- Biomedical Research (2010) Volume 21, Issue 1
Role of Beta-Carotene on the Histological Features of Neonatal Albino Rat Cardiocytes Treated with Monosodium Glutamate
Mohamed Samir Ahmed Zaki*Department of Histology, College of Medicine, Zagazig University, Egypt (Department of Anatomy, College of Medicine, King Khalid University, Saudi)
- *Corresponding Author:
- Mohamed Samir Ahmed Zaki
Department of Anatomy
College of Medicine
King Khalid University
Saudi Arabia
Tel: +9660508446077
E-mail: mszaki1@hotmail.com
Accepted date: August 07 2009
Abstract
The objective of this study was to analyze the cardiac effects of exposure to monosodium glutamate for a period of 7 days alone and in combination with beta-carotene supplementa-tion. Rats were allocated into: control (Ι), experimental group (ΙΙ) exposed to 4 mg of mono-sodium glutamate for 7 days and experimental group (ΙΙΙ) exposed to monosodium gluta-mate and 300 mg/kg/diet beta-carotene for 7 days. After three days of randomization, car-diac structure was assessed by electron microscopy. The myocardial cells after exposure to monosodium glutamate illustrated branching cytoplasmic network of cardiac muscle with cross striations which are retracted around the nuclei. These nuclei showed dense and elec-tron dense heterochromatin that associated with nuclear covering and dense chromatin dis-tributed in the nucleoplasm. Delicate supportive tissue filling the intercellular spaces con-taining blood was also seen. Ultrastructurally, vacuolation of the mitochondria with frag-mentation of its cristae with loss of its striations were observed. The myofibrils were disso-ciated with irregularities of its sarcomere. Empty spaces in the cytoplasm with disrupted in-tercalated disc were observed. In beta-carotene plus monosodium glutamate, most fibers showed normal morphological aspects. In conclusion: One week monosodium glutamate ex-posure induces morphological cardiac alterations and beta-carotene supplementation at-tenuates this ventricular remodeling process.
Keywords
Beta-carotene, Monosodium Glutamate, Histology of cardiocytes, Neonatal Albino Rats
Introduction
Monosodium glutamate (MSG), a widely used food addi-tive, is known to induce neural damage in the immature nervous system [1]. The amino acid glutamate is abun-dantly present in the mammalian central nervous system (CNS) and is the principal excitatory neurotransmitter in the brain [2]. Glutamate receptors (GluRs) have been im-plicated in brain function and pathology. Their presence in peripheral tissues suggests a vital role in the patho-physiology of various organ systems [3]. Gill et al. [4] have hypothesized that GluRs play a role in mediating the cardiac effects of these glutamate analogues.
There are many other exogenous substances acting as glu-tamate analogues that possess excitatory properties and potential excitotoxic effects. Glutamate and its analogues may enter the food supply during food preparation and food processing, as contaminants and/or additives [5].
Domoic acid is one of the most potent excitatory amino acids and neurotoxins that can enter the food chain. It is produced by a phytoplankton that is ingested and accumu-lated in the digestive system of seashells such as mussels [6]. A variety of excitatory amino acids can also be pre-sent in foods including aspartate and monosodium gluta-mate [7]. The association of arrhythmias and other cardio-vascular symptoms in humans intoxicated with DA and on individuals susceptible to MSG prompted us to focus our attention to the investigation of GluRs in heart [8].
The antioxidant nutrients such as carotenoids [9] are at-tractive agents that could protect against cardiovascular injury, assuming that they are capable of preventing oxi-dative damage. Therefore, several observational and pro-spective epidemiological studies have consistently shown an inverse relationship between dietary intakes or blood evels of beta-carotene (BC) and cardiovascular diseases [10].
Therefore, the objective of this study was to investigate the effects of monosodium glutamate injection, isolated or in association with dietary BC, on the neonatal cardiac muscle fibers in rats.
Materials and Methods
Twelve neo-natal male Sprague-Dawley rats were housed with their mothers individually with food and water avail-able ad libitum. All animals were housed in clear plastic cages in a colony room. The neo-natal rats were divided into three groups: Group (Ι) (control; n = 4), control rats neither exposed to MSG nor supplemented with BC; ex-perimental group (ΙΙ) (n = 4) were pretreated by four mil-ligrams of monosodium glutamate per gram body weight was injected subcutaneously in the neonatal rats, every day for 7 days and experimental group (ΙΙΙ ) (n = 4) fed with the standard diet, supplemented with BC (obtained from International Vitamin Corporation) received orally (300 mg/kg/diet) dissolved in saline daily and MSG in-jected as in the experimental group (ΙΙ) simultaneously for one week. The rats were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium and sacri-ficed. The cardiac muscles from the left ventricle were removed. Cardiac muscle tissue was perfused with normal saline solution followed by phosphate-buffered 2% glu-taraldehyde and 4% paraformaldehyde.
The cardiac muscle was carefully removed and immersed in the same fixative, and 2-mm coronal slices were pre-pared. Each tissue sample was then post-fixed in 1% po-tassium-ferrocyanide reduced osmium tetroxide, dehy-drated in graded acetones, and embedded in Epon 812. Semi-thin (0.5 um) sections were cut from tissue blocks and stained with 0.5% toluidine blue. The sections were stained with 0.25% lead citrate and 5% uranyl acetate in 50% methanol and were observed and photographed by an electron microscopy technician using a JEOL 100CX electron microscope [11].
Results
Group Ι (control)
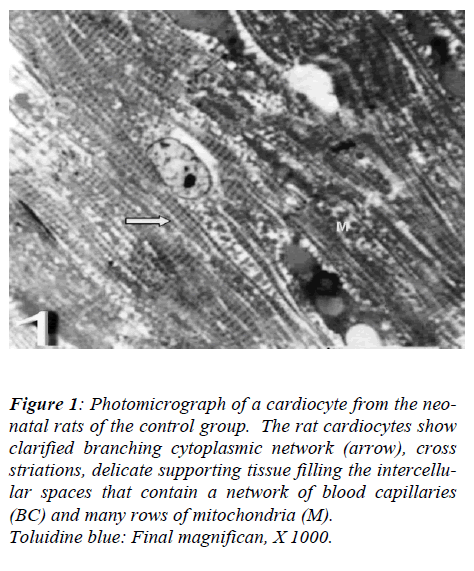
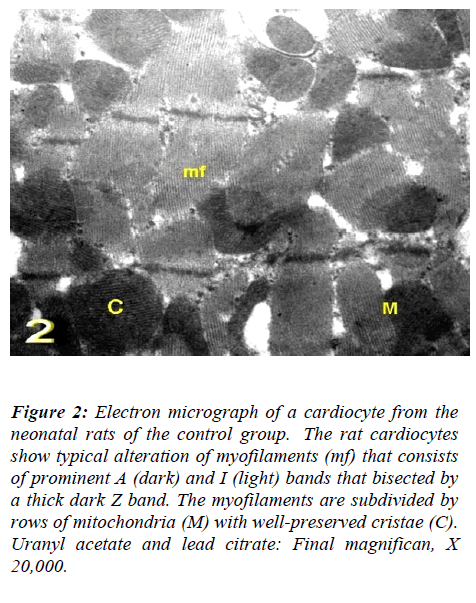
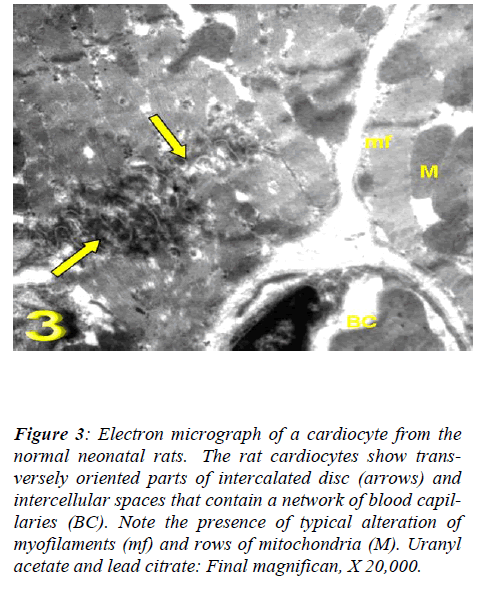
With regard to touilidine blue stain, the rat cardiocytes showed clarified branching cytoplasmic network, cross striations, delicate supporting tissue filling the intercellu-lar spaces that contain a network of blood capillaries and many rows of mitochondria. Ultrastructurally, the rat cadiocytes were noticed the striation pattern, typical al-teration of myofilaments, normal appearance of mito-chondria with well-preserved mitochondrial cristae and transversely oriented parts of intercalated disc. (Figs. 1, 2 and 3).
Figure 1: Photomicrograph of a cardiocyte from the neo-natal rats of the control group. The rat cardiocytes show clarified branching cytoplasmic network (arrow), cross striations, delicate supporting tissue filling the intercellu-lar spaces that contain a network of blood capillaries (BC) and many rows of mitochondria (M).
Toluidine blue: Final magnifican, X 1000.
Figure 2: Electron micrograph of a cardiocyte from the neonatal rats of the control group. The rat cardiocytes show typical alteration of myofilaments (mf) that consists of prominent A (dark) and I (light) bands that bisected by a thick dark Z band. The myofilaments are subdivided by rows of mitochondria (M) with well-preserved cristae (C). Uranyl acetate and lead citrate: Final magnifican, X 20,000.
Figure 3: Electron micrograph of a cardiocyte from the normal neonatal rats. The rat cardiocytes show trans-versely oriented parts of intercalated disc (arrows) and intercellular spaces that contain a network of blood capil-laries (BC). Note the presence of typical alteration of myofilaments (mf) and rows of mitochondria (M). Uranyl acetate and lead citrate: Final magnifican, X 20,000.
Experimental groups II (the rats treated with MSG alone)
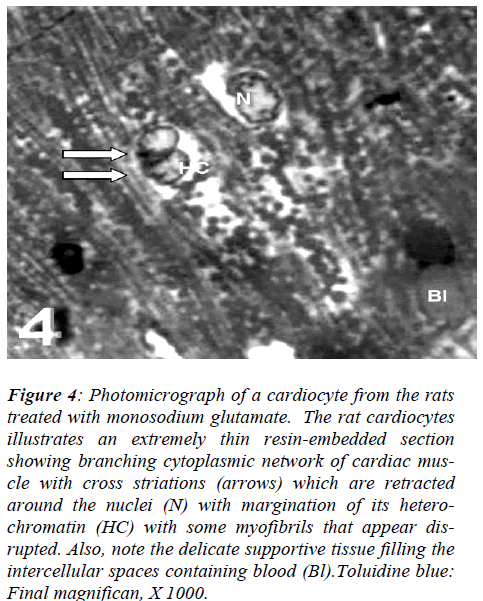
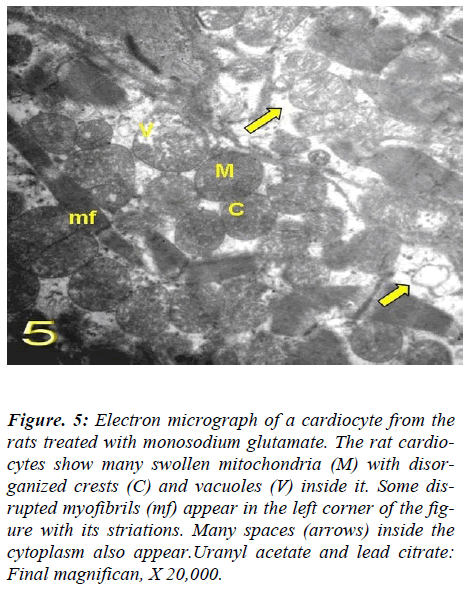
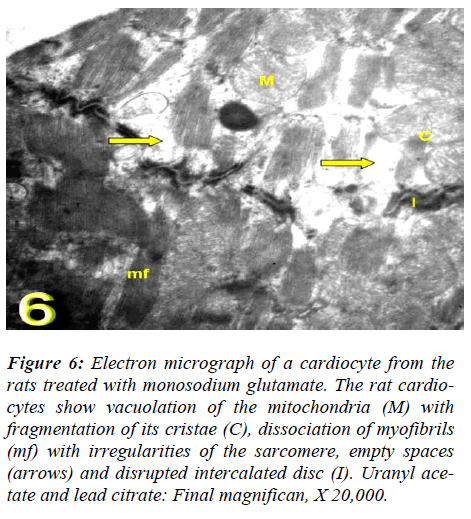
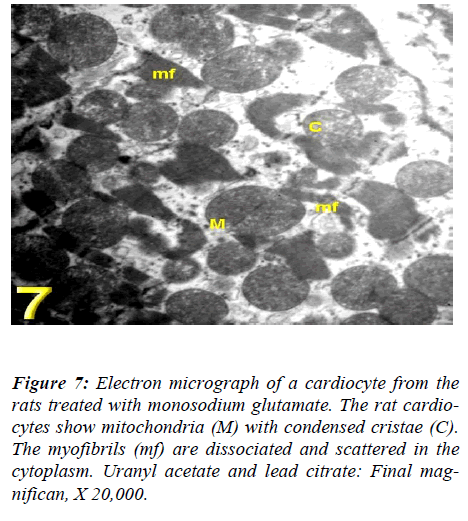
With regard to touilidine blue stain, the rat cardiocytes illustrated branching cytoplasmic network of cardiac muscle with cross striations which are retracted around the nuclei with dense and electron dense heterochromatin, associated with nuclear covering and dense chromatin distributed in the nucleoplasm and delicate supportive tissue filling the intercellular spaces containing blood. Ultrastructurally, vacuolation of the mitochondria with fragmentation of its cristae myofibrils with irregularities of the sarcomere, empty spaces in the cytoplasm and dis-rupted intercalated disc were observed (Figs. 4, 5, 6, and 7).
Figure 4: Photomicrograph of a cardiocyte from the rats treated with monosodium glutamate. The rat cardiocytes illustrates an extremely thin resin-embedded section showing branching cytoplasmic network of cardiac mus-cle with cross striations (arrows) which are retracted around the nuclei (N) with margination of its hetero-chromatin (HC) with some myofibrils that appear dis-rupted. Also, note the delicate supportive tissue filling the intercellular spaces containing blood (Bl).Toluidine blue: Final magnifican, X 1000.
Figure 5: Electron micrograph of a cardiocyte from the rats treated with monosodium glutamate. The rat cardio-cytes show many swollen mitochondria (M) with disor-ganized crests (C) and vacuoles (V) inside it. Some dis-rupted myofibrils (mf) appear in the left corner of the fig-ure with its striations. Many spaces (arrows) inside the cytoplasm also appear.Uranyl acetate and lead citrate: Final magnifican, X 20,000.
Figure 6: Electron micrograph of a cardiocyte from the rats treated with monosodium glutamate. The rat cardio-cytes show vacuolation of the mitochondria (M) with fragmentation of its cristae (C), dissociation of myofibrils (mf) with irregularities of the sarcomere, empty spaces (arrows) and disrupted intercalated disc (I). Uranyl ace-tate and lead citrate: Final magnifican, X 20,000.
Figure 7: Photomicrograph of a cardiocyte from the rats treated with beta-carotene and monosodium glutamate. The rat cardiocytes show most of the fibers has normal morphological aspects (arrows) except some disorganiza-tion of myofilaments (arrow heads) and margination of heterochromatin (HC) of the nucleus still present. Tolu-idine blue: Final magnifican, X 1000.
Experimental group III (the rats treated with MSG and BC)
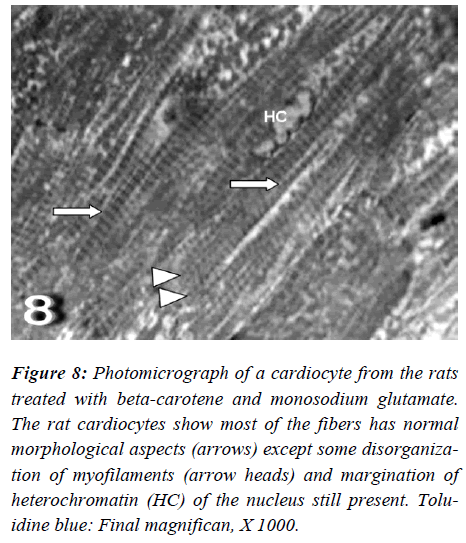
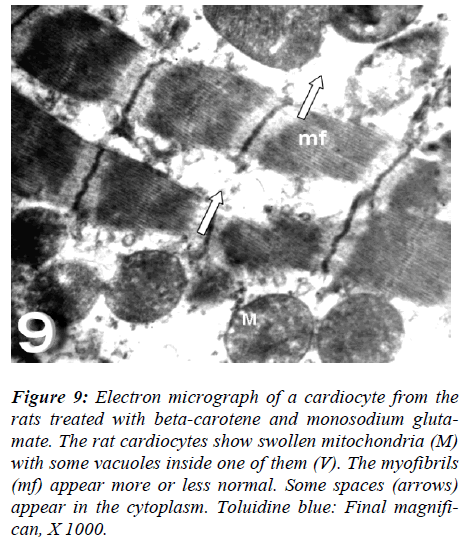
With regard to touilidine blue stain, the rat cardiocytes were noticed most of fibers had normal morphological aspects except disorganization of myofilaments with ir-regular-shaped nuclei are evident. Ultrastructurally, the rat cardiocytes show vacuolation of the mitochondria with fragmentation of its cristae, dissociation of myofibrils with irregularities of the sarcomere, empty spaces (Figs. 8 and 9).
Figure 8: Photomicrograph of a cardiocyte from the rats treated with beta-carotene and monosodium glutamate. The rat cardiocytes show most of the fibers has normal morphological aspects (arrows) except some disorganiza-tion of myofilaments (arrow heads) and margination of heterochromatin (HC) of the nucleus still present. Tolu-idine blue: Final magnifican, X 1000.
Figure 9: Electron micrograph of a cardiocyte from the rats treated with beta-carotene and monosodium gluta-mate. The rat cardiocytes show swollen mitochondria (M) with some vacuoles inside one of them (V). The myofibrils (mf) appear more or less normal. Some spaces (arrows) appear in the cytoplasm. Toluidine blue: Final magnifi-can, X 1000.
Discussion
An investigation was carried out on the effects of MSG, a commonly used as food additive, on the cardiac muscle of neo-natal rats [12].
In the present study, the ultra-structural results revealed that the cardiocytes submitted to MSG showed vacuola-tion of the mitochondria with fragmentation of its cristae with loss of its striations. The myofibrils were dissociated with irregularities of its sarcomere. Empty spaces in their cytoplasm with disrupted intercalated disc were observed. The presence of vacuoles inside the mitochondria of group II is indicative of irreversible injury, however on-cocytic or apoptotic cells were not found, although with-out receiving oxygen to maintain the aerobic metabolism, the structures remained in a quiescent state [13]. How-ever, Kennedy and Rice [14] found sublethally damaged cardiocytes that had lysed contractile material; vacuolated sarcoplasm; altered mitochondria and pyknotic nuclei. Necrosis was followed by macrophage invasion and phagocytosis of necrotic debris. Repair of the lesions was by deposition of collagen and elastic fibers.
Regarding the current work, the experimental group III (the rats treated with MSG & BC), the rat cardiocytes revealed that most of the cardiac muscle fibers had nor-mal morphological aspects except disorganization of myofilaments with irregular-shaped nuclei are evident with touilidine blue stain. Ultrastructurally, the rat cardio-cytes of this group were noticed vacuolation of the mito-chondria with fragmentation of its cristae, dissociation of myofibrils with irregularities of the sarcomere and empty spaces in the cytoplasm also appeared.
Antioxidants have been studied for their capacity to pre-vent the chronic disease. However, the use of antioxidants in cardiovascular diseases remains controversial. Discrep-ancy among results from their use may be related to pa-tient characteristics or doses of antioxidants [15].
Beta-carotene and other carotenoids are mainly consid-ered as belonging to the group of micronutrients. As they are contained in fruit and vegetables and thus part of hu-man diet, a regular low-dose intake from natural sources is normally assured. In the last decade high-dose supple-mentation with synthetic carotenoids has been used suc-cessfully in the treatment of diseases believed to be asso-ciated with oxidative stress. However, in a few clinical studies harmful effects have been observed as well, e.g., a higher incidence of lung cancer after BC was given in high doses to smokers [16]. Beta-carotene has been better characterized with regard to its antioxidant capabilities; observational and prospective epidemiologic studies have shown an inverse relationship between cardiovascular disease and dietary intake of carotenoids and/or blood levels [17, 18]. Four large interventional trials were de-signed to test the hypothesis that BC protects against can-cer and/or cardiovascular disease development in humans. In these studies, doses of BC were higher than those that could be achieved from the habitual diet, and the BC blood levels were 26 times higher than the 95th percentile level of BC in the Health and Nutrition Examination Sur-vey of the United States [19, 20, 21, and 22]. Three out of these four intervention trials did not show any protective effect of BC against cancer or cardiovascular disease; actually, some adverse effects were found.
In fact, the risk of fatal coronary heart disease was in-creased in the groups that received either beta-carotene or the combination of alpha-tocopherol and beta-carotene [23] and the relative risk of death by lung cancer also in-creased by 17% in the beta-carotene-supplemented heavy smokers vs. placebo group [21]. Then, it can be specu-lated that BC is health promoting when taken at a physi-ologic level, but may take on circumstantially adverse properties when given at a high dose or in the presence of highly oxidative conditions [24]. Antioxidant vitamins reduce cardiac oxidative stress and cardiomyocyte apop-tosis produced by exogenous norepinephrine and attenu-ate cardiac dysfunction in animals with pacing-induced congestive heart failure [25]. The findings observed by Qin et al. [26] suggest that NE-induced myocyte apop-tosis is mediated by oxidative stress, and that antioxidant vitamins may be beneficial in heart failure in which car-diac NE release is increased.
In conclusion, our findings suggest that one-week MSG exposure induces morphological cardiac alterations and that BC supplementation attenuates this ventricular re-modeling process in rats.
References
- Morhenn VB, Waleh NS, Mansbridge JN, Unson D, Zolotorev A, Cline P, Toll L. Evidence for an NMDA receptor subunit in human keratinocytes and rat cardio-cytes. Eur. J. Pharmacol 1994; 268, pp. 409-414.
- Miller S, Kesslak J, Romano C, Cotman C. Roles of metabotrophic receptors in brain plasticity and pathol-ogy. Ann N Y Acad Sci 1999; 57,460-474.
- Santokh G, Barker M Pulido O. Neuroexcitatory Tar-gets in the Female Reproductive System of the Non-human Primate (Macaca fascicularis) Toxicologic Pa-thology 2008; Vol. 36, No. 3, 478-484.
- Gill S, Pulido O, Mueller R, McGuire P. Immunologi-cal characterization of the metabotropic glutamate re-ceptors in the rat heart. Brain Res Bull 1999; 48, 143-146.
- Gill S, Pulido, O. Glutamate receptors in peripheral tissues, Distribution and implications for toxicology. In Glutamate Receptors in Peripheral Tissue: Excitatory Transmission Outside the CNS (S. Gill and O. Pulido, eds.), Kluwer Academic/Plenum Publishers, New York 2005.
- Silvagni PA, Lowenstine LJ, Spraker T, Lipscomb TP, Gulland FM. Pathology of domoic acid toxicity in Cali-fornia sea lions (Zalophus californianus). Vet Pathol 2005; 42: 184-191.
- Gulland F. Domoic acid toxicity in California sea lion (Zalophus californianus) stranded along the central California coast, May-October 1998. Report to the Na-tional Marine Fisheries Service Working Group on Un-usual Marine Mammal Mortality Events. U.S. Dep. Commer., MOAA Tech. Memo 2000; NMFS-OPR-17, 145-152.
- Mueller R, Gill S, and Pulido O The monkey (Macaca fascicularis) heart neural structures and conducting sys-tem: an immunochemical study of selected neural bio-markers and glutamate receptors. Toxicol Pathol 2003; 31: 227–234.
- Anand U, Anand CV, Chandrika C, and Agarwal R. Interventional therapy with mega dose of antioxidant vitamins in patients with acute myocardial infarction: Could we throw caution to the winds? Am. J. Cardiol 1997; 80: 823–824.
- Knekt P, Reunanen A, Jarvinen R, Seppanen R, Helio-vaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am. J. Epidemiol 1994; 139: 1180–1189.
- Bancroft JD, Stevens A. Theory and Practice of Histo-logical Techniques. 4th ed., pp. 175-190. Churchill Liv-ingstone, New York, Edinburgh and London 1996
- Anyanwu GE, Ezeugwuorie EO, Anibeze EC, Ngokere UA. Effects of Monosodium Glutamate (MSG) On the Histological Features of the Spinal Cord of Adult Wis-ter Rat. Journal of Experimental and Clinical Anatomy 2005; 4 (1): 6-8
- Lima-Oliveira AP, Maria Azeredo-Oliveira TV, Ta-boga SR, Godoy MF, Braile2lima DM. Cardioplegia using low volumic cardioplegic agents: morphological study in isolated rabbit hearts Rev Bras Cir Cardiovasc 2003; 18 (3): 227-234
- Kennedy S, Rice DA. Histopathologic and ultrastruc-tural myocardial alterations in calves deficient in vita-min E and selenium and fed polyunsaturated fatty ac-ids. Toxicologic Pathology 2001; 29 (2): 208-223.
- Jiala I, Devaraj S. Antioxidants and atherosclerosis: Don't throw out the baby with the bath water. Circula-tion 107, 926: 928-2003.
- Siems W, Wiswedel I, Salerno C, Crifò C, Augustin W, Schild L, Langhans CD, Sommerburg O. Beta-carotene breakdown products may impair mitochondrial func-tions--potential side effects of high-dose beta-carotene supplementation. J Nutr Biochem 2005; 16 (7): 385-397.
- Albanes D Beta-carotene and lung cancer: A case study. Am. J. Clin. Nutr 1999; 69: 1345S-1350S
- Ford ES, Giles WH. Serum vitamins, carotenoids, and angina pectoris: Findings from the National Health and Nutrition Examination Survey III. Ann. Epidemiol 2000; 10: 106-116.
- Albanes D, Virtamo J, Taylor PR, Rautalahti M, Pieti-nen P, Heinonen OP. Effects of supplemental beta-carotene, cigarette smoking, and alcohol consumption on serum carotenoids in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr 1997; 66, 366-372.
- Hennekens CH, Buring J E, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, et al. Lack of effect of long-term sup-plementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J 1996; Med. 334, 1145-1149.
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams, JH, Jr, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Can-cer Inst 1996; 88: 1550-1559/
- Vogel S, Contois JH, Tucker KL, Wilson PW, Schaefer EJ, Lammi-Keefe CJ. Plasma retinol and plasma and lipoprotein tocopherol and carotenoid concentrations in healthy elderly participants of the Framingham Heart Study. Am. J. Clin. Nutr 1997; 66: 950-958
- Rapola JM, Virtamo J, Ripatti S, Huttunen JK, Albanes D, Taylor PR, and Heinonen OP. Randomised trial of alpha-tocopherol and beta-carotene supplements on in-cidence of major coronary events in men with previous myocardial infarction. Lancet 1997; 349: 1715-1720.
- Paiva SA, Russell RM. Beta-carotene and other carote-noids as antioxidants. J. Am. Coll. Nutr 1999; 18: 426-433.
- Qin F, Shite J, Liang CS. Antioxidants attenuate myo-cyte apoptosis and improve cardiac function in CHF: association with changes in MAPK pathways. Am J Physiol Heart Circ Physiol 2003; 285 (2): H822-832
- Qin F, Rounds NK, Mao W, Kawai K, Liang CS. Anti-oxidant vitamins prevent cardiomyocyte apoptosis pro-duced by norepinephrine infusion in ferrets. Cardiovasc Res 2001; 51(4): 736-748