chronic renal failure (CRF) patients. Several factors contribute to cardiovascular disease in patients with chronic renal failure (CRF), notable among the CRF-induced risk factors are lipid disorders, oxidative stress, inflammation, endothelial dysfunction, and depressed nitric oxide availability which plays an important role in the development of cardiovascular disease [1, 2]. The CRF patients who undergo to dialysis; are subjected to an oxidative stress, dialysis induced oxidative stress is one of the possible reason which creates atherosclerotic changes in hemodialysis patients. Over last few years, there has been increasing focus on the relationship of novel risk factors such as inflammation, oxidative stress, hyperhomocysteinemia, and high hs-CRP levels are associated with cardiovascular risk in CRF [3, 4].
Oxidative stress arises due to the imbalance between the reactive oxygen species (ROS) and reactive nitrogen species (RNS) production and impairment of antioxidant defense mechanism [5]. Reactive oxygen species may cause lipid peroxidation and damage macromolecules resulting in the impairment in the superoxide dismutase activity [6]. There is increasing evidence which supports that ROS are involved in the pathophysiology of uremic complications in CRF patients and mainly those patients who are undergoing hemodialysis treatment. Elevated RNS may lead to oxidative stress, as indicated by the fact that an impaired nitric oxide (NO•) synthetic pathway plays a key role in mediating renal injury. It has also been suggested that oxidant exposure may contribute to the high rate of cardiovascular disease in these patients [7, 8].
C - reactive protein, an acute–phase reactant synthesized in the liver in response to the cytokine activation and may act as a marker of inflammatory mediators involved in the development of atherosclerotic plaque [9]. Chronic inflammation is an important component in the development and progression of atherosclerosis, and numerous epidemiological studies have demonstrated that increased serum CRP concentrations are positively associated with a risk of future coronary artery disease, or peripheral arterial disease [10]. Recent studies suggested that chronic inflammatory state could account for the high risk of ischemic heart disease in patients with End stage renal disease (ESRD)[11]. Homocyseine , a non essential sulphur- containing amino acid and it’s accumulation in CRF leads to impair it’s metabolism and reduced renal excretion [12]. Hyperhomocysteinemia is an additional factor that increases the risk of vascular diseases in renal failure patients [13].
Hemodialysis (HD) is the treatment which is preferably given to the CRF patients who do not undergo renal transplantation therapy. In recent years hemodilalysis has been successful in extending life span of renal patients and is effective in correcting the metabolic abnormalities related to renal oxidative stress that contributes to morbidity in hemodialysis patients. Factors like dialysis membrane, purity of dialysis water and dietary limitations make dialysis patients on dialysis susceptible to more oxidative stress and inflammation [14, 15].
Hence we thought it worthwhile to determine the alterations of oxidant-antioxidant status and vascular inflammation in pre and post hemodialysis of CRF patients. For this, we planned to determine the levels of Lipid peroxidation (LPO), superoxide dismutase (SOD), Nitric Oxide (NO•), Homocysteine and high sensitivity C-reactive protein (hs - CRP) in pre and post hemodialysis patients.
Material and Methods
In the present study, 30 CRF patients and 50 healthy controls matching in age and sex were enrolled. The patients were selected on the basis of their estimated glomerular filtration rate (eGFR) according to National Kidney Foundation guidelines, for the classification of kidney disorder. All the selected patients were in the 4th and 5th stages of CRF, undergoing hemodialysis [16, 17].
All control subjects were free of infection and were taking no medication during last two weeks. Institutional Ethical Committee approval was taken before starting this study and informed concent was obtained from each subject before they were included in the study. Plain heparin dose 1000 IU was administered during HD process. Hemodialysis was carried out three times per week for four hours per session using polysulphone hemodialysis membrane. These samples were analyzed to observe the immediate effect of hemodialysis process on the biochemical parameter.
5-8ml of blood samples were obtained from the patients just before and after the hemodialysis by using aseptic precautions, in pain tubes. Separated sera were processed for the assay of biochemical analytes. Lipid Peroxidation was measured as MDA in serum by the method of Kei Satoh [18]. The colour produced by the reaction of thiobarbituric acid with MDA was measured at 530 nm with the help of spectrophotometer and the results were expressed as nmol/ml. Superoxide Dismutase was assayed by the Nischal HK et.al, this method is based on the ability of superoxide dismutase to inhibit autoxidation of pyrogallol under specific conditions. Spectrophotometric readings were taken at 420 nm and results were expressed as units/ml [19]. Nitric Oxide was assayed by the method of Najwa K.Cortas and Nabil W. Wakid by cadmium reduction method and colour complex produced was measured at 540nm and result results were expressed as μmol/L [20]. Homocysteine was assayed by chemiluminescence and results were expressed as μmol/l [21]. Serum hs-CRP levels were measured by turbidometric method and values were expressed as mg/L [22] .
Statistical Analysis
The statistical analysis included paired and unpaired “t” test and regression analysis for correlation coefficient. All data were presented as Mean and standard deviation. P< 0.05 was considered significant.
Results
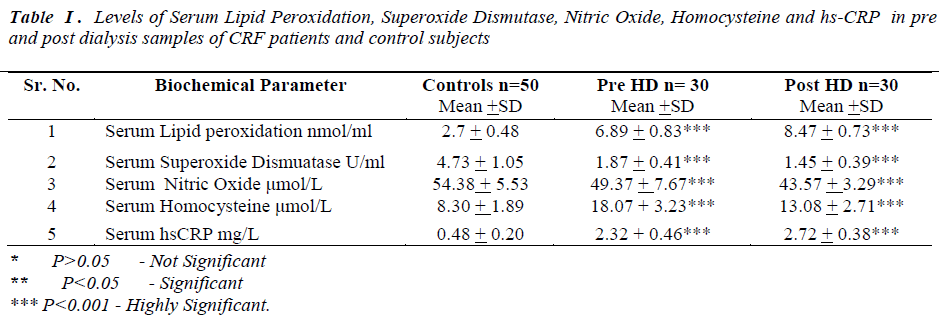
Laboratory data showed a significant change in the levels of biochemical parameters in pre and post hemodialysis groups in comparison to normal controls (Table I).
All the data was presented as mean and standard deviation unpaired‘t’ test was used to compare prehemodialysis groups with control groups. Paired‘t’ test was used for comparing post hemodialysis groups with a prehemodialysis groups. P value of < 0.005 was considered statistically significant.
In the present study prehemodialytic samples showed significant rise (P<0.001) in serum lipid peroxidation (LPO), serum homocysteine and high sensitivity C reactive Protein (hs-CRP) as compared to controls. Mean values of serum superoxide dismutase (SOD) and serum nitric oxide (NO•)were found to be significantly reduced (P<0.001)in prehemodialytic samples as compared to controls. In post hemodialytic samples mean values
Discussion
Oxidative stress and inflammation recently came in foucs as nonconventional risk factors of cardiovascular morbidity and overall mortality in end stage renal disease. Oxidative stress occurring due to decreased antioxidant defences and an increase in pro-oxidant factors is a well recognized phenomenon in hemodialysis patients.
Hemodialysis is an effective treatment in most renal failure patients and forms an alternative to renal transplantation. Adequate dialytic treatment has prolonged the survival of patients with quality of life. Cardiovascular disease was found to be the most frequent cause of mortality in majority of patients on maintenance haemodialysis [23].
In our study we found mean values of lipid peroxidation were significantly elevated and superoxide dismutases were significantly reduced in post hemodialytic samples, as compared to prehemodialytic samples. There are two potential mechanisms by which hemodialysis could stimulate the production of reactive oxygen species. First, interactions between blood and the dialyzer membrane could lead to the generation of inflammatory mediators, such as the complement components C3 and C5a or platelet activating factor (PAF), which may then stimulate neutrophils. Second, bacterial products in the dialysate might cross the dialyzer membrane and directly or indirectly stimulate release of reactive oxygen species by neutrophils [24]. Disturbances in enzymatic mechanisms of free radical detoxification may be responsible to elevate the LPO level in post HD sample [25]. Endothelial cells also contain some superoxide dismutase enzyme to the cell surface, which probably serves to counterbalance the local formation of oxidants [26]. Previous studies have shown that overproduction of H2O2 because of decreased GPx activity. Due to increased oxidative stress, H2O2 may inhibit the SOD activity [27]. During the hemodialysis process the dialysis membrane is subjected to immunologic response by low molecular weight plasma constituents such as IgG and complement components to make the membrane active for granulocytes. Activation of blood granulocytes can increase reactive oxygen species. Oxygen radicals generated may interact with neutropils and further stimulate ROS production and it can lead to the imbalance between production of free radicals and alter the different antioxidant enzyme. This can increase the LPO level and depress the SOD activity in hemodialysed samples [28, 29]. Previous study have shown that trace elements are being increasingly recognized as key mediators of progression of kidney disease [30]. During the hemodialysis process the levels of trace elements like Cu and Zn, the cofactors of SOD might be reduced. This is one more possible cause to reduce SOD activity post HD [31].
There is considerable evidence from earlier studies to suggest that multiple pathways lead to NO• deficiency as a result of chronic kidney disease (CKD). In our study we found mean values of nitric oxide were significantly decreased in post hemodialytic samples as compared to prehemodialytic samples. Many mechanism are likely to be responsible, including substrate limitation, L-arginine is the precursor in the biosynthesis of nitric oxide . During hemodialysis process, L-arginine can be removed decreasing its concentration in blood [32]. Dyslipidemia, increased oxidative stress and impaired endothelial func tion may cause decrease in nitric oxide level in posthemodialytic samples [33, 34].
Homocysteine, a non conventional risk factor for CVD has involved much interest in dialysis patients. There are multiple plausible mechanisms by which homocysteine may promote endothelial dysfunction, oxidative stress and vascular disease [35]. In post hemodialytic samples mean values of homocysteine were significantly decreased, as compared to prehemodialytic samples. The homocysteine lowering effect may be due to the high flux dialyzer. High flux dialyzer membrane has a high efficacy of dialytic removal of both small and middle molecules, during hemodialysis procedure. Removal of uraemic inhibitor of homocysteine metabolism may play a significant role in the intradialytic lowering of homocysteine [36].
C-reactive protein (CRP) not only biomarker of inflammation as it has been found in atherosclerotic plaques and shown to cause endothelial cell dysfunction, oxidant stress in experimental model [37]. In earlier studies observed that inlfammaiton is among the strongest predictors of poor clinical outcome CKD patients [38]. Chung et al, have demonstrated a significant association between markers of inflammation and changes in residual renal function in post hemodialysis patients [39]. CRP is the leading of acute phase protein and easily measured biomarkers of inflammation as high sensitive assay (hs- CRP). In the present study we observed, in post hemodialytic samples mean values of hs-CRP were significantly elevated as compared to prehemodialytic samples. Hemodialysis may induce a state of inflammation and increase in hs-CRP may be the result of inflammatory response suggesting that dialysis procedure with it’s extracorporeal circulation of blood, may itself be the cause of inflammation [40].
We observed significant elevation of serum LPO and hs- CRP and significant reduction was observed in the mean serum SOD, NO• and homocysteine levels in post HD. The causes of oxidative stress and inflammation in CKD are definitely multiple . Our results reflects the primary mechanisms of oxidative stress and inflammation during hemodialysis which may cause of CVD development in hemodialysed patients. Due to adverse effect of HD which is demonstrated by our results, the clinicians may plan to advice antioxidant therapy and use of antioxidant bounded membrane for HD will be new approach to overrule oxidative stress and inflammation during HD session.
References
- Wever R, Boer P, Hijmering M, Stores E, Verhaar M, Kastelein J, Varsluis K. Nitric oxide production is reduced in patients with chronic renal failure. Arteriosclerosis, Thrombosis, and Vascular Biology 1999; 19:1168-1172.
- Cruz DN, Soni SS, Polanco N, Bobek I, Corradi V, Cal MD, Rohco C. Markers of inflammation and oxidative stress in periotoneal dialysis: a comparison between high and low peritoneal transporters. J Nephrol 2010; 23(4): 453-458.
- Locatelli F, Bommer J, London GM, Martin-Malo A, Wanner C, Yaqoob M. Cardiovascular disease determinants in chronic renal failure : Clinical approach and treatment. Nephrol Dial Transplant 2005; 16 : 459-68.
- Bitla AR, Reddy PE, Manohar SM, Vishnubhotla SV, Srinivasa Rao PVLN. Effect of a single hemodialysis session on inflammatory markers. Hemodialysis International 2010; 14(4): 411-417.
- Reddy EP, Suchitra MM, Bitla AR, Sivakumar V, Srinivasa Rao PVLN. Antioxidant enzymes status in south Indian hemodialysis patients. Int J Biol Med Res. 2011; 2(3): 682-687.
- El-Khawaga O.Y and El-Sayed I.H. Evaluation of trace elements and antioxidants in pre and post hemodialysis of chronic renal failure patients. International Journal of Science and Nature 2012; 3(3): 617-6
- Clermont G , Lecour S , Lahet J, Siohan P , Vergely C, Chevet D. Alteration in plasma antioxidant capacities in chronic renal failure and cardiovascular risk in these patients. Cardiovascular Research 2000; 47: 618-623.
- Aiello S, Noris M, Remuzzi G. Nitric Oxide/ Larginine in uremia. Mineral Electrolyte Metab 1999; 25: 384-390.
- Wang AY, Woo J, Lam CW. Is a single time point Creactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol 2003; 14: 1871- 1879.
- Delaney MP, Price CP, Newman DJ. Kidney disease. In : Burtis CA, Ashwood ER, Bruns DE, editor. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed. Missouri : Elsevier; 2006:1671-1725. 11.
- Tarakcroglu E, Binnur A, Erbagic, Usalan C, Deveci R and Kocabas R. Acute effect of hemodialysis on serum levels of the proinflammatory cytokines. Mediators of Inflammation 2003; 12: 15-19.
- Zoungas S, McGrath BP, Branley P, Kerr PG, Muske C, Wolfe R, Atkins RC, Nichols K, Fraenkel M, Hutchison BG, Walker R, McNeil JJ. Cardiovascular morbidity and mortality in the atherosclerosis and folic acid supplementation trial (ASFAST) in chronic renal failure. Journal of the American Collegge of Cardiology2006; 47(6): 1108-16.
- Moustapha A, Naso A, Nahlwai M, Gupta A, Arheart KL, Jacobsen DW. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation 1998, 97: 138-141.
- Jackson P, Loughrey CM, Lightbody J H, Mc Namee PT, Young IS. Effect of haemodialysis on total antioxidant capacity and serum antioxidant in patients with chronic renal failure. Clin Chem 1995; 41(8), 1135- 1138.
- Durak I, Akyol O, Basesme E, Canbolat O, Kavutcu M. Reduced defence mechanism against free radical toxicity in patients with chronic renal failure. Nephron1994; 66: 76-80.
- National kidney foundation. K/DOQRI : clinical practice guidelines for chronic kidney disease : evaluation classification, and stratification Kidney Disease outcome Quality Initiative. Am J kidney Dis. 2002;39:1- 246.
- Levey AS, Bosch JP, Lewis JB, Green T, Rogers N, Roth D. A more accurate method to estimate glomerular filteration rate from serum creatinine : a new prediction equation modification of diet in renal disease study group. Ann Intern Med 1999; 130(6): 461-470.
- Satoh K. Plasma lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clinica Chimica Acta 1978; 90: 37-43.
- Nischal HK, Sharma MP, Goyal RK, Kaushik GG. Serum superoxide dismutase levels in diabetes mellitus with or without microangiopathic complications. JAPI 1998; 46:853-855.
- Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmimum method. Clin Chem 1990; 36(8):1440-1443.
- Estimation of homocysteine by automated chemiluminescence’s Bayer Health Care (Company System Manual).
- Chenilot O. Clin Chem Lab Med 2000; 38: 1003-1011.
- Varan HI , Dursun B , Dursun E, Ozben T, Suleymanlar G . Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparision of two dialysis membranes. International Journal of Nephrology and Renovascular Disease 2010: 3; 39-45.
- Tarng DC, Huang TP, Wei YH, Lin TY, Chen HW, Wen Chen T. 8-Hidroxy-2’-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patients. Am J Kidney Dis 2000; 36(5): 934-944.
- Annuk M, Zilmer M, Lind L, Linde T, Fellstrom B. Oxidative stress and endothelial function in chronic renal failure. J Am Soc Nephrol 2001;12:2747-2752.
- Miric D, Kisic B, Stolic R, Miric B, Mitic R and Janicijevic- Hudomal S. The role of Xanthine oxidase in hemodialysis-induced oxidative injury: Relationship with Nutritional status. Oxidative Medicine and Cellular Longevity 2013;1-8.
- Nouri M, Nober MR, Argani H, Rokhforooz. Superoxide dismutase and glutathione peroxide in hemodialyzed patients and renal transplant recipients and their relationship to osmotic fragility. Medical Journal of Islamic Academy of Sciences 1999; 12(2): 33-38.
- Demirtas S, Nergisoglu G, Akbay A, Karaca L. The relation between low-density lipoprotein (LDL) oxidation and hemodialysis with respect to membrane types. Turk J Med Sci 2002; 32: 93-100.
- Meerashivashekar, W.Ebenezer W, Revathi R, Padmanabhan. Effect of oxidative stress in pre and post hemodialysis in chronic renal failure patients. Int J Biol Med Res. 2012; 3(1): 1335-1337.
- Ramprasad N and Al-Ghoniam Mohammed I. Role of trace elements and lipid peroxidation levels in pre and post hemodialysis of chronic renal failure patients. International Journal of Research in Biochemistry and Biophysics 2013; 3(1): 1-6.
- Shelgikar PJ, Deshpande KH, Sardeshmukh AS, Katkam RV, Suryakar AN. Role of oxidants and antioxidants in ARF patients undergoing hemodialysis. Indian J Nephrol 2005, 15:73-76.
- Sperschneider H, Deppisch R, Beck W, Wolf H , Stein G. Impact of membrane choice and blood flow pattern on Coagulation and heparin requirement- potential consequences on lipid concentration. Nephrol Dial Transplant 1997; 12:2638-2646.
- Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000; 58: 1261-1266.
- Cross JM, Donald A, Vallance PJ, Deanfield JE, Woolfson RG, Macallister RJ. Dialysis improves endothelial function in humans. Nephrol Dial Transplant 2001; 16: 1823-1829.
- Filiopoulos V and Vlassopoulos D. Inflammatory syndrome in chronic kidney disease : Pathogenesis and influence on outcmes. Inflammation and Allergy- Drug Target 2009;8: 369-382.
- Massy ZA. Potential strategies to normalize the levels of homocysteine in chronic renal failure patients. Kidney Int. 2003; 63:134-136.
- Nakou ES, Elisaf MS and Liberopoulos EN. High- Sensitivity C-Reactive Protein: to measure or not to measure? The Open Clinical Chemistry Journal 2010;3:10-18.
- Suliman ME, Stenvinkel P. Contribution of inflammation to vascular disease in chronic kidney disease patients. Saudi J Kidney Dis Transplant 2008; 19(3): 329- 345.
- Chung SH, Heimburger O, Stenvinkel P, Bergstrom J, Lindholm B. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant 2001; 16(11): 2240-5.
- Calearfield MB. C-Reactive protein: A new risk assessment tool for cardiovascular Disease. JAOA. 2005; 105(9):409-416.