Keywords
|
| Schistosoma mansoni, Selenium nano-particles, Liver, Mice. |
Introduction
|
| Developing countries such as “Africa, South America and Asia” are suffering from morbidity and mortality due to schistosomiasis. The main agent of human schistosomiasis is Schistosoma mansoni [1]. Predominantly, eggs of Schistosoma mansoni are deposited in the liver and intestines which resulting in parasitic disease [2]. In addition; Mahmoud et al. [3] reported that the clinical symptoms include hepatosplenomegaly, liver fibrosis, portal hypertension, and liver cirrhosis. Acute symptoms of schistosomiasis include fever, diarrhea, abdominal pain, weight loss, and eosinophilia [4]. |
| Chang et al., [5] reported that eggs are transported to the liver by portal circulation. Acute schistosomiasis is associated with heavy primary infections and with the initiation of egg production [4]. In the portal venous system eggs are deposited while eggs are trapped in the peri-sinusoidal spaces of the liver, thus causing periportal granulomatous inflammation and the deposition of scar tissue around the eggs trapped inside the liver [6]. Granulomas are formed of inflammatory cells “eosinophils, macrophages, and lymphocytes” [7,8]. |
| Current treatment of schistosomiasis depends on praziquantel (PZQ), which was developed in the late 1970s [9]. PZQ has been widely used as an effective means to control schistosomiasis. However, PZQ does not treat early infection or prevent reinfection [10]. In addition, available evidence indicates the appearance of PZQ resistance by schistosomes [11,12,13,14]. |
| Heretofore controlling schistosomiasis, there is an urgent necessity to discover a new effective drug. According to National Science Foundation of USA, nanotechnology deals with controlling or restructuring of the material dimension more or less between 1 and 100 nm. For various reasons related to their small size, e.g., better solubility, absorption and uptake, nanoparticle-based medicines can get across cell membranes and reach specific targets more easily than bulk form agents [15]. |
| The field encompasses nanomedicine, which strives to utilize nanotechnology to improve health care [16]. Various nanoparticles have applications for diagnosis and treatment [17]. Armstead and Li [18] recently summarized the range of intracellular infectious diseases that nanomedicines may be more effective in treating than conventional bulk form drugs, such diseases include leismaniasis and malaria [19,20]. |
| Nano-elemental selenium (Se) has attracted wide spread attention due to its high bioavailability and low toxicity because nanometer particulates exhibit novel characteristics, such as great specific surface area, high surface activity, and a lot of surface active centers, high catalytic efficiency and strong adsorbing ability and the character of low toxicity of routine Se0 [21]. |
| Science the nano-elemental selenium (Se) has high bioavailability, low toxicity and nanometer particulates; it has attracted wide spread attention. Moreover, it showed new features, such as great specific surface area, high surface activity, high catalytic efficiency and strong adsorbing ability and the character of low toxicity of routine Se0 [22]. |
| Many investigations have shown that Se in nano-form has novel in vitro and in vivo antioxidant properties, which acts through the activation of seleno-proteins [23]. |
| So far, there is not any information concerning Se nanoparticles role in treatment of schistosome infected mice, thus the aim of this paper is to investigate the anti-schistosomal effect of Se nanoparticles on liver of infected mice. |
Materials and Methods
|
Animals
|
| Male Swiss albino mice weighing 20 ± 2 g were obtained from the Experimental Animal Research Unit of the Schistosome Biological Supply Program at Theodor Bilharz Research Institute (TBRI), Al-Giza, Egypt and fed a standard diet and water ad libitum. All experimental protocols were approved by the legal and ethical guidelines of the Medical Ethics Committee of TBRI, Giza, Egypt (Approval No. 4018/2011). |
Selenium nanoparticles
|
| Selenium nanoparticles (50-100 nm particle size) were obtained from Nano-tech Lab in 6 October City, Egypt, as a sterilized solution, as they were dispersed in phosphatebuffered saline (PBS) and ready for use. In brief, a simple wet chemical method has been developed to synthesize selenium nanoparticles, by the reaction of sodium selenosulphate precursor with deferent organic acids in aqueous medium, under ambient conditions. Polyvinyl alcohol has been used to stabilize the selenium nanoparticles. The synthesized nanoparticles can be separated from its sol by using a highspeed centrifuge and can be re-dispersed in aqueous medium with a sonicator [24]. |
| Transmission electron microscope (TEM) was used for characterization of nanoparticles (shape and size) (Figure1).TEM were performed on JEOL JEM-2100 high resolution TEM at an accelerating voltage of 200 kV, respectively to characterize the size and shape of Se nanoparticles. |
 |
Mice infection
|
| Mice were injected subcutaneously by 100 ± 10 S. mansoni cercariae per mouse according to Oliver and Stirewalt [25]. The procedures of cercaria injection were done in Schistosome Biological Supply Center at Theodor Bilharz ResearchInstitute, Imbaba, Giza, Egypt. |
Experimental design
|
| Forty eight mice were divided into four groups (12 mice/ group), as follows: Group I normal, non-infected control group received vehicle (0.5 ml/mice PBS by intrapretoneal (i.p) injection for 7 consecutive days this group was injected b. Groups II, III and IV were infected with S. mansoni cercariae (100 ± 10). On day 46 post infection (pi) with S. mansoni, the animals of group III were i.p injected 0.5 mg/kg SeNPs dispersed in 0.5 ml PBS; for 7 consecutive days [26]. Infected animals of group IV were orally administered 0.5 ml of PZQ (600 mg/kg body weight) on day 46 pi at an interval of 24 h for 2 days. |
Egg count in infected hepatic tissue
|
| According to Pelligrino et al. [27]; the eggs in the hepatic tissue of infected mice were counted. Concisely, 0.1 g of liver was divided into 4 fragments; each one was crushed between a slide and a cover slip. The slides were examined by light microscope. |
Liver histopathology
|
| After animal dissection; mice hepatic tissue samples from each of the groups were immediately fixed in 10% neutral buffered formalin, then dehydrated and processed for paraffin sectioning. Sections were then deparaffinised and stained with hematoxylin and eosin. In addition; the diameter of tissue granuloma was determined by measuring the mean diameter (µm) for 30 granulomas were chosen from different sections and different mice in each group. |
Biochemical analysis
|
| Glutathione level |
| By using Ellman’s reagent glutathione (GSH) level was determined in the hepatic homogenates. This method is based on the reduction of Ellman’s reagent (5,5' dithiobis (2- nitrobenzoic acid) with GSH to produce a yellow compound. The chromogen is directly proportional to the GSH concentration and its absorbance was measured at 405 nm [28]. |
Nitrite/nitrate level
|
| According to the method of Green et al. [29]; the level of nitrite/nitrate was determined; where, nitrous acid diazotize sulfanilamide was formed in an acid medium and in the presence of nitrite. Then nitrous acid diazotize sulfanilamide is coupled with N-(1-naphthyl) ethylene diamine forming Azo dye (a bright reddish-purple color) and it can be measured at 540 nm. |
Malondialdehyde level
|
| In liver homogenate malondialdehyde (MDA) level was determined by using trichloroacetic acid (1 ml;10%) and of thiobarbituric acid (1 ml;0.67%).In a boiling water bath; the mixture was heated for 30 min. Thiobarbituric acid-reactive substances were measured at 535 nm [30]. |
Statistical analysis
|
| The statistical comparisons among the groups were carried out by using one-way ANOVA (Duncan’s test); (SPSS version 17.0). P<0.05 was considered as significant for all statistical analysis in this study. |
Results
|
| From the histological view; schistosomiasis resulted in a significant destructive lesions and distorted architecture of hepatic tissue. Distinct granulomatous inflammation and eosinophilic infiltration were noticed. In addition, S. mansoni induced parenchyma disorganization, cell vacuolization and liver necrosis as compared to non-infected group. Furthermore, treatment with SeNPs revealed improvement in the histological picture versus infected group (Figure 2). |
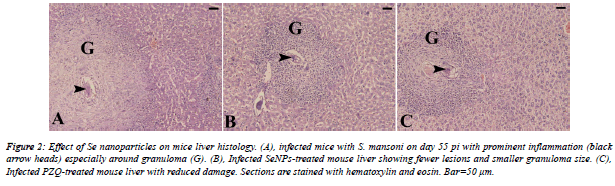 |
| In the same manner, SeNPs treatment (0.5 mg/kg b.wt.) for 7 successive days reduced the number of ova in hepatic tissue of infected mice significantly. Moreover, the granuloma size (granuloma diameter) recorded a significant reduction (400 ± 31 µm) and (370 ± 29 µm) as a result of SeNPs and PZQ injection, respectively versus schistosome-infected group (750 ± 55 µm) as shown in figure 3. |
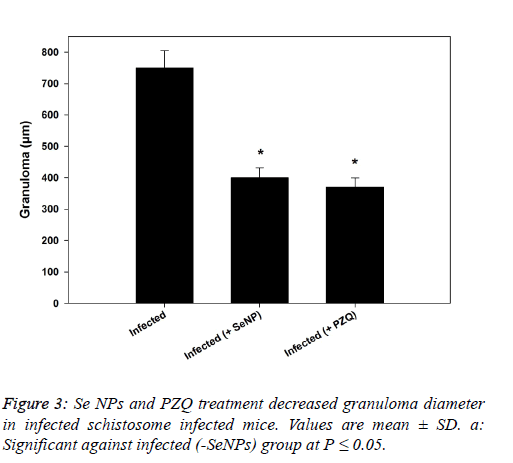 |
| The data presented in figure 4, revealed that the treatment of schistosomiasis by SeNPs and PZQ induced a significant reduction in eggs count in liver as compared to infected mice. |
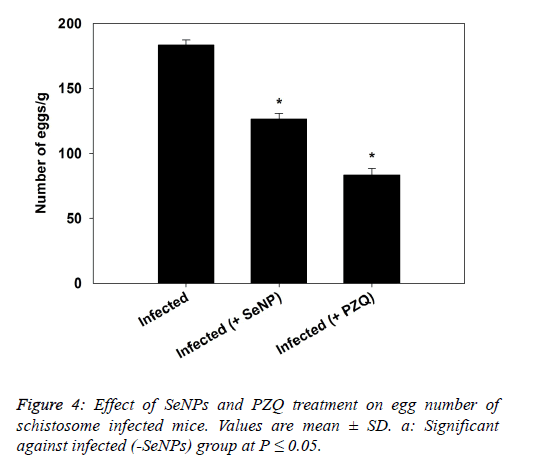 |
| Hepatic GSH level showed a significant reduction as a result of Schistosomiasis mansoni. On contrary, the infection induced a significant increment in both levels of nitrite/nitrate and MDA as compared to non-infected values. On the other hand, SeNPs treatment to the schistosome infected mice increased hepatic GSH level significantly and reduced nitrite/nitrate and MDA levels significantly versus infected group (Table1). |
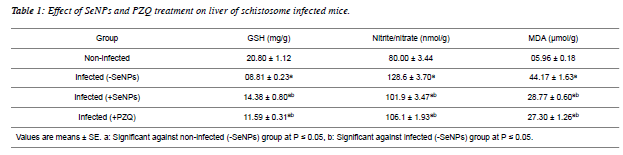 |
Discussion
|
| Ferrari et al. [31] cleared that in Schistosomiasis mansoni; there is a marked correlation between worm burden and disease severity in endemic areas. Moreover, severe hepatosplenic forms were developed in approximately 4% of untreated parasitized people. |
| Ferrari [32] studied the medical applications of nanotechnology and concluded that while the risks are very small, the potential benefits are huge in comparison. As a consequence; in toxoplasmosis an alternative treatment involved gold nanospheres. In addition, several nano sized delivery systems have already proved their effectiveness in animal models for the treatment and prophylaxis of malaria [33]. Likewise, the use of nanotechnology for treatment of leishmaniasis showed promising results and thus could be the pavement for curing this disease [34]. In addition, Soflaei et al. [35] revealed that selenium NPs and SeO2 have dosedependent anti-leishmanial activities. Also, selenium NPs have more anti-leishmanial activities with less cytotoxic effects than SeO2. |
| The liver pathology of S. mansoni revealed a significant distorted architecture and altered liver parenchyma. As well as, the parasite infection caused granulomatous inflammatory response; our histological findings go hand in hand with that of Amer et al. [36]; Kadry et al. [37] and Dkhil [38]. |
| Granulomas were remarkable by concentric fibrosis and many fibroblasts encircled the trapped eggs [36,37]. Moreover; Dkhil [38] reported that infection with S. mansoni caused a severe hepatic granulomatous inflammatory response which appears in form of inflammatory cellular infiltration, cytoplasmic vacuolation and degeneration of hepatocytes. The presence of huge number of granulomas resulted in disorganization of the hepatic strands and lobular structure where granulomas are surrounded by a cuff of aggregated lymphocytes, epitheloid cells, eosinophils and collagenous fibres. Also, the hepatic sinusoids were dilated and apparently contained more Kupffer cells. Meanwhile, our treatment with SeNPs improved all the histological disturbances of liver of infected mice. |
| From the present results and previous studies; Schistosma parasite induced a marked hepatic oxidative stress in schistoseme-infected mice [36,37,38,39]. Amer et al. [36] and Dkhil [38] deduced that S. mansoni altered the levels of free radicals and enzymatic/non-enzymatic antioxidands significantly. In the same manner, Fahmy et al. [39] reported that schistosome infected mice elevated the level of MDA, while decreased the GSH level and catalase activity significantly in hepatic tissue. A significant elevation was noticed in MDA and nitrite/nitrate levels, meanwhile; a significant reduction was tabulated in an antioxidant markers (GSH, glutathione reductase, catalase, thioredoxine reductase) of infected liver [37]. |
| SeNPs injection (0.5 mg/kg b.wt.) increased GSH level on contrary; it reduced the levels of nitrite/nitrate and MDA as compared to infected mice. Previous studies revealed that Se at nano-size can serve as antioxidant [40,41]; with lower toxic effects than Se [41,42,43]. |
| From our results we can conclude that the SeNPs (0.5 mg/kg body weight) injection for 7 sequent days caused ameliorating effects of hepatic disturbances, histopathology and oxidative stress. Ultimately, SeNPs have anti-schistosomal activities in hepatic tissue of schistosome infected mice. |
Conflict of interest
|
| The authors declare that they have no conflict of interest. |
Acknowledgement
|
| The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No (RG-198). |
|
|
References
- dos Santos AF, de Azevedo DP, Mata RS, de Mendonc DD, Sant’Ana AE. The lethality of Euphorbia conspicua to adults of Biomphalariaglabrata, cercaria of Schistosomamansoni and larvae of Artemiasalina. BioresourTechnol 2007; 98:135139.
- Allam G. Vasoactive intestinal peptide inhibits liver pathology in acute murine schistosomiasismansoni and modulates IL-10, IL-12 and TNF-a production. Immunobiol 2007; 212:603–612.
- Mahmoud MR, El-Abha HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosomamansoni infection in mice. J Ethnopharmacol 2002; 79:1-11.
- David JR, Vadas MA, Butterworth AE, De Brito PA, CarvalhoEM,etal.Enhancedhelminthotoxic capacity of eosinophils from patients with eosinophilia. N Engl J Med 1980; 303:1147-1152.
- Chang D, Ramalho LN, Ramalho FS, Martinelli AL, Zucoloto S. Hepatic stellate cells in human schistosomiasismansoni: A comparative immunohistochemical study with liver cirrhosis. ActaTropica 2006; 97:318–323.
- Andrade Z. Schistosomiasis and liver fibrosis: Review Article. ParasitolImmunol 2009; 31:656-663.
- Boloukhere M, Baldo-Correa E, Borojevic R. Experimental schistosomiasismansoni: characterization of connective tissue cells in hepatic periovular granulomas. J SubmicroscopCytolPathol 1993; 25:505–517.
- Brito JM, Borojevic R. Liver granulomas in schistosomiasis: mast cell-dependent induction of SCF expression in hepatic stellate cells is mediated by TNF-ß. J LeukocBiol 1997; 62:389–396.
- Seubert J, Pohlke R, Loebich F. Synthesis and properties of Praziquantel, a novel broad spectrum anthelmintic with excellent activity against Schistosomes and Cestodes. Experientia 1977; 33: 1036-1037.
- Magnussen P. Treatment and re-treatment strategies for schistosomiasis control in different epidemiological settings: a review of 10 years’ experiences. Acta Trop 2003; 86:243-254.
- Cioli D, Pica-Mattoccia L, Archer S. Drug resistance in schistosomes. Parasitol Today 1993; 9:162–166.
- Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosomamansoni in mice is drug specific. Am J Trop Med Hyg 1994; 51:83-88.
- Ismail M, Botros S, Metwally A, William S, Farghally A, et al. Resistance to praziquantel: direct evidence from Schistosomamansoni isolated from Egyptian villagers. Am J Trop Med Hyg 1999; 60:932- 935.
- Zhang SM, Coultas KA. Identification and characterization of five transcription factors that are associated with evolutionarily conserved immune signaling pathways in the schistosome-transmitting snail Biomphalariaglabrata. MolImmunol 2011; 48:1868–1881.
- Roduner E. Size matters: Why nanomaterials are different. ChemSoc Rev 2006; 35:583-592.
- Jia L. Nanoparticle Formulation Increases Oral Bioavailability of Poorly Soluble Drugs: Approaches Experimental Evidences and Theory. CurrNanosci 2005; 1:237-243.
- Ambrogio MW, Thomas CR, Zhao YL, Zink JI, Stoddart JF. Mechanized silica nanoparticles: a new frontier in the ranosticnanomedicine. AccChem Res 2011; 44: 903-913.
- Armstead AL, Li B. Nanomedicine as an emerging approach against intracellular pathogens. Int J Nanomedicine 2011; 6:3281-3293.
- Nnamani PO, Scoles G, Krol S. Preliminary characterization of N-trimethylchitosan as a nanocarrier for malaria vaccin. J Vector Borne Dis 2011; 48:224-230.
- Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BP, Nakamura CV. Recent advances in leishmaniasis treatment. Int J Infect Dis 2011; 15:e525-e532.
- Y Wang, X Yan, L Fu. Effect of selenium nanoparticles with different sizes in primary cultured intestinal epithelial cells of crucian carp, Carassiusauratusgibelio. Int J Nanomedicine. 2013; 8: 4007–4013.
- Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. Biofactors 2001; 15:27–38.
- Chen T, Wong YS, Zheng W, Bai Y, Huang L. Selenium nanoparticles fabricated in Undariapinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf B 2008; 67:26–31.
- Dwivedi C, Shah CP, Singh K, Kumar M, Bajaj PN. An organic acid-induced synthesis and characterization of selenium nanoparticles. J Nanotechnol 2011; 1-6.
- Oliver L, Stirewalt MA. An efficient method for the exposure of mice to cercaria of Schistosomamansoni. J Parasitol 1952; 38:19–23.
- Hassanin KM, Abd El-Kawi SH, Hashem KS. The prospective protective effect of selenium nanoparticles against chromium-induced oxidative and cellular damage in rat thyroid. Int J Nanomed 2013; 8:1713–1720.
- Pelligrino J, Oliveria CA, Faria JA, Cunha AC. New approach to the screening of drugs in experimental Schistosomamansoni in mice. Am J Trop Med Hyg 1962; 11:201–215.
- Ellman GL. Tissue sulfhydryl groups. Arch BiochemBiophys 1959; 82:70–77.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982; 126:131-138.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95:351–358.
- Ferrari ML, Coelho PM, Antunes CM, Tavares CA, da Cunha AS. Efficacy of oxamniquine and praziquantel in the treatment of Schistosomamansoni infection: a controlled trial. Bull WHO 2003; 81:190–196.
- Ferrari M. Symposium on: Nanotechnology for Cancer Prevention, Diagnosis and Treatment. May 3-7, 2009; George R. Brown Convention Center, Houston, Texas, USA.
- 33. El-Tonsy MM. Nanotechnology and Nanomedicine Applications in Parasitic Diseases. PUJ 2010; 3:19-26.
- Goura JK, Srivastavaa A, Kumara V, Bajpai S, Kumar H. Nanomedicine and Leishmaniasis: Future prospects. Digest J NanomatBiostr 2009; 4: 495-499.
- Soflaei S, Dalimi A, Abdoli A, Kamali M, Nasiri V, et al. Anti-leishmanial activities of selenium nanoparticles and selenium dioxide on Leishmaniainfantum. Comp ClinPathol 2014; 23:15–20.
- Amer OS, Dkhil MA, Al-Quraishy S. Antischistosomal and Hepatoprotective Activity of Morus alba Leaves Extract. Pak J Zool 2013; 45: 387-393.
- Kadry SM, Mohamed AM, Farrag EM, Fayed DB. Influence of some micronutrients and Citharexylum quadrangular extract against liver fibrosis in Schistosomamansoni infected mice. Afri J PharmaPharmacol 2013; 7: 2628-2638.
- Dkhil MA. Role of berberine in ameliorating Schistosomamansoni-induced hepatic injury in mice. Biol Res 2014; 47: 1-7.
- Fahmy SR, Rabia I, Mansour EM. The potential role of mefloquine against Schistosomamansoni infection by prohibition of hepatic oxidative stress in mice. J Basic ApplZool 2014; 67:40-47.
- Huang B, Zhang J, Hou J, Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free RadicBiol Med 2003; 35:805-813.
- Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free RadicBiol Med 2007; 42:1524-1533.
- Zhang JH, Yan X, Zhang L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci 2005; 76:1099-1109.
- Shakibaie M, Khorramizadeh MR, Faramarzi MR, Sabzevari O, Shahverdi AR. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. BiotechnolApplBiochem 2010; 56:7 -15.
|




